1. Introduction
Martian meteorites are very important samples for determining the chemical and mineralogical composition of Mars. There are three types of Martian meteorites: Shergottites, Nakhlites and Chassignites, which differ in their mineralogical composition, among other aspects. Nakhlites are particularly important due to the presence of minerals that cannot be found in other types of Martian meteorites, such as smectite (phyllosilicate minerals), iron oxyhydroxides (FeOOH), silica (SiO2) and other types of salts like carbonates, sulfates or halides. These alteration minerals from olivine, together with the remnant olivine, combine to form a microcrystalline rock called iddingsite (Lee and others, Reference Lee, Tomkinson, Hallis and Mark2015; Udry and others, Reference Udry2020).
To conduct a thorough mineralogical characterization of a meteorite, it is crucial to determine and distinguish between original and alteration products. This allows determining the origin and the history of a meteorite. The minerals in a meteorite can form various alteration phases from their formation to their discovery on the Earth surface. Some of these alteration phases can be produced due to the exposition of the sample to the terrestrial weathering that differs depending on the location where the meteorite was collected. The two main locations where meteorites are normally found on Earth are Antarctica and hot deserts (Bland and others, Reference Bland, Zolensky, Benedix and Sephton2006).
Meteorites are commonly found on Antarctica as they are easily detected on the snow/ice. Hence, the Antarctic Search for Meteorites group (ANSMET) from NASA carries out several expeditions in different Antarctic locations searching for meteorites. Since its foundation in 1976, ANSMET has found more than 25 000 meteorites in different 6-week meteorite collection campaigns (Love and Harvey, Reference Love and Harvey2014; Mitzi, Reference Mitzi2014).
Different studies examined the weathering alteration in the Miller Range (MIL) Martian paired Nakhlites (MIL 090030, MIL 03346, MIL 090032 and MIL 090136). Paired meteorites have similar chemical composition and have been formed by the same entry event of a stony meteor that broken in several fragments that can be dispersed in areas of several kilometers. Gypsum (CaSO4⋅2H2O) and jarosite (KFe3(SO4)2(OH)6) were detected as terrestrial weathering alteration products in all of them (Hallis and Taylor, Reference Hallis and Taylor2011). In MIL 090032, other terrestrial alteration minerals, such as altered olivine, iron oxides and iron hydroxides, were also found (Hallis and others, Reference Hallis, Ishii, Bradley and Taylor2014).
Calcium sulfate and iron oxyhydroxides are among the weathering alteration minerals found in other types of Martian meteorites in Antarctica (Wentworth and Gooding, Reference Wentworth and Gooding1993; Lee and Bland, Reference Lee and Bland2004). Comparing the weathering alteration minerals in Nakhlite meteorites from Antarctica with other types of Martian meteorites, it can be concluded that the mineralogical alterations resulting from the exposition of the sample to the Antarctic environment are quite similar.
Comparing the alteration products detected in meteorites collected in Antarctica with meteorites found in hot deserts, it is evident that there are fewer alteration products in Antarctica meteorites. There are similarities and differences in the alteration compounds detected in meteorites from both regions. In both cases, the main alteration products are related to Fe-oxides and oxyhydroxides, but with different outcomes. Troilite (FeS) is the main alteration product found in samples from hot deserts, whereas this mineral is not commonly found in meteorites found in the Antarctica. The Fe-phase alteration products in Antarctica's meteorites are goethite (FeOOH), jarosite and akaganéite (FeO(OH,Cl)). Other alteration minerals found in meteorites collected in hot deserts are Fe-poor sulfates, carbonates and silica (Buchwald and Clarke, Reference Buchwald and Clarke1989; Lee and Bland, Reference Lee and Bland2004).
The MIL 090030 meteorite stands out as one of the least explored meteorite in terms of weathering alteration products. This meteorite was selected for this study to demonstrate that meteorites collected in a polar region, as it is the Miller Range region in Antarctica, can be contaminated with some compounds from this region. It is important to identify those compounds in order to determine which minerals from the meteorite are original from Mars.
2. Materials and methods
2.1. Sample description
The meteorite analyzed in this study is the so-called Miller Range 090030 (MIL 090030). It is a Martian meteorite belonging to the Nakhlite group. This meteorite is paired to other three, the MIL 03346, the MIL 090032 and the MIL 090136. All of them were found in the Miller Range region in the Transantarctic mountains (Righter, Reference Righter2018), hence their names.
The main minerals that constitute the MIL 090030 meteorite are pyroxene (Fs22–49Wo34–43) and olivine (with a Fa57 content; although in some outer zones of the meteorite the content of fayalite can be up to Fa89). Pyroxene is the main mineral and corresponds to the 66.3 modal vol% of the modal abundances (Udry and McSween Jr., Reference Udry and McSween2012; Udry and others, Reference Udry and McSween2012). Olivine is the second main mineral and its modal abundance is 9.8 modal vol% (Udry and McSween Jr., Reference Udry and McSween2012; Udry and others, Reference Udry and McSween2012). Less-abundant minerals that occur in MIL 090030 meteorite are magnetite (Fe3O4), titanomagnetite (Fe(Fe,Ti)2O4), pyrrhotite (Fe(1−x)S, where x is between 0 and 0.2) or cristobalite (SiO2) (Hallis and Taylor, Reference Hallis and Taylor2011; Righter, Reference Righter2018). These minor compounds correspond to a modal abundance of 23.9 modal vol% (Udry and McSween Jr., Reference Udry and McSween2012; Udry and others, Reference Udry and McSween2012).
Antarctica is a region with cold temperatures. Specifically, as is shown in Fig. S1a, in the Miller Range region during winter the temperature ranges between −30 °C and −50 °C and during summer the temperature is between −20 °C and −10 °C (Barry and Hall-McKim, Reference Barry and Hall-McKim2018). These low temperatures mean that the relative humidity in this region is very high, between 70 and 88% throughout the year (Fig. S1b) (Gettelman and others, Reference Gettelman, Walden, Miloshevich, Roth and Halter2006). However, if the absolute humidity is considered, the region is really dry. The weathering conditions of this region can produce a characteristic mineralogical alteration in meteorites found in the Antarctic region.
The MIL 090030 meteorite found weighed 452.630 g and the dimensions were 8.0 cm × 7.5 cm × 4.0 cm (Righter and NASA-JSC, Reference Righter2010). In this study, a fragment weighing 0.9746 g was analyzed. Among the four paired meteorites, the MIL 090300 displayed the lowest proportion of Antarctica's weathering alteration (Hallis and others, Reference Hallis2012).
2.2. Instrumentation
2.2.1. Raman spectroscopy
The molecular analyses were performed using high-resolution micro-Raman spectroscopy. Single-point analyses and spectral imaging were conducted using an InVia confocal micro-Raman instrument (Renishaw, UK). The single-point spectra were acquired in the 100–1400 cm−1 range for the 785 nm laser and 100–1835 cm−1 for the 532 nm laser, using the laser power attenuated to 5%, an acquisition time between 5 and 10 s to increase the Raman intensity and between 3 and 8 accumulations.
For Raman spectroscopy images, the same spectrometer was used employing the high-resolution StreamLine technology (Renishaw) that it is a spectral collection that varies lateral sample position. Raman spectroscopy images were obtained using the 532 nm laser with 100× objective, laser power attenuated to 5%, 10 s of exposure time, 3 accumulations and 0.5 μm step size.
In both cases, the spectrometer was calibrated using a silicon sample and its band at 520.5 cm−1. The treatment of the spectra was performed using Wire™ software (Renishaw).
2.2.2. Energy-dispersive X-ray fluorescence
The elemental characterization was performed using the energy-dispersive X-ray fluorescence spectrometer M4 TORNADO (Bruker Nano GmbH, Berlin, Germany).
The instrument was connected to an XFlash SSD detector and to an MV 10 N VARIO-B pump to work under vacuum for the analysis of the lightest elements, with Z < 16. For the analysis of MIL 090030 meteorite, a step distance of 20 μm was used. The spectrometer was calibrated every day using the Kα line of Zr and the results were interpreted using M4 TORNADO software.
3. Results and discussion
3.1. Calcium sulfates
There are different types of calcium sulfates depending on the water proportion in their structure. These minerals include gypsum (CaSO4⋅2H2O), bassanite (CaSO4⋅0.5H2O) and anhydrite (CaSO4) (Wang and others, Reference Wang, Zeng, Zhou, Wu and Yin2015). The stability of these phases depends on the environmental conditions. For instance, at temperatures below 42 °C and humidity levels above 60%, gypsum is the most stable phase. However, between 42 °C and 97 °C and with humidity values higher than 40%, bassanite is metastable compared to gypsum. Both phases are metastable with respect to anhydrite at temperatures above 42 °C. Finally, at temperatures higher than 97 °C, anhydrite is the stable phase regardless of relative humidity (Charola and others, Reference Charola, Pühringer and Steiger2007).
In addition, three types of anhydrite can be distinguished based on their crystallization structure. Anhydrite I has a cubic structure, anhydrite II presents an orthorhombic structure and anhydrite III a hexagonal one. The temperature formation for these three types of anhydrites differs. Anhydrite I is formed ~1180 °C, anhydrite II at 300 °C and anhydrite III at 110 °C (Prieto-Taboada and others, Reference Prieto-Taboada, Gómez-Laserna, Martínez-Arkarazo, Olazabal and Madariaga2014).
In the MIL 090030 meteorite, gypsum and a mixture of bassanite and anhydrite was found by Raman spectroscopy (Figs 1a and b respectively). Gypsum was identified by its main band at 1008 cm−1. Besides, in Figure 1b a mixture of bassanite and anhydrite II can be observed, with the main bands of both compounds overlapping (1015 cm−1 for bassanite and 1017 cm−1 for anhydrite II). In addition, secondary bands of both sulfates were detected, with bands at 489 and 1149 cm−1 corresponding to bassanite and Raman bands at 629 and 1128 cm−1 corresponding to a mixture of bassanite and anhydrite II (Prieto-Taboada and others, Reference Prieto-Taboada, Gómez-Laserna, Martínez-Arkarazo, Olazabal and Madariaga2014).

Fig. 1. (a) Raman spectrum of gypsum (black) with diamond (blue) and (b) Raman spectrum of anhydrite II and bassanite (black) with olivine (blue). Diamond is a contamination due to the polishing pretreatment and olivine is an original Martian mineral.
In other studies conducted on the four paired Nakhlites, gypsum, anhydrite and bassanite were identified as original minerals from Mars (Righter, Reference Righter2018). Other scientists (Hallis and Taylor, Reference Hallis and Taylor2011) suggested that gypsum found in veins of the outer regions of the MIL paired meteorites is a result of the exposition to terrestrial environments. However, in the inner section of the same meteorites, gypsum was found in veins and it has probably a preterrestrial origin (Hallis and Taylor, Reference Hallis and Taylor2011).
In this study, two hypotheses were proposed to explain the presence and origin of the three sulfates in the meteorite. The first hypothesis suggests that both minerals are original compounds from Mars, as gypsum and anhydrite have been found on the Martian surface (Vaniman and others, Reference Vaniman2018). On the other hand, gypsum forms anhydrite when it is exposed to high temperatures and pressures (Mirwald, Reference Mirwald2008). The MIL 090030 meteorite suffered from high temperatures and pressures during its formation, travel and arrival to the Earth. This is evidenced by the presence of different SiO2 polymorphs detected in the meteorite using Raman spectroscopy (Fig. S2). On the one hand, cristobalite, which is a high-temperature polymorph formed at 1470 °C, was identified (Gomez-Nubla and others, Reference Gomez-Nubla2017). On the other, coesite, a high-pressure polymorph formed at 3 GPa (Lakshtanov and others, Reference Lakshtanov, Sinogeikin and Bass2007) was detected as well. Therefore, it can be concluded that the meteorite was subjected to temperatures of at least 1470 °C and pressures of 3 GPa. Under these conditions, all calcium sulfate originally from Mars would have arrived to the Earth as anhydrite.
Thus, the presence of gypsum in the meteorite can be explained as the subsequent transformation of anhydrite due to the environmental conditions to which the meteorite was exposed on Earth, in this case in Antarctica. As the region experiences low temperatures and high relative humidity throughout the year (Fig. S1; Barry and Hall-McKim, Reference Barry and Hall-McKim2018; Gettelman and others, Reference Gettelman, Walden, Miloshevich, Roth and Halter2006), anhydrite can be hydrated to form gypsum. During this process, bassanite, detected by Raman spectroscopy, was also formed as an intermediate mineral.
The second hypothesis considered that not all the meteorite mass was exposed to the same temperature and pressure conditions. Therefore, not all gypsum originally from Mars would have been transformed to anhydrite. As a result, part of the gypsum would have formed bassanite and some would have remained as gypsum. Upon reaching the surface of Antarctica, anhydrite and bassanite could have been hydrated to form gypsum. Considering these factors, gypsum found in MIL 090030 meteorite could be a mixture of Martian gypsum and hydrated gypsum from anhydrite and bassanite formed in the Antarctica region.
Taking into account all the minerals detected as weathering alteration products, calcium sulfates were the most abundant alteration minerals found in both faces of this fragment of the meteorite. They were found in a grain form of <5 μm.
3.2. Halite
Halite (NaCl) is the most common compound found in sea water. This mineral was found in the MIL 090030 meteorite through micro-energy-dispersive-X-ray fluorescence (μED-XRF) analysis. As depicted in Figure 2, a correlation between Na and Cl was observed. However, the correlation of this mineral in the meteorite was lower compared to that of calcium sulfates. The NaCl grains found in the meteorite measured ~0.5 mm in size.
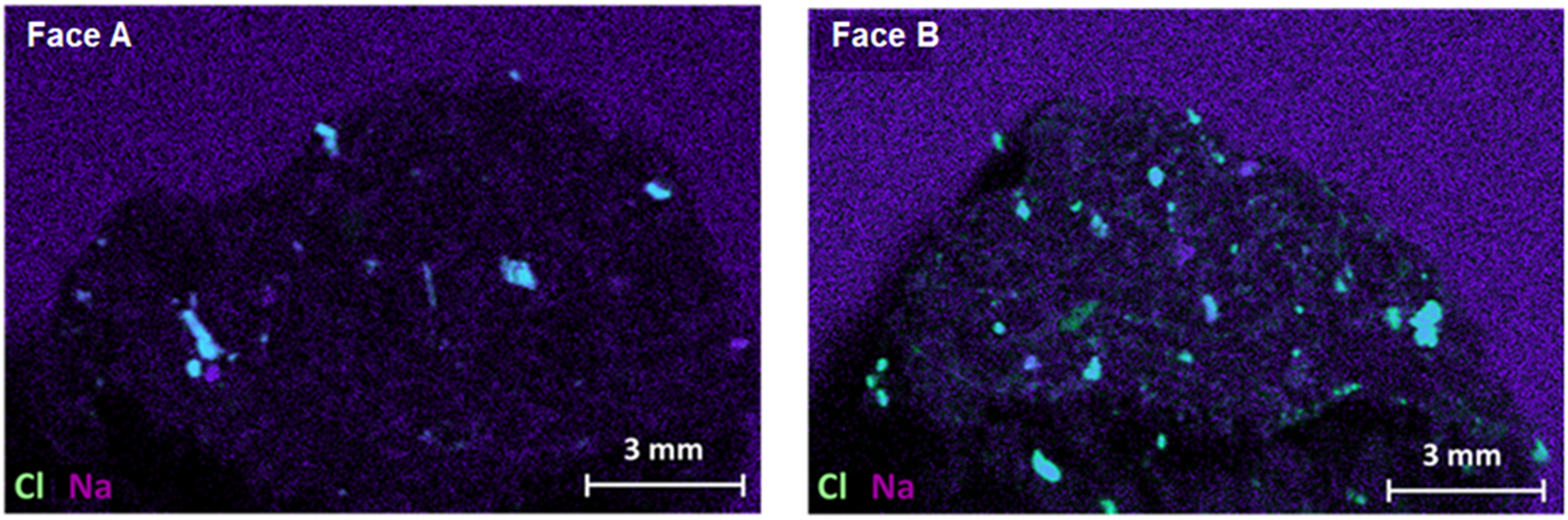
Fig. 2. μED-XRF images from Cl distribution (green) and Na (purple). NaCl corresponds to superposition of Cl and Na colors (cyan).
In certain regions of Antarctica's coastline and interior, the level of sea spray aerosol is notably high. Sodium is the main element of this aerosol, while calcium, magnesium and chlorine are secondary elements (Udisti and others, Reference Udisti2012). Therefore, it is plausible that halite precipitated on the meteorite as a result of sea spray evaporation.
3.3. Nitrates
Nitrates are common compounds found in Antarctica because of windborne aerosol from South America (Marina-Montes and others, Reference Marina-Montes2022). In the MIL 090030 meteorite two types of nitrates were found by Raman spectroscopy: nitratine (NaNO3) and niter (KNO3). A spectrum obtained from the MIL 090030 meteorite is shown in Figure 3a. However, these minerals were detected in a lower proportion compared to calcium sulfates in both faces of the meteorite.
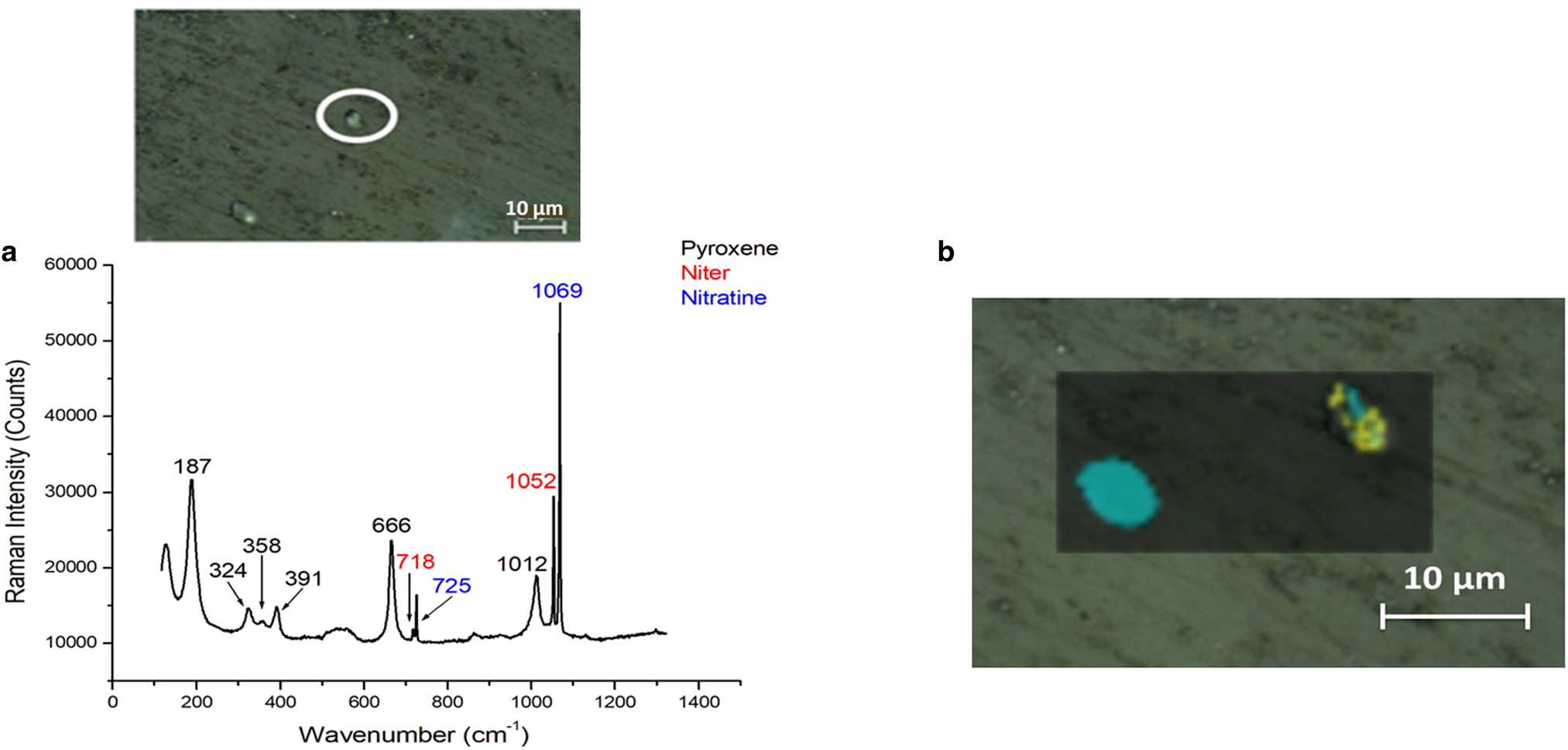
Fig. 3. (a) Raman spectrum of nitratine (blue) and niter (red) with pyroxene (black). Pyroxene is an original Martian mineral. (b) Raman image of nitratine (blue) and niter (yellow).
The origin of these compounds was confirmed through the study of the structure and arrangement of the nitrate grains using Raman imaging (Fig. 3b). It was determined that nitrates were in a grain form, with an approximate size of 5 μm, and they were not evenly distributed along cracks and fissures. These grains were deposited on the meteorite matrix surface and did not form an integral part of it. For this reason and considering the significant presence of nitrates in Antarctica's environment, the nitrates detected in MIL 090030 were attributed to terrestrial weathering alteration.
It is important to consider potential cross-contamination resulting from the meteorite curation process, which involves cutting, embedding the thick section in a resin and polishing. Silicon carbide, diamond and resins are the most common compounds found in meteorites due to the meteorite curation process. However, this is the first time that nitrates were found in a meteorite subjected to the same curation process. For this reason, these compounds cannot be attributed to meteorite curation contamination.
3.4. Goethite
The latest mineral found in the MIL 090030 meteorite due to weathering alteration was goethite (α-FeOOH). This mineral has also been found in Antarctica in the analysis of different icebergs because of the ferrihydrite (Fe2O3⋅0.5H2O) alteration into more stable compounds such as goethite (Raiswell and others, Reference Raiswell, Benning, Tranter and Tulaczyk2008).
In the MIL 090030 meteorite goethite was found by Raman spectroscopy (Fig. 4) based on its Raman bands at 241, 299, 387, 480 and 549 cm−1. This mineral was found in a smaller proportion compared to other weathering minerals detected.
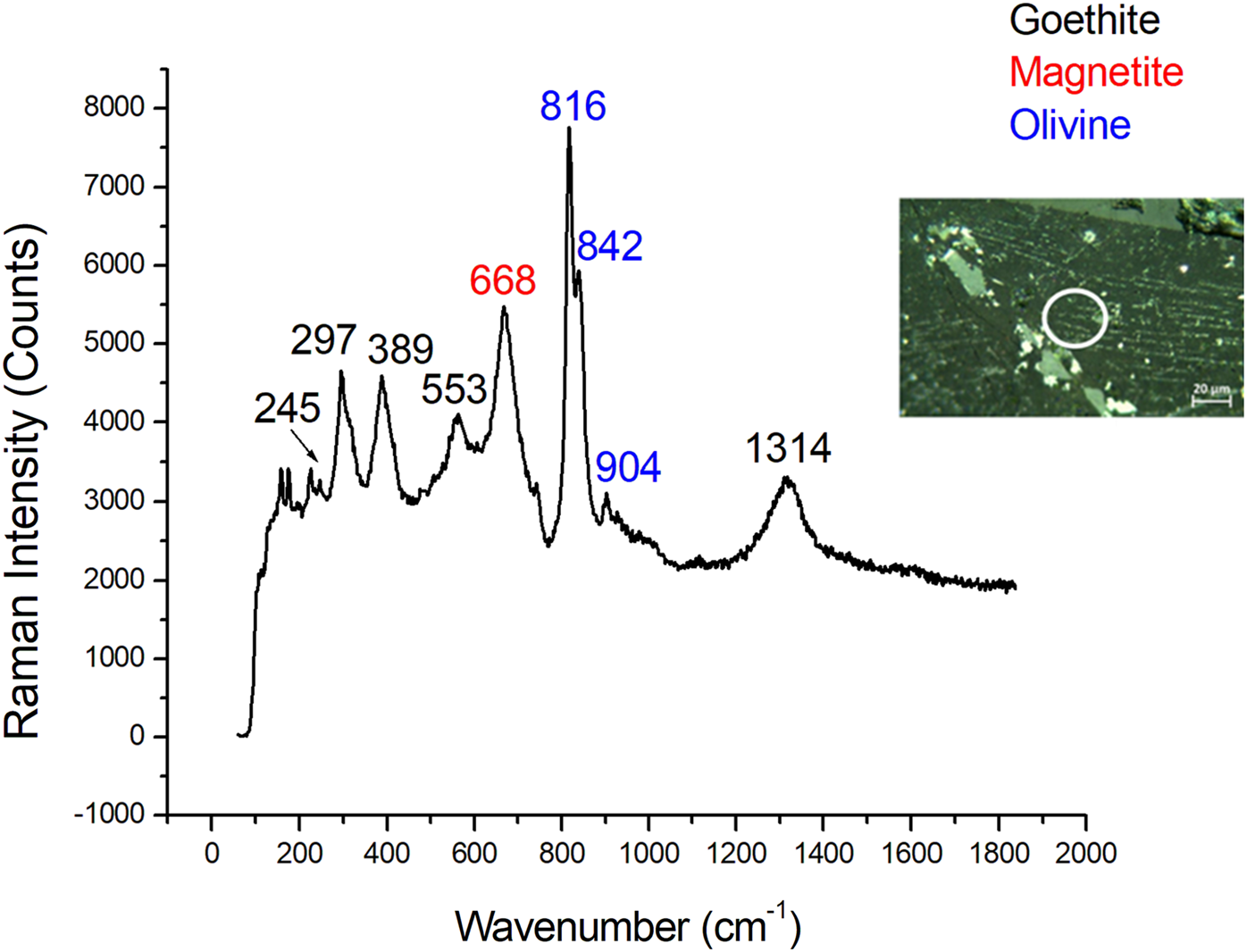
Fig. 4. Raman spectrum of goethite (black) with magnetite (red) and olivine (blue). Magnetite and olivine are original Martian minerals.
Goethite was considered a terrestrial weathering product because when it is exposed to high temperatures (more than 600–800 °C), hematite is formed as follows (Gialanella and others, Reference Gialanella2010):
As mentioned previously, the MIL 090030 meteorite was exposed to higher temperatures. Therefore, goethite should have been completely or partially converted to hematite. The total absence of hematite associated with goethite grains confirmed the terrestrial origin of the latter.
4. Conclusions
This study provides information for the first time about the weathering processes suffered by MIL 090030 meteorite in a polar region. However, the possible weathering paths that meteorites suffer under these conditions are not widely referenced in literature.
In the specific case of MIL 090030 meteorite, although it is one of the least altered meteorites, several mineral phases of terrestrial alteration were found in this study. The most common ones were gypsum, anhydrite and bassanite. Together with those, halite and goethite were also found. It is worth highlighting the presence of different nitrates. Nitrates are not a very commonly found in meteorites and, in this study, two different kinds of nitrates were detected.
Comparing the obtained results with the literature, in this study only gypsum was detected. However, this study detected for the first time other minerals like nitrates, halite or goethite, which were not described before in literature for these meteorites. Considering the possible presence of these compounds in the location where they were collected, the most probable source for them is through terrestrial alteration.
In addition, the suitability of non-destructive analytical techniques for mineralogical meteorite characterization was demonstrated. The use of these techniques provides information about mineralogical composition and history of the sample studied. Furthermore, these techniques allow us to determine the spatial distribution of the minerals detected in the sample. Knowing the spatial distribution, we can determine different relationships between minerals and establish their origin.
The importance of using Raman spectroscopy must be highlighted because it can be used to determine polymorphs present in meteorites. These polymorphs can determine the minimum temperature and pressure that the meteorite suffered, and this can help in the identification of the origin of the minerals detected.
Taking all these into account, it has been demonstrated that the Antarctic environmental conditions can carry out a specific weathering process in meteorites under cold conditions. There are different minerals that were detected as weathering alteration products and some of them, like nitratine or niter, have not been referenced in the literature before.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/aog.2024.4.
Acknowledgements
All the authors are grateful to NASA for providing access to the MIL 090030 Martian meteorite sample through the loan agreement between NASA's JSC and the UPV/EHU. US Antarctic meteorite samples are recovered by the Antarctic Search for Meteorites (ANSMET) program which has been funded by NSF and NASA, and characterized and curated by the Department of Mineral Sciences of the Smithsonian Institution and Astromaterials Curation Office at NASA Johnson Space Center. This study has been supported through the PAMMAT project ‘Alteration processes in Mars and Moon Meteorites, and Terrestrial Analogues at different environments: Mars2020, Rosalind Franklin and Returned Samples from Mars and Moon’ (Grant No. PID2022-142750OB-I00), funded by the Spanish Agency for Research (through the Spanish Ministry of Science and Innovation, MCIN, and the European Regional Development Fund, FEDER), and the Strategic Project ‘Study of Alteration Processes in Terrestrial and Planetary Materials’ (Grant No. UPV/EHU PES21/88), funded by the UPV/EHU. J. Aramendia is grateful to the University of the Basque Country and the Ministry of Universities for her post-doctoral Maria Zambrano position. Open Access funding by University of Basque Country.







