Insulin resistance is a growing and significant health problem strongly associated with obesity, dyslipidaemia and type 2 diabetes mellitus. An increase in the consumption of high-sugar diets, especially of fructose, correlates positively with the development of hyperinsulinaemia, insulin resistance, hypertension and hypertriacylglycerolaemia(Reference Gerrits and Tsalikian1). Emerging evidence clearly indicates that chronic feeding of a fructose-enriched diet markedly alters insulin signal transduction involving receptor autophosphorylation and tyrosine kinase activity, and results in post-receptor defects at the level of phosphorylation substrates or effector molecules such as GLUT4, leading to increased blood circulation of glucose(Reference Chidambaram and Carani Venkatraman2, Reference Qin, Polansky and Harry3). Recently, it has been reported that long-term high-fructose feeding is associated with a decrease in plasma adiponectin levels and defects in adiponectin receptor and AMP-activated protein kinase (AMPK) expression in skeletal muscles and adipocytes, leading to impaired GLUT4 translocation to the plasma membrane(Reference Havel4, Reference Bonnard, Durand and Vidal5). In addition, impairment in adiponectin and AMPK signalling has also been shown to contribute to alterations in glucose metabolism and impairment of insulin sensitivity(Reference Kadowaki and Yamauchi6).
Recently, it has been found that the consumption of plant foods has been associated with a reduced risk of developing chronic diseases such as diabetes mellitus and CVD. In the prevention or treatment of a variety of diseases, grape seed extract (GSE) has been shown to have various beneficial pharmacological effects, including anti-hyperlipidaemic(Reference Moreno, Ilic and Poulev7), anti-inflammatory(Reference Terra, Montagut and Bustos8) and antibacterial activities(Reference Mayer, Stecher and Wuerzner9). GSE and its bioactive compounds exhibit favourable anti-diabetic effects by stimulating glucose uptake in insulin-sensitive cell lines(Reference Pinent, Blay and Bladé10), inhibiting intestinal α-glucosidases, pancreatic α-amylase activities and the process of glycation(Reference Adisakwattana, Jiphimai and Prutanopajai11). A recent study has clearly shown that oligomeric procyanidins in GSE stimulate glucose uptake related to Akt, p44/42 and p38 mitogen-activated protein kinase function, which differs from insulin activation and results in differences in downstream signalling(Reference Montagut, Onnockx and Vaqué12). Our previous study demonstrated that GSE improved glucose tolerance and prevented insulin resistance in high-fructose diet (HF)-induced diabetic rats. In addition, GSE significantly increased the activities of hepatic superoxide dismutase and catalase and significantly suppressed lipid peroxidation in HF-fed rats(Reference Suwannaphet, Meeprom and Yibchok-Anun13). On the basis of these findings, it can be speculated that GSE may improve insulin sensitivity by regulating the insulin signalling or adiponectin signalling pathway in HF-induced diabetic rats. To reveal the underlying mechanisms at the molecular level, the present study was undertaken to investigate the protein expression of the insulin signalling pathway and the mRNA expression of the adiponectin signalling pathway in high-fructose-fed rats.
Materials and methods
Chemicals
o-Dianisidine dihydrochloride and peroxidase-glucose oxidase (PGO) enzymes were purchased from Sigma-Aldrich Company (St Louis, MO, USA). Cholesterol, TAG and HDL-cholesterol kits were obtained from Human Diagnostics (Wiesbaden, Germany). Anti-insulin receptor-β (IRβ), anti-insulin receptor substrate-1 (IRS-1), anti-Akt, anti-GLUT4 and anti-IgG horseradish peroxidase-linked antibodies were purchased from Cell Signaling Technology (Beverly, MA, USA). TRIzol reagent and DNase1 were obtained from Invitrogen (Carlsbad, CA, USA) and Promega (Madison, WI, USA), respectively. All PCR primers and oligo dT primers were obtained from Pacific Science (Anaheim, CA, USA). The Rat Adiponectin ELISA kit was purchased from Adipogen (Incheon, Korea). All other chemical reagents used in the present study were of analytical grade.
Preparation of grape seed extract
The extraction and phytochemical analysis of grape seeds were performed according to a previously established method(Reference Saito, Hosoyama and Ariga14). The chemical composition of GSE included 50·1 % total flavanols, 49·08 % procyanidins and 1·02 % monomeric flavanols.
Animals and experimental design
Male Wistar rats (180–200 g) were obtained from the National Laboratory Animal Center, Mahidol University, Salaya, Thailand. All animal experiments were conducted according to the ethical guidelines outlined in the Guide for Care and Use of Laboratory Animals. Animal facilities and protocols were approved by the Laboratory Animal Care and Use Committee at the Faculty of Veterinary Science, Chulalongkorn University, Thailand. Wistar rats were housed in individual, stainless-steel cages in a room maintained at 25 ± 1°C on a 12 h light–12 h dark cycle. They were fed standard laboratory chow with water ad libitum and fasted overnight before the experiments.
Rats were randomly divided into four groups of six animals each. As shown in Tables 1 and 2, the composition of the normal diet (ND; American Institute of Nutrition-93 diet) and the HF was prepared following reported procedures(Reference Guo, Ling and Wang15). For 8 weeks, groups 1 and 2 received the ND and HF, respectively, whereas groups 3 and 4 were fed the HF supplemented with 0·5 and 1·0 % GSE, respectively. Body weight was monitored weekly. At the end of the experiment, the rats were fasted overnight before blood sampling. Blood samples were obtained from the tail vein and collected in tubes with or without heparin-containing solution. These samples were immediately centrifuged (2500 g), and the plasma was separated and frozen at − 20°C until analysed for blood chemistry. The animals were euthanised; the soleus muscle was immediately removed, frozen in liquid N2 and stored at − 70°C.
Table 1 Composition of the experimental diets (g/kg diet)
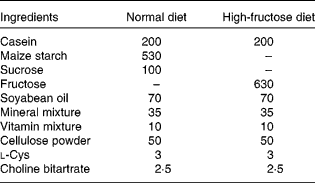
Table 2 Fructosamine, lipid profiles, and kidney and liver function parameters in rats at the end of the experimental periods (8 weeks)
(Mean values with their standard errors, n 6)

ND, normal diet; HF, high-fructose diet; GSE, grape seed extract; ALT, alanine transaminase; AST, aspartate transaminase; ALP, alkaline phosphatase.
* Mean values were significantly different from those of the ND group (P < 0·05).
† Mean values were significantly different from those of the HF group (P < 0·05).
Biochemical analysis
Fructosamine was determined using the nitroblue tetrazolium assay. Plasma adiponectin levels were determined by ELISA. Total serum cholesterol, TAG and HDL-cholesterol levels were assayed by using enzymatic methods. Blood urea N, creatinine, and the activities of alanine and aspartate transaminases (AST) and alkaline phosphatase were determined using an autoanalyser at Service Unit, Faculty of Allied Health Sciences, Chulalongkorn University.
Tissue homogenisation
The frozen soleus muscles were cut into small pieces (0·2 g) and homogenised in 0·8 ml homogenising buffer containing 250 mm-sucrose, 20 mm-Tris (pH 7·5), 2 mm-EDTA, 0·5 mm-ethylene glycol tetraacetic acid, leupeptin (20 μg/ml), aprotinin (10 μg/ml), phenylmethylsulfonyl fluoride (174·2 μg/ml), 20 mm-dithiothreitol and 5 % Triton X-100. The homogenate was then centrifuged at 10 000 g for 10 min at 4°C. The Bradford assay was used to determine the protein concentration of the supernatant, which was then stored at − 80°C until subjected to Western blot analysis(Reference Liu, Tzeng and Liou16).
Western blot analysis
For detection of anti-IRβ, muscle homogenates containing protein (5 μg/μl) were subjected to immunoprecipitation with an anti-IRβ subunit antibody at 4°C overnight, followed by shaking with protein A Sepharose beads according to the manufacturer's instructions. The protein (35 μg) was separated by 10 % SDS-PAGE at a constant 70 and 130 V for the stacking and separating phases, respectively, and transferred by electroblotting onto a PolyScreen polyvinylidene fluoride transfer membrane using a semi-dry transfer cell (Bio-Rad, Hercules, CA, USA) at a constant 100 V for 2 h at 4°C (Laemmli, 1970). The membrane was then treated sequentially with blocking solution (Tris-buffered saline Tween-20 containing 5 % non-fat, skimmed milk). After three 5 min washes in Tris-buffered saline Tween-20, the membrane was incubated with the primary antibody against IRβ at 4°C overnight, washed and then incubated with the horseradish peroxidase-linked secondary antibody at room temperature for 1 h. After washing, the blotted proteins were visualised using the enhanced chemiluminescence detection system.
For the detection of anti-IRS-1, anti-Akt and anti-GLUT, proteins (35 μg) were prepared from muscle homogenates, subjected to SDS-PAGE, transferred to a polyvinylidene fluoride membrane as described earlier and blotted with anti-IRS-1, anti-Akt or anti-GLUT4 antibodies according to the manufacturer's instructions at 4°C overnight, washed and then incubated with the horseradish peroxidase-linked secondary antibody at room temperature for 1 h. After washing, the blotted proteins were visualised using enhanced chemiluminescence. Band intensities were quantified by the Bio-Rad ChemiDoc XRS Gel Documentation system(Reference Liu, Tzeng and Liou16). The mean value for samples from the ND group on each immunoblot, expressed in densitometry units, was adjusted to a value of 1·0. All experimental sample values are then expressed relative to this adjusted mean value.
Total RNA extraction and RT-PCR
Total RNA from the soleus muscle was isolated with TRIzol reagent according to the manufacturer's directions. The concentration and purity of the RNA extracted were checked by measuring the absorbance at 260 and 280 nm. Total RNA (1 μg/μl) was incubated with 10 μm-Oligo dT primer at 70°C for 5 min and then reverse transcribed to Complementary DNA in a reaction mixture of 1 × ImProm-II reaction buffer, RNase inhibitor (1 U/μl), 0·5 mm-dNTP Mix and ImProm-II RT at 42°C for 60 min. The mixture was heated at 70°C for 15 min to terminate the reaction. PCR was performed in a mixture (20 μl) containing 2·5 μl of 10 × Taq buffer, 5 μl reverse-transcribed template solution, 1 mm-MgCl2, 10 μm each of sense and antisense primers, 0·2 mm-dNTP mix and 1·25 U Taq DNA polymerase.
The specific primer sequences were as follows: hypoxanthine guanine phosphoribosyl transferase (Hprt; GenBank accession no. NM_012583.2), 5′-CAG TCC CAG CGT CGT GAT TA-3′ (sense) and 3′-AGC AAG TCT TTC AGT CCT GTC-5′ (antisense); adiponectin (GenBank accession no. NM_144744.2), 5′-AAT CCT GCC CAG TCA TGA AG-3′ (sense) and 3′-CAT CTC CTG GGT CAC CCT TA-5′ (antisense); adiponectin receptor R1 (AdipoR1; GenBank accession no. NM_207587.1), 5′-CTT CTA CTG CTC CCC ACA GC-3′ (sense) and 3′-TCC CAG GAA CAC TCC TGC TC-5′ (antisense); AMPK-α1 (GenBank accession no. NM_019142.1), 5′-TGT GAC AAG CAC ATT TTC CAA-3′ (sense) and 3′-CCG ATC TCT GTG GAG TAG CAG-5′ (antisense); glycogen synthase 1 (GS1; GenBank accession no. NM_001109615.1), 5′-TTT TCC TTG GTC CAG TGT CC-3′ (sense) and 3′-CGG TGG GCT CAC TTT TGT AT-5′ (antisense); glycogen synthase kinase-3 (GSK-3α; GenBank accession no. NM_017344.1), 5′-ACC TAC CCT CAC TAA CTC TTC CTG-3′ (sense) and 3′-GTA GAA GGT CCT CAT ACC CCA AAC-5′ (antisense). Taq DNA polymerase was activated by a 95°C incubation step for 3 min. After an initial denaturation at 95°C for 60 s, reactions were performed as follows: for Hprt detection, 60°C for 30 s, thirty-five cycles (139 bp); for adiponectin detection, 60°C for 30 s, thirty-five cycles (215 bp); for adiponectin receptor 1 detection, 60°C for 30 s, thirty-five cycles (139 bp); for AMPK-α1 detection, 55°C for 30 s, thirty-five cycles (134 bp); for GS1 detection, 57°C for 30 s, thirty-five cycles (190 bp); for GSK-3α, 57°C for 30 s, thirty-five cycles (266 bp). The final extension was performed at 72°C for 1 min. The products were electrophoresed on a 2 % agarose gel. After the gel was stained with ethidium bromide, the relative density of the bands was measured using the Bio-Rad ChemiDoc XRS Gel Documentation system and Bio-Rad Quantity One 1-D Analysis software. Relative quantification of the PCR products was done after normalisation to the amount of Hprt mRNA.
Glycogen accumulation
The frozen soleus muscles were homogenised in ice-cold 0·6 m-HClO4. The mixture was immediately centrifuged at 3000 g and analysed for the determination of free glucose in the tissue by the glucose oxidase method. Amyloglucosidase solution (10 U/ml) in 0·2 m-sodium acetate buffer (pH 4·8) was then mixed and incubated in the mixture at 40°C for 2 h. After incubation, the pH of the mixture was adjusted to 7 and assayed for the determination of total glucose. Free glucose was subtracted from total glucose to obtain glycogen content. Glycogen concentration is expressed as mg/g wet tissue(Reference Adisakwattana, Roengsamran and Hsu17).
Statistical analysis
Data are expressed as means with their standard errors of the mean for animals under each diet. Statistical significance was evaluated using one-way ANOVA followed by Duncan's multiple range test. A P value < 0·05 was considered statistically significant.
Results
Biochemical parameters
Rats fed the HF alone had a markedly elevated plasma fructosamine level. Moreover, the plasma fructosamine level was significantly reduced in the HF groups supplemented with 0·5 and 1·0 % GSE. Furthermore, plasma adiponectin concentration was reduced by 40 % in the HF group, compared with that in the ND group. However, its concentration in rats fed the HF with 0·5 and 1·0 % GSE was increased by approximately 42 and 32 %, respectively. There was a significant (71 %) increase in the serum TAG level of the HF group compared with the ND group, whereas the GSE-fed groups (0·5 and 1·0 %) had serum TAG levels that were reduced by 23·0 and 25·0 %, respectively, relative to the HF group. Fructose feeding caused a significant increase of 15 % in the total cholesterol level. However, there was no significant difference in total cholesterol levels between the HF group and the HF groups supplemented with GSE. No change in HDL-cholesterol levels, alanine transminase and alkaline phosphatase was observed among the groups at the end of the experiments. Meanwhile, the GSE treatment significantly decreased the AST levels in HF rats supplemented with 0·5 and 1·0 % GSE, compared with the HF group.
Pathway-related proteins in the skeletal muscle
The expression of proteins in the insulin-signalling pathway for skeletal muscle was measured for IR, IRS-1, Akt and GLUT4. As shown in Fig. 1, HF rats had impaired protein expression for the skeletal muscle insulin signalling pathway as shown by the decrease in the expression of IR by 54 %, IRS-1 by 41 %, Akt by 52 % and GLUT4 by 56 %. After 4 weeks of GSE supplementation, there was no increase in the protein expression of IR and IRS-1 among the HF group and the HF ones supplemented with GSE. The Akt protein expression for HF rats supplemented with 0·5 and 1·0 % GSE increased 1·41- and 1·47-fold when compared with that of the HF group. The HF group supplemented with 0·5 % GSE was slightly increased for GLUT4 protein expression. Moreover, the GLUT4 protein content for the HF group supplemented with 1 % GSE was significantly increased by 1·82-fold when compared with that of the HF group.

Fig. 1 Effect of grape seed extract (GSE) on the protein expression of (a) insulin receptor, (b) insulin receptor substrate 1, (c) Akt and (d) GLUT4 in the soleus muscle isolated from rats after 8 weeks of the dietary experiment. Values are means, with their standard errors represented by vertical bars (n 6). * Mean values were significantly different from those of the normal diet (ND) group (P <0·05). † Mean values were significantly different from those of the high-fructose diet (HF) group (P <0·05).
Pathway-related mRNA expression in the skeletal muscle
The mRNA expression levels of adiponectin, AdipoR1 and AMPK-α1 in the soleus muscle are shown in Fig. 2. After being normalised to Hprt1, the relative mRNA level of adiponectin in the HF group was significantly decreased by 50 % when compared with that in the ND group. GSE supplementation (0·5 and 1·0 %) significantly ameliorated the changes in the mRNA levels of adiponectin, with the levels close to those of the ND groups. The HF feeding also decreased the skeletal muscle mRNA expression of AdipoR1 by 30 %, and supplementation with GSE (0·5 and 1·0 %) enhanced this expression by 26 % compared with the HF group. Moreover, HF feeding also suppressed the mRNA expression of AMPK-α1 by 22 % when compared with the ND. For the HF groups supplemented with 0·5 and 1·0 % GSE, this expression was increased by 34 and 39 %, respectively, relative to the HF group.

Fig. 2 Effect of grape seed extract (GSE) on the mRNA expression of (a) adiponectin, (b) adiponectin receptor R1 (AdipoR1) and (c) AMP-activated protein kinase-α (AMPK-α) in the soleus muscle isolated from rats after 8 weeks of the experiment. Values are means, with their standard errors represented by vertical bars (n 6). * Mean values were significantly different from those of the normal diet (ND) group (P <0·05). † Mean values were significantly different from those of the high-fructose diet (HF) group (P <0·05). Hprt, hypoxanthine guanine phosphoribosyl transferase.
Glycogen synthesis
As shown in Fig. 3, the mRNA expression of GS1 was lower in the HF group than in the ND group, whereas the supplementation of GSE significantly increased the mRNA levels of GS1 in the HF group. There was no significant difference between the mRNA expression of GSK3α in the ND group and the HF group. The HF groups supplemented with GSE exhibited significantly lower mRNA expression of GSK3α when compared with the HF group. In the skeletal muscle, glycogen accumulation in the HF group was lower than that in the ND group by 19 %, whereas the HF supplemented with 1·0 % GSE showed a significant increase in glycogen accumulation by 35 %.

Fig. 3 Effect of grape seed extract (GSE) on the mRNA expression of (a) glycogen synthase-1 (GS1), (b) glycogen synthase kinase-3 (GSK3-α) and (c) glycogen accumulation in the soleus muscle isolated from rats after 8 weeks of the experiment. Values are means, with their standard errors represented by vertical bars (n 6). * Mean values were significantly different from those of the normal diet (ND) group (P <0·05). † Mean values were significantly different from those of the high-fructose diet (HF) group (P <0·05). Hprt, hypoxanthine guanine phosphoribosyl transferase.
Discussion
We have recently shown that supplementing the diet of HF rats with GSE attenuated hyperglycaemia and hyperinsulinaemia and improved insulin sensitivity(Reference Suwannaphet, Meeprom and Yibchok-Anun13). In that study, 8 weeks after the diet, the body weight had significantly decreased in rats on an HF with 0·5 % and 1·0 % GSE, relative to that of HF rats. Plasma glucose and plasma insulin significantly increased in these rats; however, 1·0 % GSE resulted in a significant decrease in these levels. Decreased HOMA-IR scores and incremental AUC for glucose were also observed in HF rats supplemented with GSE, compared with untreated HF rats(Reference Suwannaphet, Meeprom and Yibchok-Anun13). Chronic high-fructose feeding in experimental animals has been reported to produce hypertriacylglycerolaemia by increasing the rate of de novo lipogenesis and TAG synthesis(Reference Basciano, Federico and Adeli18). Increased delivery of TAG to the skeletal muscle interferes with glucose utilisation, ultimately leading to hyperglycaemia and hyperinsulinaemia. The results of the present study indicate that GSE might have improved insulin resistance in rats by preventing HF-induced hypertriacylglycerolaemia.
Amadori products such as fructosamine result from the reaction between the carbonyl group of a glucose or fructose and the amino group of a protein to form a Schiff base(Reference Ahmed19). Thereafter, fructosamine undergoes further protein cross-linking reactions to form advanced glycation end products, which play an important role in diabetic complications through a series of pathological changes such as decreased insulin binding to its receptors(Reference Takagi, Kashiwagi and Tanaka20). Recent findings have revealed that advanced glycation end products directly induce the impairment of insulin action in the muscle by mediating the formation of a multi-molecular complex that consists of receptor for advanced glycation end products (RAGE)/IRS-1 Src and protein kinase C-α (PKC-α)(Reference Cassese, Esposito and Fiory21). Generally, fructosamine levels are used to access glycaemic control since they can indicate the accumulation of early (Amadori) glycation products in diabetic patients. The present findings demonstrate that plasma fructosamine was markedly increased in the HF group but decreased in the HF groups supplemented with GSE. Recently, GSE has been shown to inhibit fructose-mediated non-enzymatic glycation in vitro (Reference Adisakwattana, Jiphimai and Prutanopajai11). The results from the previous study support that the fructosamine-lowering effect may account for the anti-glycation activity of GSE. The inhibitory effect of GSE might decrease advanced glycation end product formation and thereby prevent the progression of diabetic complications.
Creatinine is a product of phosphocreatine degradation and is excreted in the urine. Increased plasma creatinine levels indicate the inability of kidneys to eliminate protein metabolic by-products. In the present study, there was no significant change in creatinine and blood urea N levels in the HF group, indicating that renal function was unaffected. Changes in liver function parameters reflect hepatocellular damage. In the present study, alanine transaminase and alkaline phosphatase levels were not altered by the HF, indicating that hepatic function in the HF group remained normal. AST levels tended to be higher in the HF group, but it was not significant when compared with those in the ND group. However, GSE significantly lowered AST levels in the supplemented HF groups relative to the untreated HF group. Several studies have described chemical components in GSE that can reverse alanine transaminase and AST alterations in hepatic injury by reducing oxidative stress(Reference Dulundu, Ozel and Topaloglu22, Reference Khoshbaten, Aliasgarzadeh and Masnadi23). Although the possible AST-lowering mechanisms of GSE in high-fructose-fed rats remain unclear, we suggest that it is possibly related to components having antioxidant activity.
Insulin resistance represents a decrease in the insulin responsiveness of target tissues, namely skeletal muscle, adipose tissue and the liver. Chronic HF feeding directly impairs insulin action on peripheral glucose uptake by reducing the expression of insulin-stimulated proteins such as IR, IRS-1, Akt and GLUT4(Reference Tsai, Wu and Hwang24). We have found that excess fructose interferes with insulin action by altering the levels of these proteins. These results are in good agreement with other studies(Reference Tsai, Wu and Hwang24, Reference Cao, Hininger-Favier and Kelly25). In general, insulin activates its specific receptor, which leads to the activation of IRS-1 and its increased association with phosphoinositide 3-kinase (PI3K), resulting in increased PI3K activity and the activation of protein kinase B or Akt. Finally, the pathway stimulates GLUT4 translocation to the plasma membrane and results in enhanced glucose uptake into the cells(Reference Musi and Goodyear26). The present study showed that GSE improves Akt and GLUT4 expression in the skeletal muscle of HF rats. This leads to improved insulin sensitivity and hyperglycaemia, providing strong evidence for the potential beneficial effects of GSE in diabetic patients.
Adiponectin, a hormone secreted by adipocytes, plays a protective role against insulin resistance by regulating glucose and lipid metabolism(Reference Lindsay, Funahashi and Hanson27). Circulating adiponectin acts primarily in muscle by binding to AdipoR1(Reference Yamauchi, Kamon and Ito28), which activates AMPK-α. AMPK-α plays an important role in the regulation of cellular energy levels and elicits glucose uptake by increasing GLUT4 translocation(Reference Long and Zierath29). The reduction in plasma adiponectin levels has been reported to be associated with impaired insulin sensitivity(Reference Stefan, Vozarova and Funahashi30). The present results are in accordance with other reports of decreased plasma adiponectin levels after an HF(Reference Shih, Lin and Lin31, Reference Qin, Polansky and Anderson32), whereas it was markedly corrected by GSE. On the basis of a previous study, we suggest that the improvement in whole-body insulin sensitivity and glycaemic control in HF rats by GSE positively correlates with a rise in plasma adiponectin level(Reference Suwannaphet, Meeprom and Yibchok-Anun13). Chronic fat and fructose consumption is associated with defects in adiponectin receptor expression in the skeletal muscle, which could contribute to the alterations in glucose metabolism(Reference Bonnard, Durand and Vidal5). The present results are concordant with reports describing that high consumption of fructose markedly impairs adiponectin receptor expression(Reference Bonnard, Durand and Vidal5). We found that GSE restores the mRNA expression of AdipoR1 and AMPK-α in HF rats. The increased AdipoR1 expression might enhance adiponectin-binding capacity to muscle cells and increase adiponectin effects. Furthermore, the up-regulation of AMPK-α by GSE is likely to promote the effect of insulin-independent stimuli for glucose uptake by activating translocation of GLUT4 to the plasma membrane. Until recently, adipocytes were thought to be the only site for adiponectin synthesis and secretion. However, several studies have now discovered that skeletal muscle cells can synthesise and secrete adiponectin, which then exerts local metabolic effects, at least in part, via elevated glucose uptake(Reference Delaigle, Senou and Guiot33–Reference Liu, Chewchuk and Lavigne35). There is now considerable evidence that a decrease in local adiponectin expression could be at least partly responsible for skeletal muscle insulin resistance(Reference Liu, Chewchuk and Lavigne35). The results demonstrated that skeletal muscle adiponectin production is altered in HF rats, and that these changes can be corrected by GSE, suggesting that increased muscle adiponectin expression might partially enhance insulin sensitivity in HF rats.
Skeletal muscle glycogen synthesis is important for blood glucose homeostasis. The ability of insulin to increase glycogen accumulation in muscle involves the stimulation of GLUT and the activation of glycogen synthase(Reference Lai, Stuenaes and Kuo36). The principal step by which insulin stimulates glycogen synthase is via the activation of the IR, which leads to the PI3K-dependent activation of protein kinase B/Akt, inactivation of GSK-3 and increased glycogen synthase (GS) activity(Reference Musi and Goodyear26). A reduction in GSK-3α expression has been shown to improve insulin action by stimulating glucose uptake and glycogen synthesis(Reference Ciaraldi, Nikoulina and Bandukwala37). In contrast, elevation of GSK3α generates impaired insulin responsiveness and insulin resistance in the skeletal muscle(Reference Ciaraldi, Nikoulina and Bandukwala37). In the present study, GSE suppressed the mRNA expression of GSK3α and the elevated mRNA expression of GS1, in agreement with the increased glycogen accumulation and resulting reduction of plasma glucose levels in HF rats.
However, the detailed mechanisms for the induction of impairment in HF rats remain unclear. It is possible that an HF may induce the formation of reactive oxygen species associated with an increase in oxidative stress, which has often been implicated in the pathology of insulin resistance(Reference Faure, Rossini and Lafond38). Prolonged oxidative stress can affect the resistance of skeletal muscle insulin signalling, glucose transport and glycogen synthesis(Reference Dokken, Saengsirisuwan and Kim39, Reference Archuleta, Lemieux and Saengsirisuwan40). Increased oxidative stress also inhibits the expression of adiponectin(Reference Furukawa, Fujita and Shimabukuro41). In the present study, GSE was found to comprise procyanidins and monomeric flavanols such as (+)-catechin and ( − )-epicatechin, which have potent antioxidant activities(Reference Yilmaz and Toledo42–Reference Shi, Yu and Pohorly44). Thus, we suggest that these active principles might be acting as antioxidants and helping to prevent the decreased expression of post-receptor-related proteins such as Akt and GLUT4 and of the corresponding mRNA.
Taken together, these results suggest that GSE ameliorates defective insulin and adiponectin signalling pathways in the skeletal muscle of HF rats. Moreover, the present study provided evidence for the molecular mechanisms by which GSE prevents high-fructose-induced insulin resistance.
Acknowledgements
The present study was financially supported by the 90th Anniversary of Chulalongkorn University Fund (Ratchadaphiseksomphot Endowment Fund). The authors would like to thank the Medical Food Research and Development Center, which has been financially and institutionally supported by the Chulalongkorn University. The authors' contributions were as follows: A. M., S. Y.-a., S. A. developed the concept and designed the study. In particular, A. M., W. So., W. Su. performed the study, collected and analysed the data; S. A. wrote the manuscript. The authors declare that they have no conflicts of interest.






