Introduction
In 2018, 56% of the more than five million hectares of cotton in the United States was planted in Texas (USDA-NASS 2018). The High Plains region is the largest contiguous cotton producing region in the nation and where 66% of Texas cotton and cottonseed production is located (Plains Cotton Growers 2020). One of the most detrimental impediments to efficient cotton production is the presence of weeds. Weeds cause an average yield loss of 34% if not properly controlled (Oerke Reference Oerke2006). Palmer amaranth is considered the most common and troublesome weed among all broadleaf crops as well as in fruit and vegetable production (Van Wychen Reference Van Wychen2019). Palmer amaranth is native to the semi-arid southwestern United States and northwestern Mexico (Sauer Reference Sauer1950) and was ranked as the most common and most troublesome weed among all broadleaf crops as well as in fruit and vegetable production (Van Wychen Reference Van Wychen2019). Palmer amaranth has the greatest leaf number, biomass, and growth rate per growing degree days than roughfruit amaranth (Amaranthus rudis Sauer), redroot pigweed (Amaranthus retroflexus L.), and prostrate pigweed (Amaranthus albus L.; Horak and Loughin Reference Horak and Loughin2000). Steckel et al. (Reference Steckel, Sprague, Stoller and Wax2004) determined that Palmer amaranth has the greatest germination rate of eight Amaranthus species studied including Powell’s amaranth (Amaranthus powelli S. Wats.), mat amaranth (Amaranthus blitoides S. Wats.), slim amaranth (Amaranthus hybridus L.), spiny amaranth (Amaranthus spinosus L.), redroot pigweed, roughfruit amaranth, and prostrate pigweed. Intensive use of herbicides has led to populations of seven different herbicide-resistant weeds in Texas (Heap Reference Heap2021). In a recent state-wide survey of Palmer amaranth in Texas, samples collected from the High Plains region of west Texas had the greatest number of populations that were resistant or less sensitive to glyphosate, pyrithiobac, and atrazine (Garetson et al. Reference Garetson, Singh, Singh, Dotray and Bagavathiannan2019).
Cotton growers rely on a number of strategies to manage weeds including cultivation, cultivar selection, cover crops, and herbicides. Controlling herbicide-resistant Palmer amaranth before it emerges can be achieved using soil-residual herbicides (Young Reference Young2006). In cotton, an 8-wk weed-free period after emergence is needed to prevent yield loss due to weed competition (Buchanan and Burns Reference Buchanan and Burns1970). Cotton lint yield linearly decreased as Palmer amaranth density increased at a rate of 7.6% for every Palmer amaranth plant present (Morgan et al. Reference Morgan, Baumann and Chandler2001). In Georgia, Palmer amaranth that emerged between the 12- and 17-leaf stage of cotton had no effect on yield, whereas earlier emerging weeds decreased lint yield (MacRae et al. Reference MacRae, Webster, Sosnoskie, Culpepper and Kichler2013). In stripper cotton, the presence of Palmer amaranth at harvest decreased efficiency and increased harvest time up to 2.5-fold (Smith et al. Reference Smith, Baker and Steele2000). Smith et al. also reported an increase in foreign matter and trash in cotton lint prior to ginning in the presence of ≥650 Palmer amaranth plants ha−1. Preemergence (PRE) herbicides are available for use in cotton; however, weed resistance and crop selectivity limit the utility of these herbicides in certain geographic areas and production systems (Heap Reference Heap2021).
Isoxaflutole is a Group 27 herbicide (categorized as such by the Weed Science Society of America) that inhibits the essential enzyme p-hydroxyphenylpyruvate dioxygenase, also known as HPPD (WSSA 2021). P-hydroxyphenylpyruvate inhibitors are part of a larger group of carotenoid biosynthesis inhibitors. Herbicides in this group deplete plastoquinones, essential electron acceptor molecules in the carotenoid biosynthetic pathway that are essential for plant life, because they protect chlorophyll molecules from photooxidation. This generates singlet oxygen in the absence of carotenoids (Beaudegnies et al. Reference Beaudegnies, Edmunds, Fraser, Hall, Hawkes, Mitchell, Schaetzer, Wendeborn and Wibley2009). Once the carotenoid biosynthesis pathway is blocked and the formation of new is carotenoids stopped, all new plant growth displays symptomology that resembles “bleaching,” or white-colored meristematic tissue (Lee et al. Reference Lee, Prisbylla, Cromartie, Dagarin, Howard, Provan, Ellis, Fraser and Mutter1997). Lipid peroxidation causes eventual plant death.
Isoxaflutole received registration by the U.S. Environmental Protection Agency in 1998 and has been used to control annual grasses and broadleaf weeds in field corn (Zea mays L.; EPA 1998). When used as part of a PRE herbicide program, isoxaflutole provided up to 95% Palmer amaranth control 3 wk after application (Meyer et al. Reference Meyer, Norsworthy, Young, Steckel, Bradley, Johnson, Loux, Davis, Kruger, Bararpour, Ikley, Spaunhorst and Butts2016). Johnson et al. (Reference Johnson, Chahal and Regehr2012) found that isoxaflutole controlled Palmer amaranth by 87% to 99% 8 wk after application. While current cotton varieties do not tolerate HPPD inhibitors, BASF Corporation has developed HPPD-resistant cotton that will allow growers to use isoxaflutole in future weed management programs pending regulatory approvals of the transgenic trait. The objectives of these studies were to determine HPPD-resistant cotton response to isoxaflutole and to evaluate season-long weed management programs that include isoxaflutole applied PRE or early postemergence (EPOST).
Materials and Methods
Cotton Response Experiments
Field experiments were conducted in 2019 and 2020 at the Texas Tech University New Deal Research Farm (33.73°N, 101.73°W) near New Deal, TX, and at the BASF Corporation Breeding and Trait Development Research Farm (33.58°N, 101.77°W) in Lubbock, TX. The soil type at the New Deal location was a Pullman clay loam (46% sand, 20% silt, and 34% clay), pH 8.1, and with <1% organic matter. The soil type at the Lubbock location was an Amarillo fine sandy loam (66% sand, 15% silt, and 19% clay), pH 8.4, and less than 1% organic matter. An experimental HPPD-resistant cotton variety with a Coker background was planted in New Deal on May 15, 2019, and May 16, 2020; and in Lubbock on May 29, 2019, and May 26, 2020. At the Lubbock site in 2020, high winds destroyed emerged cotton on June 9, so cotton was replanted on existing beds with minimal soil disturbance on June 11. The target planting density at both locations was 145,000 plants ha-1. Each location received supplemental irrigation throughout the season. At New Deal, trifluralin (479 g ai L−1; Agri Star, Ankeny, IA) at 1.12 kg ai ha−1 was applied and incorporated twice to a depth of 5 cm using a rolling cultivator on April 9, 2019, and March 25, 2020. Plots were maintained weed-free throughout the season by hand-weeding, cultivation, and use of clethodim (Select®, 240 g ai L−1; Valent, San Ramon, CA) at 0.25 kg ai ha−1 plus 1% vol/vol crop oil concentrate. At Lubbock, a blanket treatment of trifluralin at 0.84 kg ai ha−1 was applied and incorporated to a depth of 5 cm using a tandem double disc lister on March 18, 2019, and April 1, 2020. To aid in weed control at the Lubbock location, S-metolachlor (Dual Magnum®, 913 g ai L−1; Syngenta Crop Protection, Greensboro, NC) at 1.07 kg ai ha−1 was applied over the entire trial area on May 28 and July 11 in 2019, and May 28 in 2020, and diuron (Direx®, 479 g ai L−1; Adama, Raleigh, NC) at 0.9 kg ai ha−1 was applied under a hooded sprayer on July 28, 2020. At New Deal, yield data were collected per plot using a two-row John Deere 7445 cotton stripper, and at Lubbock with a John Deere 7460 cotton stripper, both equipped with calibrated load cells to determine plot yield.
Plot size was four 101.6-cm rows, by 7.6 m in length with the center two rows receiving herbicide treatments. Treatments were arranged in a randomized complete block design with four replication (Table 1). All herbicides were applied using a CO2-pressurized backpack sprayer equipped with AIXR 11002 nozzles (TeeJet® Technologies, Glendale Heights, IL) calibrated to deliver 140 L ha−1 at 4.85 kph using 220 kPa.
Table 1. Herbicide treatments, rates, and application timings used in crop response and non-crop weed control experiments at all sites in 2019 and 2020.a
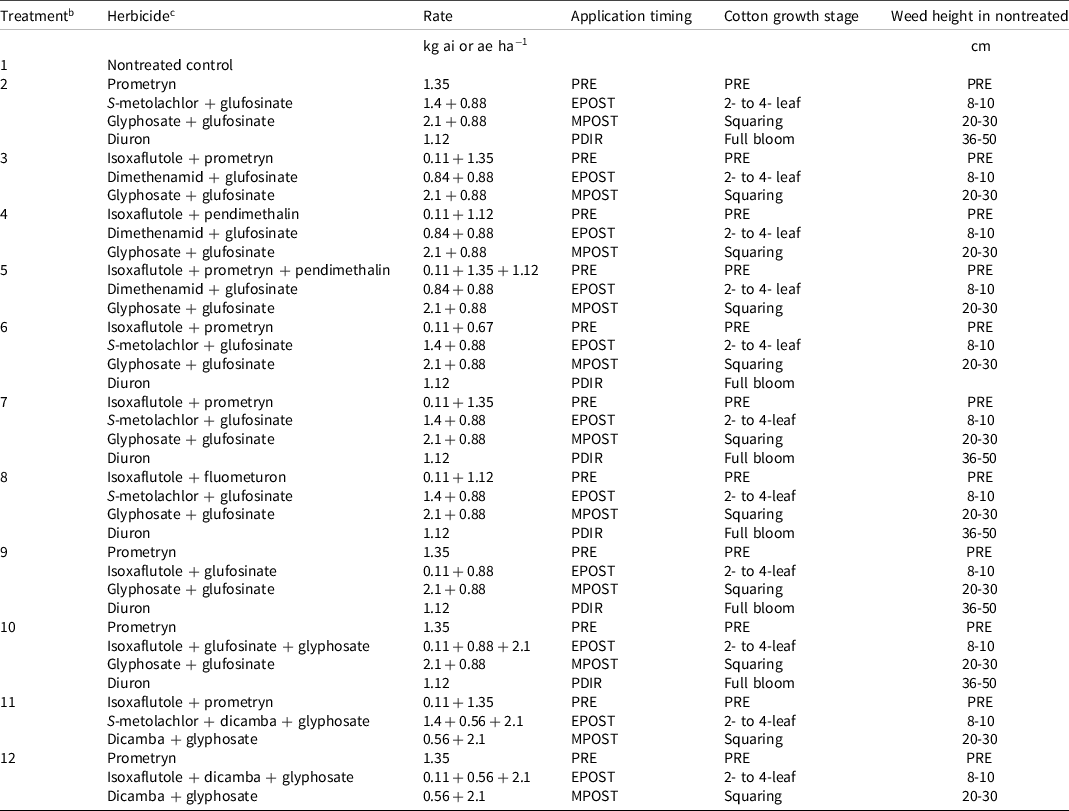
a Abbreviations: EPOST, early postemergence; MPOST, mid-postemergence; PDIR, postemergence-directed; PRE, preemergence.
b Treatments 11 and 12 were used in weed control experiments only.
c Ammonium sulfate (2.82 kg ha−1) was added to all treatments containing glufosinate.
Visual cotton response was evaluated on a 0% to 100% scale (0% being no visual response and 100% being all plants dead; Frans et al. Reference Frans, Talbert, Marx, Crowley and Camper1986) at both locations 14, 7, and 10 d after PRE, EPOST, and postemergence directed (PDIR) applications, respectively. Cotton stand was recorded in 2 m from the center two rows 21 d after the PRE application except in Lubbock in 2020, where stand was recorded 21 d after the replanting, which was 37 d after the initial PRE application. The height of six plants chosen randomly per plot (three from each of the center two rows) was recorded by measuring plants to the tallest part of the growing point 14 d after the EPOST application and just prior to harvest.
A 25-boll sample was collected at random from the center two rows of each plot just prior to mechanical harvest. Samples were ginned on a 20-saw tabletop gin to calculate lint percentage. Fiber samples were sent to Texas Tech’s Fiber and Biopolymer Institute in Lubbock, TX, in 2019, and to BASF Corporation’s internal laboratory in Leland, MS, in 2020, for fiber quality analysis using high-volume instrument testing. Lint yield was calculated on a per plot basis by multiplying plot yield by the lint percentage from the 25-boll sample.
Weed Management Studies
In 2019 and 2020, non-crop field experiments were conducted at the Texas A&M AgriLife Research Center (34.18°N, 101.94°W) in Halfway, TX. The soil was a Pullman clay loam (22.5% sand, 44.5% silt, and 33% clay), pH 8.4, and less than 1% organic matter. A blanket treatment of trifluralin at 1.12 kg ai ha−1 was applied and incorporated to a depth of 5 cm using a field cultivator on March 5, 2019, and March 6, 2020. All herbicide treatments (Table 1) were applied using a CO2-pressurized backpack sprayer equipped with AIXR 11002 nozzles (TeeJet® Technologies) calibrated to deliver 140 L ha−1 at 4.82 kph using 220 kPa. Overhead sprinkler irrigation was used to supplement rainfall and activate preemergence herbicides within 48 h of application using 1.9 cm of water.
Palmer amaranth control was evaluated on a 0% to 100% scale (0% being no control and 100% being no Palmer amaranth present; Frans et al. Reference Frans, Talbert, Marx, Crowley and Camper1986) 14 and 21 d after the PRE application, 21 d after the EPOST and mid-postemergence applications, and 10 d after the PDIR application. Palmer amaranth density was recorded by counting the total number of plants present between the center two rows of four 101.6-cm rows, by 102 cm in length 21 d after the EPOST applications. Total in-season irrigation was 95 mm and 398 mm in 2019 and 2020, respectively. Total in-season rainfall was 233 mm in 2019 and 123 mm in 2020.
Data Analysis
Data were analyzed using the GLIMMIX procedure with SAS software (version 9.4; SAS Institute Inc., Cary, NC) for analysis of variance and Tukey’s highly significant difference at α = 0.05. For cotton response experiments, locations were analyzed separately. For experiments at the Lubbock site, years also were analyzed separately because of the need to replant cotton in 2020. Year was considered a random effect at the New Deal site and in the weed management experiments at the Halfway site to broaden the inference space and account for environmental variability when making a recommendation (Blouin et al. Reference Blouin, Webster and Bond2011; Carmer et al. Reference Carmer, Nyquist and Walker1989; Moore and Dixon Reference Moore and Dixon2014).
Results and Discussion
Cotton Response Experiments
At 14 d after the PRE treatment, cotton response (stunting and chlorosis) was ≤6% for all treatments at the New Deal site (Table 2). At the Lubbock site in 2019, cotton response ranged from 1% to 14%, with isoxaflutole plus fluometuron or pendimethalin applied PRE resulting in 1% injury to cotton and isoxaflutole plus prometryn PRE resulting in 14% cotton injury. Synergism has been observed between the herbicides that inhibit photosystem II and those that inhibit p-hydroxyphenylpyruvate dioxygenase, which could explain the increase in cotton response following the application of isoxaflutole and prometryn in combination (Abendroth et al. Reference Abendroth, Martin and Roeth2006; Woodyard et al. Reference Woodyard, Bollero and Riechers2009). However, in 2020 at the Lubbock site, cotton response was <3% 14 d after replanting (31 d after the PRE application) and was similar for all treatments. Similarly, in HPPD-resistant soybean (Glycine max L. Merr.), Schultz et al. (Reference Schultz, Weber, Allen and Bradley2015) observed ≤2% injury following PRE application of isoxaflutole at rates up to 0.14 kg ai ha−1. Cotton response and densities were similar among treatments and the nontreated control at all locations and years (data not shown).
Table 2. Cotton response 14 d after planting at the New Deal and Lubbock sites in 2019 and 2020.

a Treatment means within a column followed by the same or no letter do not statistically differ according to Tukey’s highly significant difference test at α = 0.05.
b Cotton response data were combined across years for New Deal but separated for Lubbock.
c All herbicides were used according to labeled rates in kg ai ha−1: prometryn at 1.35, isoxaflutole at 0.11, fluometuron at 1.12, and pendimethalin at 1.12, except where ½ prometryn (0.675 kg ha−1) is specified.
At the New Deal site, cotton height in the nontreated control was 25 cm, while cotton height in herbicide-treated plots ranged from 23 to 24 cm 14 d after the EPOST treatments (Table 3). Isoxaflutole plus prometryn, pendimethalin, and prometryn plus pendimethalin followed by (fb) dimethenamid plus glufosinate as well as prometryn fb isoxaflutole plus glufosinate resulted in a 1-cm decrease in cotton height. In 2019 at the Lubbock site, cotton height in the nontreated control was 22 cm. Isoxaflutole plus prometryn plus pendimethalin fb dimethenamid plus glufosinate resulted in a 3-cm decrease in cotton height. In 2020, cotton height was 28 cm in the nontreated control, and it was decreased by 4 cm with the use of isoxaflutole plus pendimethalin fb dimethenamid plus glufosinate, isoxaflutole plus prometryn fb S-metolachlor plus glufosinate, and prometryn fb isoxaflutole plus glufosinate.
Table 3. Cotton heights 14 d after the EPOST application at the New Deal and Lubbock sites in 2019 and 2020. a
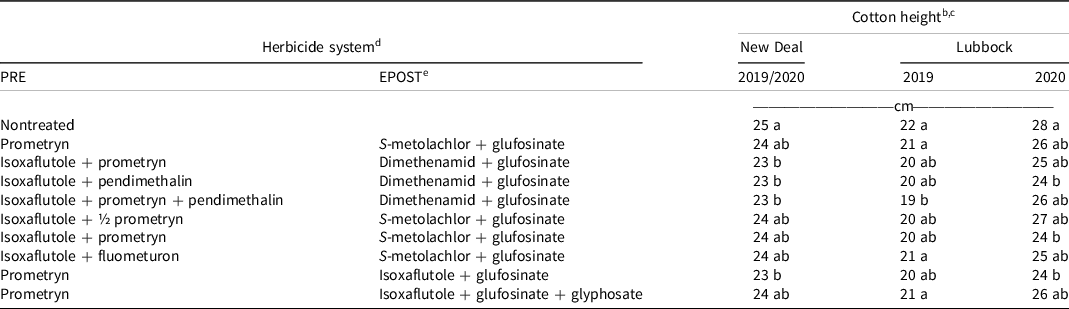
a Abbreviations: EPOST, early postemergence; PRE, preemergence.
b Cotton height data were combined across years for the New Deal site but separated for Lubbock.
c Treatment means within a column followed by the same or no letter do not statistically differ according to Tukey’s honestly significant difference test at α = 0.05.
d All herbicides were used according to labeled rates in kg ai ha−1: prometryn at 1.35, isoxaflutole at 0.11, fluometuron at 1.12, pendimethalin at 1.12, S-metolachlor at 1.4, dimethenamid at 0.84, glufosinate at 0.88, and glyphosate at 2.1, except where ½ prometryn (0.675 kg ha−1) is specified.
e Ammonium sulfate (2.52 kg ha−1) was included in all treatments containing glufosinate.
At the New Deal site, cotton lint yield ranged from 1,214 to 1,425 kg ha−1 and did not differ from that of the nontreated control, which yielded 1,416 kg ha−1 (Table 4). At the Lubbock site in 2019, cotton lint yield ranged from 675 to 758 kg ha−1, and yields following all herbicide treatments did not differ from that of the nontreated control (730 kg ha−1). In 2020, lint yield ranged from 1,544 to 1729 kg ha−1, and yields for all herbicide treatments were similar to that of the nontreated control (1,729 kg ha−1).
Table 4. Cotton lint yield for the New Deal and Lubbock sites in 2019 and 2020 trials. a
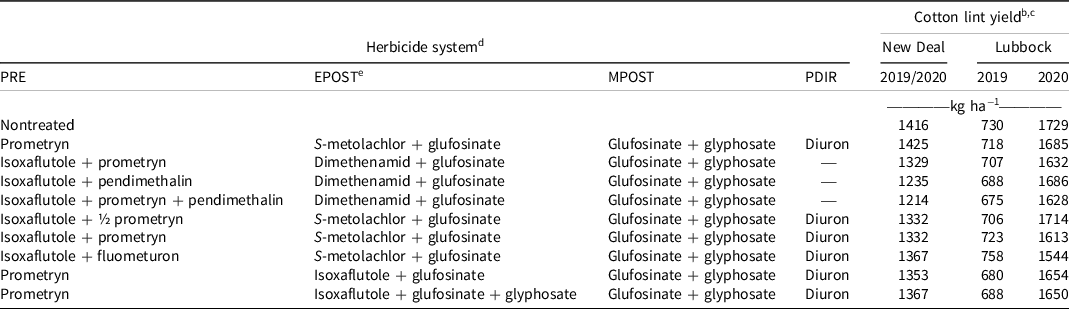
a Abbreviations: EPOST, early postemergence; MPOST, mid-postemergence; PDIR, postemergence-directed; PRE, preemergence.
b Cotton lint yield data were combined across years for the New Deal site but separated for Lubbock.
c Treatment means within a column followed by the same or no letter do not statistically differ according to Tukey’s honestly significant difference test at α = 0.05.
d All herbicides were used according to labeled rates in kg ai ha−1: prometryn at 1.35, isoxaflutole at 0.11, fluometuron at 1.12, pendimethalin at 1.12, S-metolachlor at 1.4, dimethenamid at 0.84, glufosinate at 0.88, glyphosate at 2.1, and diuron at 1.12, except where ½ prometryn (0.675 kg ha−1) is specified.
e Ammonium sulfate (2.52 kg ha−1) was included in all treatments containing glufosinate.
Weed Management Studies
At 21 d after the PRE application, all treatments containing isoxaflutole controlled Palmer amaranth by ≥94%, while prometryn provided 89% control of Palmer amaranth (Table 5). Isoxaflutole plus fluometuron and isoxaflutole plus prometryn plus pendimethalin provided complete control of Palmer amaranth. All treatments controlled Palmer amaranth by ≥94% 21 d after the EPOST application (Table 6). Palmer amaranth density 21 d after the EPOST treatment ranged from 269 to 4,777 plants ha−1 in herbicide-treated plots and 40,365 plants ha−1 in the nontreated control. When compared to the nontreated control, all systems that included isoxaflutole applied PRE decreased Palmer amaranth density by 96% to 99%, whereas treatments with isoxaflutole applied EPOST decreased Palmer amaranth density by 88% to 94%. Prometryn fb S-metolachlor plus glufosinate decreased Palmer amaranth density by 90%, which was similar to all isoxaflutole treatments and density was greatly reduced when compared to the nontreated control. End-of-season weed control was similar among all herbicide combinations (Table 7). It is important to note that this weed management research was conducted on bareground in the absence of a crop to shade the ground, and should be considered a worst-case scenario and may not fully reflect these weed management programs when used with crops.
Table 5. Palmer amaranth control 21 d after PRE application at the Halfway, TX, site in 2019 and 2020 trials. a

a Abbreviation: PRE, preemergence.
b Treatment means within a column followed by the same or no letter do not statistically differ according to Tukey’s honestly significant difference test at α = 0.05.
c All herbicides were used according to labeled rates in kg ha−1: prometryn at 1.35, isoxaflutole at 0.11, fluometuron at 1.12, and pendimethalin at 1.12, except where ½ prometryn (0.675 kg ha−1) is specified.
d Palmer amaranth control data were combined across years.
Table 6. Palmer amaranth control and counts 21 d after the EPOST application at the Halfway, TX, site in 2019 and 2020 trials. a
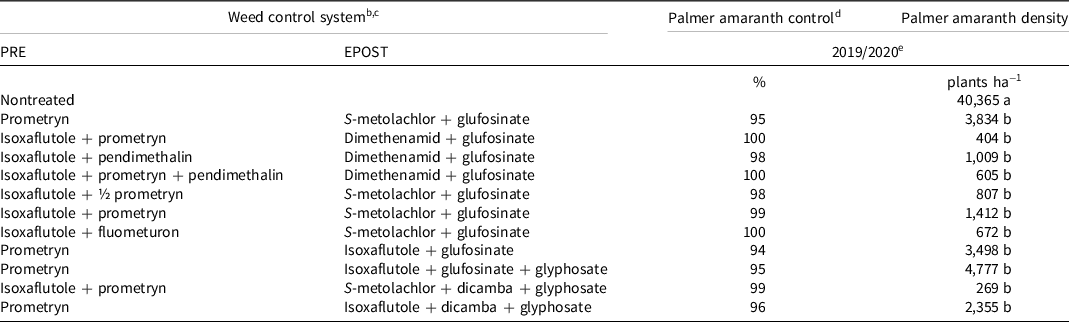
a Abbreviations: EPOST, early postemergence; PRE, preemergence.
b All herbicides were used according to labeled rates in kg ai or ae ha−1: prometryn at 1.35, isoxaflutole at 0.11, fluometuron at 1.12, pendimethalin at 1.12, S-metolachlor at 1.4, dimethenamid at 0.84, glufosinate at 0.88, glyphosate at 2.1, and dicamba at 0.56, except where ½ prometryn (0.675 kg ha−1) is specified.
c Ammonium sulfate (2.52 kg ha−1) was included in all treatments containing glufosinate.
d Treatment means within a column followed by the same or no letter do not statistically differ according to Tukey’s honestly significant difference test at α =0.05.
e Palmer amaranth control and density data were combined across years.
Table 7. Palmer amaranth control 10 d after the POST-directed application at the Halfway, TX, site in 2019 and 2020 trials. a
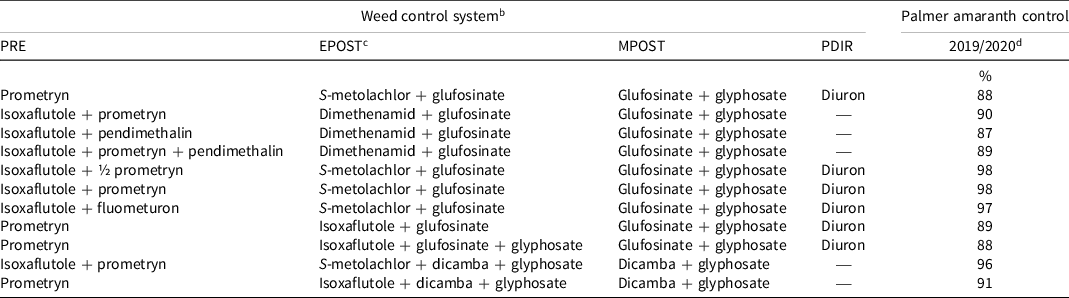
a Abbreviations: EPOST, early postemergence; MPOST, mid-postemergence; PDIR, postemergence-directed; PRE, preemergence.
b All herbicides were used according to labeled rates in kg ai or ae ha−1: prometryn at 1.35, isoxaflutole at 0.11, fluometuron at 1.12, pendimethalin at 1.12, S-metolachlor at 1.40, dimethenamid at 0.84, glufosinate at 0.88, glyphosate at 2.10, dicamba at 0.56, and diuron at 1.12, except where ½ prometryn (0.675 kg ha−1) is specified.
c Ammonium sulfate (2.52 kg ha−1) was included in all treatments containing glufosinate.
d Palmer amaranth control data were combined across years.
When applied to corn fields, isoxaflutole applied PRE alone fb glufosinate POST controlled Palmer amaranth by 91% at the end of the season (Stephenson and Bond Reference Stephenson and Bond2012). Similar to this new technology in cotton, a variety of soybean tolerance to isoxaflutole also has been developed. When used in soybean fields, isoxaflutole at 105 g plus metribuzin, a common PRE treatment in soybean, provided full-season residual control of Amaranthus species (Smith et al. Reference Smith, Soltani, Kaastra, Hooker, Robinson and Sikkema2019). In cotton, fluometuron and prometryn are common PRE herbicides, both of which increased season-long broadleaf weed control compared to a POST-only system (Porterfield et al. Reference Porterfield, Wilcut and Askew2002; Scroggs et al. Reference Scroggs, Miller, Griffin, Wilcut, Blouin, Stewart and Vidrine2007). Similarly, Grichar et al. (Reference Grichar, Besler, Brewer and Minton2004) found that mixing PRE herbicides in cotton increased season-long control of Amaranthus species while at the same time diversifying weed control programs.
Conclusions
These studies suggests that the opportunity to use isoxaflutole in HPPD-resistant cotton weed management systems will present no adverse effects on cotton yield and fiber quality. Cotton density and lint yield were not affected by treatments of isoxaflutole applied PRE or EPOST when compared to the nontreated control. When used as part of a weed management program, whether applied PRE or EPOST, isoxaflutole effectively controlled Palmer amaranth. The opportunity to use isoxaflutole will add a novel mode of action in cotton and will provide season-long control of Palmer amaranth when integrated as part of an overall weed management program without risk of negative crop response.
Acknowledgments
We thank BASF Corporation and the Texas State Support Committee – Cotton Incorporated for funding this project. No other conflicts of interest are noted.










