Bronchopulmonary dysplasia (BPD) is still a frequent complication in infants born very prematurelyReference Gregoire, Lefebvre and Glorieux1. Factors that are known to influence the metabolic rate in the preterm neonate include illness, activity, composition of food and thermal environmentReference Romera, Figueras, Rodriguez-Miguelez, Ortega and Jimenez2. Preterm neonates with neither intrauterine growth retardation nor BPD usually show catch-up growth before the age of 2 years. In later childhood, preterm children without BPD have normal nutritional status and normal resting energy expenditure (REE) even if a part of preterm still demonstrated positive metacholine provocation testReference Halvorsen, Skadberg, Eide, Hoksund, Carisen and Bakko3. Conversely, children with BPD often suffer from undernutrition and several studies have demonstrated higher REE in infants with BPD compared with controls, suggesting that this could be one factor in the development of the malnutritionReference Wahlig, Gatto, Boros, Mammel, Mills and Georgieff4–Reference Abrams6. A variety of studies have attempted to answer the question as to whether or not preterm infants with chronic lung disease have higher rates of energy expenditureReference Shennan, Dunn, Ohlsson, Lennox and Hoskins7–Reference Bauer, Maier, Muehlbauer, Poeschl and Linderkamp9. From the data presented, it is still difficult to come to a definitive conclusion about the elevation of energy expenditure in these infants. The purpose of this study was to determine whether or not the pulmonary status of children who had BPD presenting in the neonatal period is associated with a higher REE and poor nutritional status at a later age.
Patients and methods
Subjects included in this study were seventy-one children aged 4–8 years with prematurely-associated BPD (34 boys and 37 girls) and thirty healthy children (20 boys and 10 girls). The characteristics of the subjects are reported in Table 1. Neonatal characteristics of children with history of BPD are summarized in Table 2.
Table 1 Anthropometric characteristics of the children
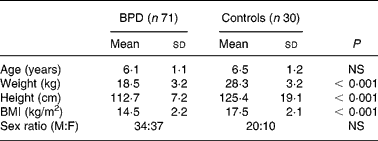
BPD, bronchopulmonary dysplasia; M, male; F, female
Table 2 Neonatal characteristics of children with bronchopulmonary dysplasia (BPD)

PMA, post-menstrual age.
At 36 weeks post-conceptional age, all infants with BPD required additional oxygen therapy to correct hypoxemia, had clinical signs of chronic respiratory distress and had an abnormal chest radiographReference Edwards, Merrit, Northway and Boynton10, Reference Bancalari, Wilson-Costello and Iben11. Body composition was assessed by bioimpedance measurements to evaluate fat mass (FM) and fat-free mass (FFM). Pulmonary function was also assessed by measuring airway resistance (R). The study was approved by the Lille University Ethical Independent Committee and written informed consent was obtained from parents.
Measurements
Auxologic characteristics
Body weight and height were measured without shoes whilst wearing light indoor clothing. To determine body weight, a standard precision hospital scale was used for all measurements. Height was measured using a wall-mounted stadiometer. BMI was calculated as weight divided by height squared (kg/m2). Growth measurements were converted to Z scores relative to the French growth references of Sempé et al. Reference Sempé, Pedron and Roy-Pernot12. The Z score was computed by taking the predicted value for the child's age (for Z score weight for age) or for the child's height (for Z score weight for height) and sex from the child's measurements, and dividing the difference by the standard deviation of the measurement in the reference group.
Resting energy expenditure
On the day of the test, each child arrived at the Clinical Investigation Centre by car. Subjects had fasted for 10–12 h from the previous day and were not treated with β-adrenergic drugs or theophiline before the test. Weight and height were measured first. The child then rested, recumbent on a hospital bed, for 15 min. REE was measured by indirect calorimetry, using an open-circuit ventilated hood system (Deltatrac II, Datex Instrumentation Corporation, Helsinki, Finland). The RQ and flow settings were calibrated, by reference to alcohol combustion every 6 months and with a reference gas mixture (95 % O2, 5 % CO2), before each measurement. Inspired O2 flow (VO2), expired CO2 flow (VCO2) and the RQ were noted. REE was calculated every minute from O2 consumption (VO2 in ml/min), as was production of CO2 (VCO2 in ml/min) using the Weir formula without protein correctionReference Weir13. After an adaptation period of 15 min under a transparent canopy system, continuous respiratory exchange measurements were initiated. The measurements were conducted for a minimum period of 30 min. CV were < 10 % for VO2 and dilution airflow, and < 5 % for RQ.
Bioimpedance analysis
Bioimpedance analysis was done with a RJL-101 analyser (BIA 101S, 5RJL System, Akern, Detroit MI, USA) using an alternating current of 800 mA and 50 kHz. The measurements were standardized (supine, empty bladder, after 10–12 h fasting;Reference Houtkooper, Going, Lohman, Roche and Van Loan14, Reference Guo, Roche and Houtkooper15.
The electrodes were placed at defined positions on the right wrist and ankle. The mean of three sequential readings was used as the measurement value for the resistance (R). The Houtkooper formulaReference Houtkooper, Going, Lohman, Roche and Van Loan14 estimates the FFM:
where W is weight (kg) and FFM is fat-free-mass in kg. We previously demonstrated that impedancemetry provides a good estimation of body composition in this population when compared to total body absorptiometryReference Bott, Béghin, Devos, Pierrat, Matran and Gottrand16.
Energy intake
An alimentary inventory using a 7-d food questionnaire was performed in all the patients. Data were analyzed with BILNUT 3 software (Nutrisoft, Paris, France) using updated national food tables.
Pulmonary function tests
All pulmonary function tests were performed after bronchodilators had been stopped for at least 2 weeks. R was measured by interruption of the airflow (software Dyn'R, Paris, France); seven measurements were performed. A variation < 14 % was needed to validate the results. Functional residual capacity was measured by the dilution of He according to American Thoracic Society standards (Medisoft, Bruxelle, Belgium); this technique required two measurements with a variation of < 10 % to validate the results. The spirometry flow–volume curve, forced expiratory volume in 1 s (FEV), and forced expiratory flow 25 to 75 % vital capacity (FEF 25–75) were evaluated. Five reproducible spirometry–flow volume curves ( < 10 %) were necessary to validate the data.
Values were expressed as the percentage of the predicted value normalized for height and sex. R < 150 %, FEV >80 %, and FEF 25–75 >80 % were considered within the normal ranges. Proximal obstruction airflow was defined as R >150 % or FEV < 80 %. We assumed a maximal expiratory flow–volume loop with a marked concavity and FEF 25–75 < 80 % to demonstrate distal flow limitation during spirometry.
Predicted resting energy expenditure
The Schofield DefaultReference Schofield17 was used to predict REE. Predicted REE was calculated for all the children and compared to the REE measured by indirect calorimetry.
Statistical analysis
Analyses were conducted to evaluate the impact of airway obstruction on REE and nutritional status in BPD. The population of children with BPD was divided into three groups: children without obstruction of the airways (R < 150 % of normal, and FEV >80 %, and normal maximal expiratory flow–volume loop and FEF 25–75 >80 %); children with moderate obstruction of the airways (R = 150–200 % of normal, and/or FEV < 80 % and/or maximal expiratory flow–volume loop with a marked concavity and/or FEF 25–75 < 80 %), and children with severe obstruction (R >200 % of normal and FEV < 80 %, and maximal expiratory flow–volume loop with a marked concavity and FEF 25–75 < 80 %).
Fisher or X 2 tests were used to compare qualitative data. The Mann–Whitney test was used to compare quantitative data. Values of P < 0·05 were considered to be significant in all two-sided tests. The relation between REE and FFM has a significant intercept resulting in higher values for REE/FFM or REE/weight of subjects with a smaller FFM or smaller weightReference Ravussin and Bogardus18. A multivariate regression analysis was therefore performed on the parameters REE, FFM and weight to balance REE for weight and FFM.
Results
Functional respiratory tests
Forty-eight children with history of BPD (67·6 %) presented with obstruction of the airways (R >150 % or FEV < 80 % or maximal expiratory flow–volume loop with a marked concavity and FEF 25–75 < 80 %). Twenty-one children (29·5 %) had moderate obstruction (R < 200 % and/or FEV < 80 %, and maximal expiratory flow–volume loop with a marked concavity and FEF 25–75 < 80 %) that was reversible by salbutamol in eleven cases. Seven children (9·8 %) had severe obstruction (R >200 % of normal and FEV < 80 %, and maximal expiratory flow–volume loop with a marked concavity and FEF 25–75 < 80 %), that was reversible in one case. Neonatal characteristics of the three groups are summarized in Table 2. No statistical difference was observed in neonatal characteristics between the three groups (P>0·05).
Among children with severe obstruction of the airways, two received inhaled steroids before the age of 2 years and one received inhaled steroids until the age of 3 years. Among children with moderate obstruction of the airways, eleven received inhaled steroids before the age of 2 years; five received inhaled steroids until the age of 3 years and were still being treated with inhaled steroids at the time of the study. Among children without obstruction of the airways, eleven children received inhaled steroids before the age of 2 years, five received inhaled steroids until the age of 3 years and two were still being treated with inhaled steroids at the time of the study.
Body composition and nutritional status
Z scores of weight for age and weight for height, and BMI of children with BPD with moderate to severe obstruction were significantly lower than those of healthy control children (Table 3). There was no difference in Z scores of weight for height or weight for age or BMI in children with BPD without obstruction when compared to controls.
Table 3 Comparison of auxologic characteristics of children with bronchopulmonary dysplasia (BPD) with severe, or moderate obstruction of the airways or without pulmonary sequelae with healthy children (controls)

Z scores of weight for age and weight for height, BMI, FM and FFM of children with BPD with severe obstruction were not significantly different from the scores of those with BPD but without obstruction. A significant decrease of FM was observed in children with BPD with severe airway obstruction while those with BPD with moderate airway obstruction or without obstruction did not demonstrate any significant change in their body composition compared with healthy children (Table 3).
Z scores of height for age were not significantly different in healthy children or children with BPD (Table 3).
Resting energy expenditure and energy intakes
Measured REE and the REE predicted by the Schofield equation were significantly lower in children with BPD (P < 0·001, Table 4) when compared to controls. However, when corrected for weight or FFM, REE was not significantly lower in the group of children with BPD when compared to the control group.
Table 4 Comparison of REE and energy intakes of children with bronchopulmonary dysplasia (BPD) with severe or moderate obstruction of the airways or without pulmonary sequelae with healthy children (controls)
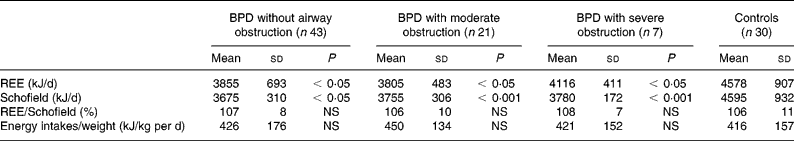
Schofield, REE predicted by the Schofield equation.
There was not a significant difference between measured and predicted REE in any groups. Energy intakes were not significantly different in BDP groups compared to controls (Table 4).
Discussion
Despite advances in neonatal critical care, including reduced O2 concentrations and less traumatic ventilatory therapies, BPD still affects one third of very low-birth-weight preterm infantsReference Gross, Anbar and Mettelman19. Earlier reports of survivors of BPD highlighted delays in growth and development in the first few years of life, but limited data are available on the long-term outcome of older children with BPDReference Kurzner, Garg, Bautista, Bader, Merritt and Warburton20–Reference Giacoia, Venkataraman, West-Wilson and Faulkner22. Several research groups have attempted to answer the question as to whether or not the higher values of REE in children with BPD could contribute to a poor nutritional status. Giacoia and co-workers investigated the outcome of school-age children with BPD in terms of nutrition, pulmonary function, REE, body composition, and intelligence. The results were compared with a preterm cohort matched for gestational age and birth weight, and with a term control groupReference Giacoia, Venkataraman, West-Wilson and Faulkner22. As in our study, they did not find any difference in the REE between children with BPD and controls. Their results on growth and mental ability support previous observations that premature-related factors may be even more important contributors to eventual outcome than those related to pulmonary diseaseReference Vrlenich, Bozynski, Shyr, Schork, Roloff and McCormick21. Conversely, our study shows that the pulmonary status of children with BPD seemed to be associated with an altered nutritional status in later childhood (lower BMI, lower Z scores of weight for age and weight for height, and reduced FM in cases of airway obstruction). Increased REE could contribute to the poorer nutritional status in these children compared with children with BPD without obstruction. Nevertheless measured REE was not higher than predicted REE in children with BPD, and was not higher in the group with severe airway obstruction after weight or FFM adjustment. However, since only seven children suffered from severe obstruction of the airways, that represents a study limitation when analyzing factors associated with pulmonary sequel in BPD. It has been shown that treatment with β-adrenergic drugs or theophylline, which our patients with BPD had previously used, increased O2 consumption and therefore could explain the high REE in these patientsReference Giacoia, Venkataraman, West-Wilson and Faulkner22, Reference Merth, De Winter, Zonderland, Borsboom and Quanjer23. To minimize this effect however, all these drugs were stopped several days before REE was measured in our patients.
A few studies have demonstrated that during the neonatal period children with BPD have a higher REE than healthy childrenReference Wahlig, Gatto, Boros, Mammel, Mills and Georgieff4, Reference De Meer, Westerterp, Houwen, Brouwers, Berger and Okken24. There are several reasons for this difference: an increase in the work involved in breathing, inflammation and/or infection, and a different body composition with consistent decrease of FM and increased metabolically active FFMReference De Meer, Westerterp, Houwen, Brouwers, Berger and Okken24, Reference Friedrich, Corso and Jones25. In adults with chronic pulmonary obstructive disease, REE was found to be positively correlated with pulmonary illnessReference Schols, Fredrix, Soeters, Westerterp and Wouters26. Patients with chronic obstructive pulmonary disease have increased respiratory muscle energy consumption secondary to an increased resistive load and impaired efficiency of the respiratory muscleReference Schols, Fredrix, Soeters, Westerterp and Wouters26. REE is known to be correlated with weight, height, age, sex, FFM or FM in both adults and childrenReference Goran, Kaskoun and Johnson27. The best predictor of REE in childhood is FFM, which represents the tissues with the highest metabolic activityReference Goran, Kaskoun and Johnson27. Tissues containing FFM have various degrees of metabolic activityReference Goran, Kaskoun and Johnson27. Organs such as the brain, liver, heart, and kidneys are made of cells with high energy expenditure levels (> 60 % of REE)Reference Goran, Kaskoun and Johnson27. Muscles have a lower metabolic rate (30 % of REE). Children with BPD, like other children with chronic obstructive pulmonary disease, have been reported to have growth failure and a decreased FMReference Kistorp, Toubro, Astrup and Svendsen28. When malnutrition occurs, the body alters the ratio of loss of body fat to muscle mass, but the highly metabolic tissues of the vital organs (brain, heart, liver and kidneys) are conservedReference Goran, Kaskoun and Johnson27. Although the body composition methods used in our study did not allow precise analysis of such highly metabolically active tissues, we did not observe any increase in REE suggesting an alteration of metabolically active tissues in children with BPD. Other factors such as chronic inflammation could also increase REE in children with a history of BPD. During the neonatal period, O2 therapy may induce lung injuries, by volo- or baro-traumatism, which may then contribute to BPD lesions. Early inflammation and angiogenic responses interfere with lung development and prevent alveolar growthReference Asikainen and White29. During the first few years of life, recurrent infections maintain inflammationReference Resch, Pasnocht, Gusenleitner and Müller30. Children with BPD tend to present with an increased incidence of recurrent infections of the lower respiratory tract (bronchiolitis and pneumonia) up to 2 years old that contributes to the persistence of lung inflammation and increases hospital admissionsReference Resch, Pasnocht, Gusenleitner and Müller30. Our results did not support such a hypothesis of lung or general inflammation in the long term for children with BPD.
In a previous study we showed that undernutrition was not correlated with pulmonary status in later childhood, but that both were associated with nutritional status before the age of 2 years. After this period, undernutrition and hyperinflation of the airways seem to be fixed sequelae and independent featuresReference Bott, Béghin, Gondon, Hankard, Pierrat and Gottrand31. In children who had bronchopulmonary dysplasia in infancy, nutritional status at 2 years of age could influence both nutrition and pulmonary outcomes in childhoodReference Bott, Béghin, Gondon, Hankard, Pierrat and Gottrand31. The present study supports the hypothesis that body composition and pulmonary function in BPD in later childhood are fixed sequelae originating from the neonatal period.
Acknowledgements
We thank Chivon Winsloe for her valuable help in editing the manuscript. We also thank Elise Mok for her valuable help in the statistical analysis described in the paper.






