Hypertensive disorders of pregnancy (HDP) are a group of conditions related to high blood pressure during pregnancy, proteinuria and in some cases convulsions( 1 ). HDP are responsible for increased morbidity and mortality in mothers and newborns, accounting for approximately 14 % of maternal deaths globally between 2003 and 2009( Reference Say, Chou and Gemmill 2 ). According to an analysis of international cohorts from six countries (Australia/New Zealand, Canada, Israel, Japan, Spain and Sweden), the incidence rate of HDP was 13 % (ranging from 10·3 to 16·4 %)( Reference Gemmell, Martin and Murphy 3 ).
Preterm birth (PTB) is the premature delivery of a neonate before 37 weeks of gestation( Reference Lawn, Gravett and Nunes 4 ). PTB is most common in low- and middle-income countries and is one of the leading causes of direct neonatal deaths and complications( Reference Lawn, Gravett and Nunes 4 ), responsible for more than 50 % of neonatal mortality in 2010( Reference Blencowe, Cousens and Chou 5 ). According to a systematic analysis and estimation of PTB, the rate of PTB was 11 % in 2010 globally, ranging from 5 % in European countries to 18 % in some African countries( Reference Blencowe, Cousens and Oestergaard 6 ). Likewise, low birth weight (LWB), which refers to a newborn birth weight of less than 2·5 kg, is common (15 %). High rates are reported in many developing countries, especially South Asia (25 %), sub-Saharan Africa (12 %)( Reference Ramakrishnan 7 ), Pakistan (35 %), Nepal (30 %) and Jordan (22 %)( Reference Mahumud, Sultana and Sarker 8 ).
Evidence has shown that dietary patterns have an influence on adverse pregnancy and birth outcomes( Reference Englund-Ogge, Brantsaeter and Sengpiel 9 , Reference Brantsaeter, Haugen and Samuelsen 10 ). When individuals consume foods, they consume a combination of nutrients, not single nutrients( Reference Jacques and Tucker 11 ). The whole diet with its expected synergistic effects may have a greater influence on the occurrence of health outcomes than single nutrients( Reference Jacques and Tucker 11 ). Hence, it appears more complete to examine the effect of the whole diet by applying a more all-inclusive method of dietary pattern analysis, because dietary patterns evaluate the usual diet as one complete dietary exposure( Reference Hu 12 , Reference Wirfält, Drake and Wallström 13 ).
Dietary pattern analysis aims to assess the usual foods consumed as one overall dietary exposure( Reference Hu 12 , Reference Kant 14 ). Dietary patterns are defined as the quantities, proportions, variety or combinations of different foods and beverages in diets and the frequency with which they are regularly consumed( 15 ). Dietary patterns can be determined by three approaches. The first is the a priori approach, which constructs dietary scores or indices based on predefined dietary recommendations( Reference Hu 12 , Reference Kant 14 , Reference Ocke 16 ). The second is the a posteriori approach, which identifies data-driven dietary patterns using statistical methods (cluster analysis and principal component analysis (PCA))( Reference Hu 12 , Reference Kant 14 , Reference Ocke 16 ). The third approach consists of hybrid methods such as reduced rank regression, which combine aspects of the a priori and a posteriori approaches( Reference Ocke 16 ).
Previous studies have indicated that dietary patterns during pregnancy have a varied effect on maternal health and pregnancy outcomes such as HDP( Reference Brantsaeter, Haugen and Samuelsen 10 , Reference Rifas-Shiman, Rich-Edwards and Kleinman 17 ), GDM( Reference De Seymour, Chia and Colega 18 , Reference He, Yuan and Chen 19 ), PTB( Reference Englund-Ogge, Brantsaeter and Sengpiel 9 , Reference Haugen, Meltzer and Brantsaeter 20 , Reference Rasmussen, Maslova and Halldorsson 21 ) and LBW( Reference Kjøllesdal and Holmboe-Ottesen 22 ). For HDP, intake of vegetables, legumes, nuts, tofu, rice, pasta, rye bread, fish, milk, green leafy vegetables and pulses/beans was associated with a lower odds of pre-eclampsia/eclampsia( Reference Brantsaeter, Haugen and Samuelsen 10 , Reference Agrawal, Fledderjohann and Vellakkal 23 ), while the consumption of meat and potatoes, processed meat, sweet drinks and salty snacks increased the likelihood of pre-eclampsia( Reference Brantsaeter, Haugen and Samuelsen 10 , Reference Borgen, Aamodt and Harsem 24 , Reference Timmermans, Steegers-Theunissen and Vujkovic 25 ). Other studies have reported contradictory findings; a cohort study in the USA( Reference Rifas-Shiman, Rich-Edwards and Kleinman 17 ) reported that a higher Alternate Healthy Eating Index (AHEI) score comprising vegetables, fruit, fibre, trans fat, high PUFA:SFA, folate, Ca and Fe from foods was not associated with pre-eclampsia. For GDM, a Western dietary pattern (high intake of red meat, processed meat, refined grain products, sweets, French fries and pizza) among pregnant women in the USA( Reference Zhang, Schulze and Solomon 26 ), a pasta–cheese–processed-meat pattern( Reference De Seymour, Chia and Colega 18 ) in a Singaporean population and a sweet and seafood pattern in China( Reference He, Yuan and Chen 19 ) have been associated with increased odds of GDM.
Regarding the birth outcomes, a ‘prudent’ dietary pattern with a high intake of vegetables, fruits, oils, water (beverage), wholegrain cereals and fibre-rich breads was associated with a reduced occurrence of PTB( Reference Englund-Ogge, Brantsaeter and Sengpiel 9 ). In contrast, a Western pattern (salty and sweet snacks, white bread, desserts and processed meat products)( Reference Englund-Ogge, Brantsaeter and Sengpiel 9 ) and a Mediterranean diet with a high intake of fish, fruit, vegetables, olive/canola oil, and a low intake of red meat and coffee had no effect on PTB( Reference Haugen, Meltzer and Brantsaeter 20 ). Contrary to this, in a Danish birth cohort study, the odds of PTB increased in women who adhered to a Western pattern (high in meat and fats and low in fruits and vegetables)( Reference Rasmussen, Maslova and Halldorsson 21 ). A study from the USA( Reference Poon, Yeung and Boghossian 27 ) revealed that birth weight and fetal growth were not associated with the maternal AHEI score (high intakes of vegetables, fruit, whole grains, nuts and legumes, long-chain (n-3) fats, polyunsaturated fats, folate, Ca and Fe).
Current epidemiological studies show some evidence for an association between dietary patterns and adverse pregnancy and birth outcomes. However, the findings are inconsistent and there is a need to identify which dietary patterns could have health benefits for pregnant women in preventing adverse pregnancy and birth outcomes. Therefore, our aim was to determine the association between dietary patterns during pregnancy and the risk of pregnancy (HDP, GDM) and birth (PTB and LBW) outcomes through a systematic review and meta-analysis.
Methods
Search strategy
Seven databases were searched, including MEDLINE, EMBASE, CINAHL, Scopus, Cochrane Library, Web of Science, and Maternity and Infant Care. The reference lists of all previous articles were hand-searched.
The following terms, words and combinations of words were searched: (‘diet’ OR ‘nutrition’ OR ‘food pattern’ OR ‘meal pattern’ OR ‘eating practice’ OR ‘food intake’ OR ‘food habits’ OR ‘eating behaviour’ OR ‘dietary pattern’ OR ‘dietary diversity score’ AND ‘pregnancy’ OR ‘pregnant women’ OR ‘gravid’ OR ‘gestation’ OR ‘prenatal care’ OR ‘antenatal care’ AND ‘gestational hypertension’ OR ‘pregnancy-induced hypertension’ OR ‘preeclampsia’ OR ‘pre-eclampsia’ OR ‘low birth weight’ OR ‘premature infant’ OR ‘premature birth’ OR ‘preterm birth’ OR ‘pregnancy in diabetics’ OR ‘gestational diabetes mellitus’).
The search was comprised of free text words, title and Medical Subject Heading for outcomes, exposure and participants, as well as applying limits including English language and human subjects.
Study selection
The studies were screened by title and then by abstract by two reviewers (K.T.K., T.K.T.). The full texts of all selected studies were critically reviewed based on the inclusion/exclusion criteria summarized in Table 1.
Table 1 Inclusion and exclusion criteria for the current systematic review and meta-analysis on maternal dietary patterns and risk of adverse pregnancy and birth outcomes

HDP, hypertensive disorders of pregnancy (gestational hypertension, pre-eclampsia and eclampsia); GDM, gestational diabetes mellitus; LBW, low birth weight; PTB, preterm birth.
Data extraction
The following variables were extracted by two reviewers (K.T.K., T.K.T.): authors, publication year, study period, study design, settings/country, sample, dietary patterns with food details, dietary assessment methods and periods, main outcomes (HDP, GDM, LBW and PTB) and adjustment for confounding factors.
Quality assessments
The quality of selected full-text articles was rated by two reviewers independently (K.T.K., T.K.T.) using the Academy of Nutrition and Dietetics quality appraisal tool( 28 ). This tool has four relevance questions and ten validity questions. The validity questions appraise the selection, comparability of groups, assessment of exposures or outcomes and statistical analysis for each study separately( 28 ). The validity of a study is assessed as the responses to all relevant questions being ‘yes’. The response for all validity questions is ‘yes’ if the criterion was fulfilled, ‘no’ if not fulfilled, ‘unclear’ if not precisely stated and ‘N/A’ (not applicable) if the criterion does not apply to the articles( 28 ). The rating scores of studies were positive (+) if the responses to the validity questions were ‘yes’ for six or more responses (including all four relevance questions). If the articles did not fulfil the relevance criterion of selection, comparability of groups and measurement of exposures or outcomes, the rating score was neutral (Ø) and if the responses for the validity questions are ‘no’ or ‘unclear’ for six or more responses, a negative (−) rating score was given( 28 ).
Statistical analysis
The data were entered into a Microsoft® Excel spreadsheet version 16 and exported to the statistical software package Stata version 13 for analysis. The OR was used as a measure of effect estimate. If an incidence of outcome variable was less than or equal to 20 %, the risk ratio (RR) and OR were pooled together in the meta-analysis; otherwise RR was converted to OR using the proposed methods of Zhang and Yu( Reference Zhang and Yu 29 ) and Cochrane( Reference Higgins and Green 30 ). If the studies did not report OR/RR but reported the coefficient (β) of the regression, it was converted into OR/RR by exponentiation of the coefficient (i.e. OR=exp(β))( Reference Wiest, Lee and Carlin 31 ).
Some articles reported OR/RR based on different references. Some used lower adherence to dietary patterns, while some used good adherence. To make this consistent and unify all results using either the higher or lower group as reference, the new OR/RR was calculated by taking the reciprocal of the reported OR/RR. The lower limit of the new OR/RR is the reciprocal of the upper limit of the old OR/RR and the upper limit of the new OR/RR is reciprocal of the lower limit of the old OR/RR( Reference Pryor 32 ).
The random-effects model was used for calculating pooled estimates. Statistical heterogeneity was evaluated by Cochran’s Q test (I 2), which shows the amount of heterogeneity between studies. An I 2 value reflects between-study variation (values of 25, 50 and 75 % refer to low, medium and high variation, respectively)( Reference Borenstein, Hedges and Higgins 33 ).
Subgroup analyses were conducted to detect potential sources of heterogeneity. The possible effects of between-study variance of dietary assessment methods (dietary diversity score (DDS), Mediterranean diet score (MDS), PCA) and dietary assessment periods/trimesters (first trimester (1st–12th weeks), second trimester (13th–27th weeks), third trimester (28th–40th weeks)) were assessed.
Dietary patterns detected in each study were different regarding to the country of origin and the approaches used for identifying dietary patterns; however, they had similarities among commonly consumed food items. For instance, most articles identified a prudent, traditional, Mediterranean or healthy dietary pattern which commonly consisted of whole grains, nuts legumes/pulses, vegetables/fruits and fish. These studies were grouped together and analysed by labelling them as ‘healthy dietary pattern’.
Similarly, those patterns comprised mostly of refined grains, processed meats or snacks, high-sugar and high-fat dairy products, eggs and white potatoes were grouped together, labelled as the ‘Western dietary pattern’ and then analysed.
Using the available articles, pooled estimates were determined for the effect of the healthy pattern on HDP, GDM, PTB and LBW. Likewise, meta-analysis was performed for a Western dietary pattern and GDM, HDP and PTB.
Results
Identified studies
Our search identified 6291 records after removal of duplicates. One hundred articles were identified for full-text review, with twenty-one articles incorporated in the systematic review and meta-analysis (Fig. 1).
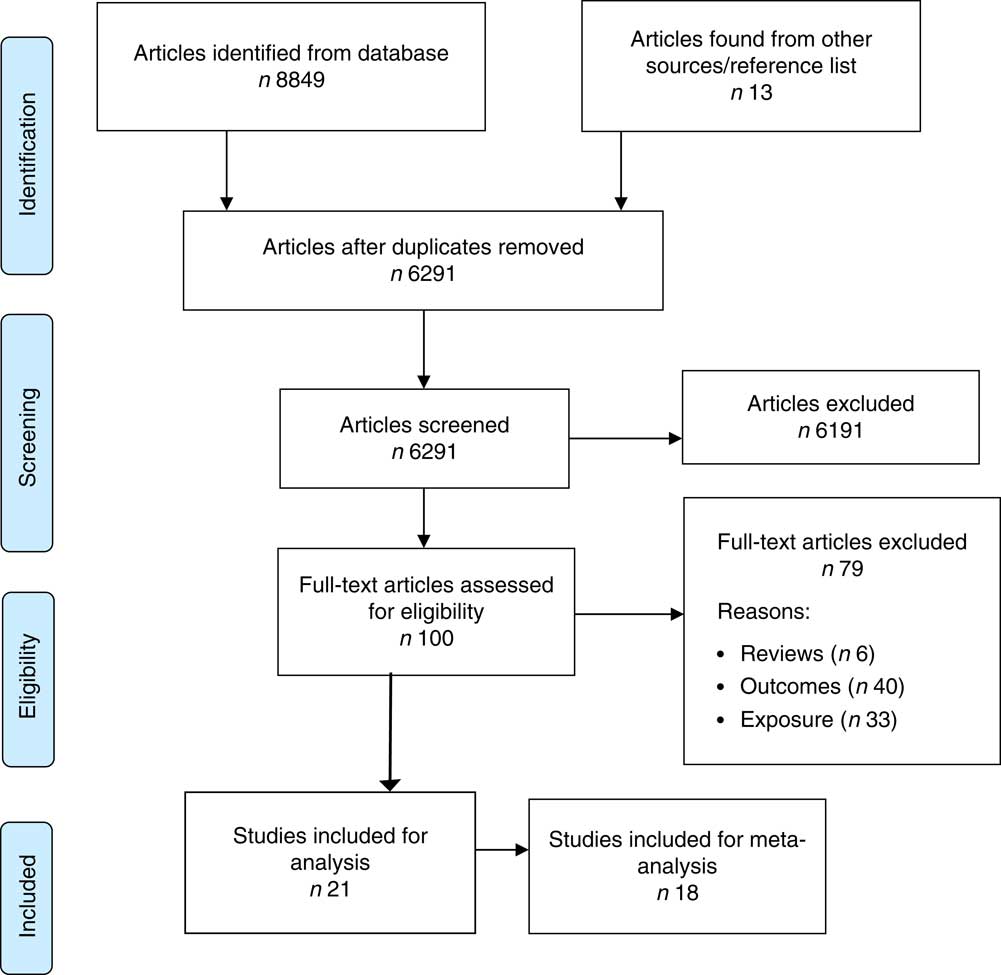
Fig. 1 (colour online) Flowchart of the study selection process for the current systematic review and meta-analysis on maternal dietary patterns and risk of adverse pregnancy and birth outcomes
Study characteristics
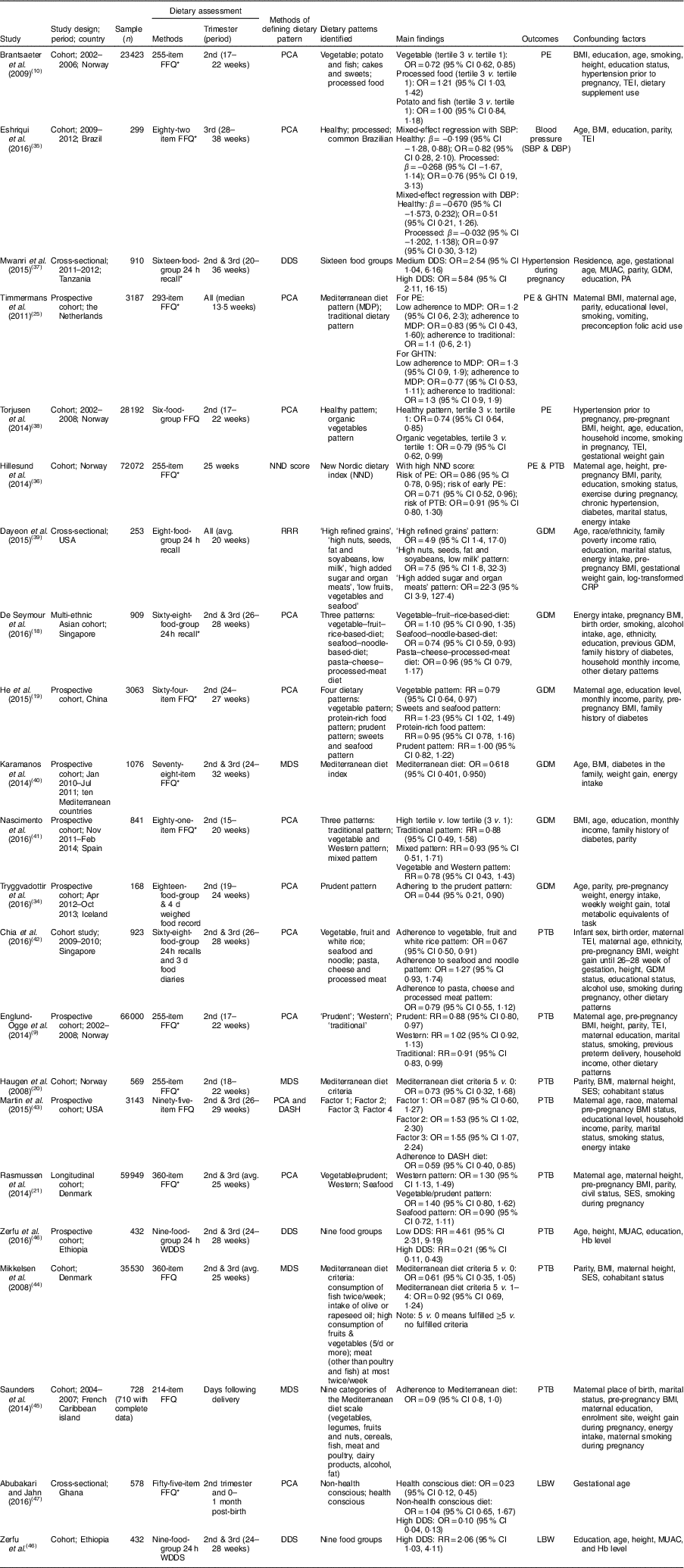
Of the twenty-one articles included, the majority (n 15) were conducted in developed countries, with the remainder in developing countries. Out of all included articles, eighteen were cohort studies and three were cross-sectional studies. The articles were published between 2008 and 2016. The sample in each study ranged from 168( Reference Tryggvadottir, Medek and Birgisdottir 34 ) to 66 000( Reference Englund-Ogge, Brantsaeter and Sengpiel 9 ) with 302 450 pregnant women in total. In the included articles, six reported the effect of dietary patterns on HDP( Reference Brantsaeter, Haugen and Samuelsen 10 , Reference Timmermans, Steegers-Theunissen and Vujkovic 25 , Reference Eshriqui, Vilela and Rebelo 35 – Reference Torjusen, Brantsaeter and Haugen 38 ), six reported on GDM( Reference De Seymour, Chia and Colega 18 , Reference He, Yuan and Chen 19 , Reference Tryggvadottir, Medek and Birgisdottir 34 , Reference Dayeon, Kyung Won and Won 39 – Reference Nascimento, Alves and Fonseca 41 ), nine reported on PTB( Reference Englund-Ogge, Brantsaeter and Sengpiel 9 , Reference Haugen, Meltzer and Brantsaeter 20 , Reference Rasmussen, Maslova and Halldorsson 21 , Reference Hillesund, Overby and Engel 36 , Reference Chia, de Seymour and Colega 42 – Reference Zerfu, Umeta and Baye 46 ) and two reported on LBW( Reference Zerfu, Umeta and Baye 46 , Reference Abubakari and Jahn 47 ) (Table 2).
Table 2 Characteristics of the articles included in the current systematic review and meta-analysis on maternal dietary patterns and risk of adverse pregnancy and birth outcomes
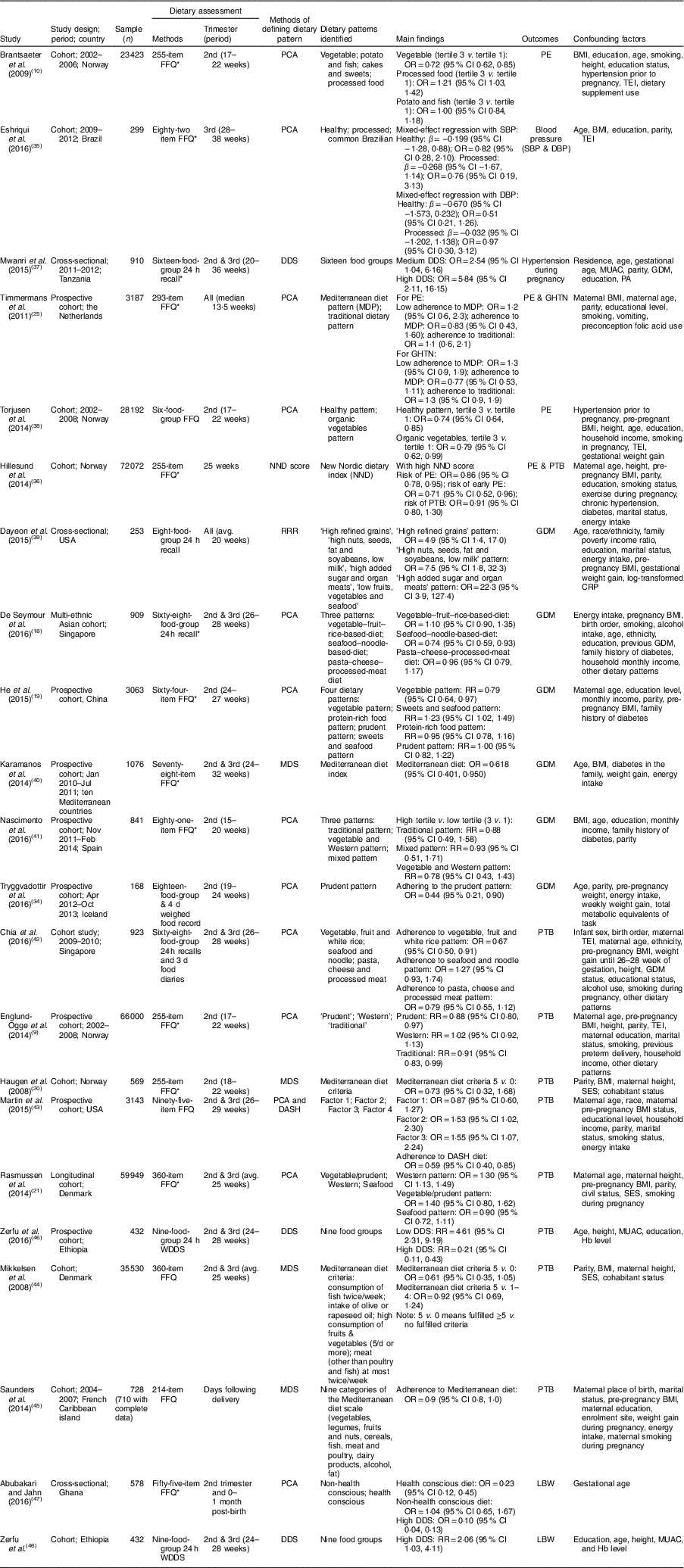
WDDS, Women Dietary Diversity Score; avg., average; PCA, principal component analysis; DDS, dietary diversity score; RRR, reduced rank regression; MDS, Mediterranean diet score; DASH, Dietary Approaches to Stop Hypertension; SBP, systolic blood pressure; β, regression coefficient; DBP, diastolic blood pressure; PE, pre-eclampsia; GHTN, gestational hypertension; RR, risk ratio; PTB, preterm birth; GDM, gestational diabetes mellitus; LBW, low birth weight; TEI, total energy intake; MUAC, mid-upper arm circumference; PA, physical activity; CPR, C-reactive protein; SES, socio-economic status.
* Validated FFQ or recall was used.
Most of the articles (n 15) used an FFQ( Reference Englund-Ogge, Brantsaeter and Sengpiel 9 , Reference Brantsaeter, Haugen and Samuelsen 10 , Reference He, Yuan and Chen 19 – Reference Rasmussen, Maslova and Halldorsson 21 , Reference Timmermans, Steegers-Theunissen and Vujkovic 25 , Reference Eshriqui, Vilela and Rebelo 35 , Reference Hillesund, Overby and Engel 36 , Reference Torjusen, Brantsaeter and Haugen 38 , Reference Karamanos, Thanopoulou and Anastasiou 40 , Reference Nascimento, Alves and Fonseca 41 , Reference Martin, Sotres-Alvarez and Siega-Riz 43 – Reference Saunders, Guldner and Costet 45 , Reference Abubakari and Jahn 47 ) as the method of dietary assessment, five studies used a 24h recall( Reference De Seymour, Chia and Colega 18 , Reference Mwanri, Kinabo and Ramaiya 37 , Reference Dayeon, Kyung Won and Won 39 , Reference Chia, de Seymour and Colega 42 , Reference Zerfu, Umeta and Baye 46 ), and one used a four-day food record( Reference Tryggvadottir, Medek and Birgisdottir 34 ) to assess the dietary intake. Various types of approaches were used to identify dietary patterns. Most studies applied the a posteriori approach (PCA; n 13)( Reference Englund-Ogge, Brantsaeter and Sengpiel 9 , Reference Brantsaeter, Haugen and Samuelsen 10 , Reference De Seymour, Chia and Colega 18 , Reference He, Yuan and Chen 19 , Reference Rasmussen, Maslova and Halldorsson 21 , Reference Timmermans, Steegers-Theunissen and Vujkovic 25 , Reference Tryggvadottir, Medek and Birgisdottir 34 , Reference Eshriqui, Vilela and Rebelo 35 , Reference Torjusen, Brantsaeter and Haugen 38 , Reference Nascimento, Alves and Fonseca 41 – Reference Martin, Sotres-Alvarez and Siega-Riz 43 , Reference Abubakari and Jahn 47 ); seven studies used the a priori method, DDS( Reference Mwanri, Kinabo and Ramaiya 37 , Reference Zerfu, Umeta and Baye 46 ) or MDS( Reference Haugen, Meltzer and Brantsaeter 20 , Reference Karamanos, Thanopoulou and Anastasiou 40 , Reference Mikkelsen, Osterdal and Knudsen 44 , Reference Saunders, Guldner and Costet 45 ) or New Nordic Diet (NND)( Reference Hillesund, Overby and Engel 36 ); and one study used the rank reduced regression method( Reference Dayeon, Kyung Won and Won 39 ) to identify the dietary pattern of the women (Table 2). All studies had a positive score for the quality assessment.
The association of dietary patterns with adverse pregnancy outcomes (hypertensive disorders of pregnancy and gestational diabetes mellitus)
Dietary pattern and hypertensive disorders of pregnancy
Six articles( Reference Brantsaeter, Haugen and Samuelsen 10 , Reference Timmermans, Steegers-Theunissen and Vujkovic 25 , Reference Eshriqui, Vilela and Rebelo 35 – Reference Torjusen, Brantsaeter and Haugen 38 ) assessed the association between dietary pattern and HDP. These articles identified a range of different dietary patterns like healthy, traditional, Mediterranean and Western patterns, and therefore the results could not be pooled in meta-analysis except the healthy dietary pattern.
Healthy dietary pattern. Four studies( Reference Brantsaeter, Haugen and Samuelsen 10 , Reference Timmermans, Steegers-Theunissen and Vujkovic 25 , Reference Hillesund, Overby and Engel 36 , Reference Torjusen, Brantsaeter and Haugen 38 ) were available for meta-analysis that reported the association between a healthy dietary pattern with a high intake of fruits, vegetables, whole-grain foods, fish and poultry and HDP. Based on this pooled analysis, study participants who adhered to a healthy dietary pattern were shown to have significantly lower odds of pre-eclampsia (OR=0·78, 95 % CI 0·70, 0·86; I 2=39·0 %, P=0·178; Fig. 2).
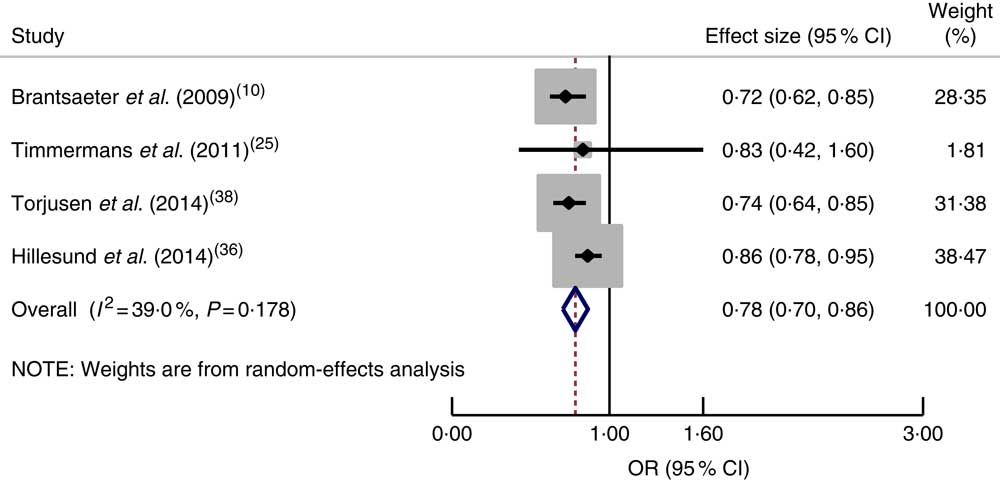
Fig. 2 (colour online) Forest plot for the pooled OR of the association between a healthy dietary pattern and pre-eclampsia. The study-specific OR and 95 % CI are represented by the black diamond and the horizontal line, respectively; the area of the grey square is proportional to the specific-study weight to the overall meta-analysis. The centre of the blue open diamond and the red dashed vertical line represent the pooled OR; and the width of the blue open diamond represents the pooled 95 % CI
However, one( Reference Mwanri, Kinabo and Ramaiya 37 ) cross-sectional study in Tanzania indicated that having a high DDS (OR=5·84; 95 % CI 2·11, 16·15) or a medium DDS (OR=2·54; 95 % CI 1·04, 6·16) was associated with increased odds of gestational hypertension. On the contrary, in a cohort study, no association was observed between gestational hypertension and adherence to a Mediterranean pattern (OR=0·77; 95 % CI 0·53, 1·11) or a traditional pattern (OR=1·3; 95 % CI 0·9, 1·9)( Reference Timmermans, Steegers-Theunissen and Vujkovic 25 ). Likewise, a cohort study from Brazil( Reference Eshriqui, Vilela and Rebelo 35 ) revealed that adherence to a healthy dietary pattern did not have an effect on systolic blood pressure (OR=0·82; 95 % CI 0·28, 2·21) or diastolic blood pressure (OR=0·94; 95 % CI 0·18, 1·28).
Western dietary pattern. In a cohort study in Norway( Reference Brantsaeter, Haugen and Samuelsen 10 ), a potato and fish dietary pattern (lean fish, cooked potatoes, processed fish; fish burgers, margarine, fish soufflé, meat spread, lean fish and poultry) was not associated with pre-eclampsia (OR=1·00: 95 % CI 0·84, 1·18). Likewise, a cohort study in Brazil( Reference Eshriqui, Vilela and Rebelo 35 ) reported that adherence to a processed food pattern was not significantly associated with the change in systolic blood pressure (OR=0·76; 95 % CI 0·19, 3·13) and diastolic blood pressure (OR=0·97; 95 % CI 0·30, 3·10) during pregnancy.
Dietary pattern and gestational diabetes mellitus
Healthy dietary pattern. Six studies( Reference De Seymour, Chia and Colega 18 , Reference He, Yuan and Chen 19 , Reference Tryggvadottir, Medek and Birgisdottir 34 , Reference Dayeon, Kyung Won and Won 39 – Reference Nascimento, Alves and Fonseca 41 ) assessed the effect of dietary patterns on GDM. A cohort study in Singapore( Reference De Seymour, Chia and Colega 18 ) indicated that a seafood–noodle-based diet was related with lower odds of GDM (OR=0·74; 95 % CI 0·59, 0·93). However, higher v. lower adherence to a vegetable–fruit–rice-based diet (OR=1·10; 95 % CI 0·90, 1·35) and a pasta–cheese–processed-meat diet (OR=0·96; 95 % CI 0·79, 1·17) was not associated with GDM. Similarly, adherence to a traditional pattern (RR=0·88; 95 % CI 0·49, 1·58) as well as adherence to a mixed pattern (RR=0·93; 95 % CI 0·51, 1·71) was not associated with the incidence of GDM among Brazilian women( Reference Nascimento, Alves and Fonseca 41 ).
The pooled estimate of a healthy dietary pattern on GDM was determined by using five studies( Reference De Seymour, Chia and Colega 18 , Reference He, Yuan and Chen 19 , Reference Tryggvadottir, Medek and Birgisdottir 34 , Reference Karamanos, Thanopoulou and Anastasiou 40 , Reference Nascimento, Alves and Fonseca 41 ). Based on this estimate, women who had higher adherence to a healthy dietary pattern had lower odds of GDM (OR=0·78; 95 % CI 0·56, 0·99), with significant heterogeneity detected between studies (I 2=68·6 %, P=0·013; Fig. 3(a)).
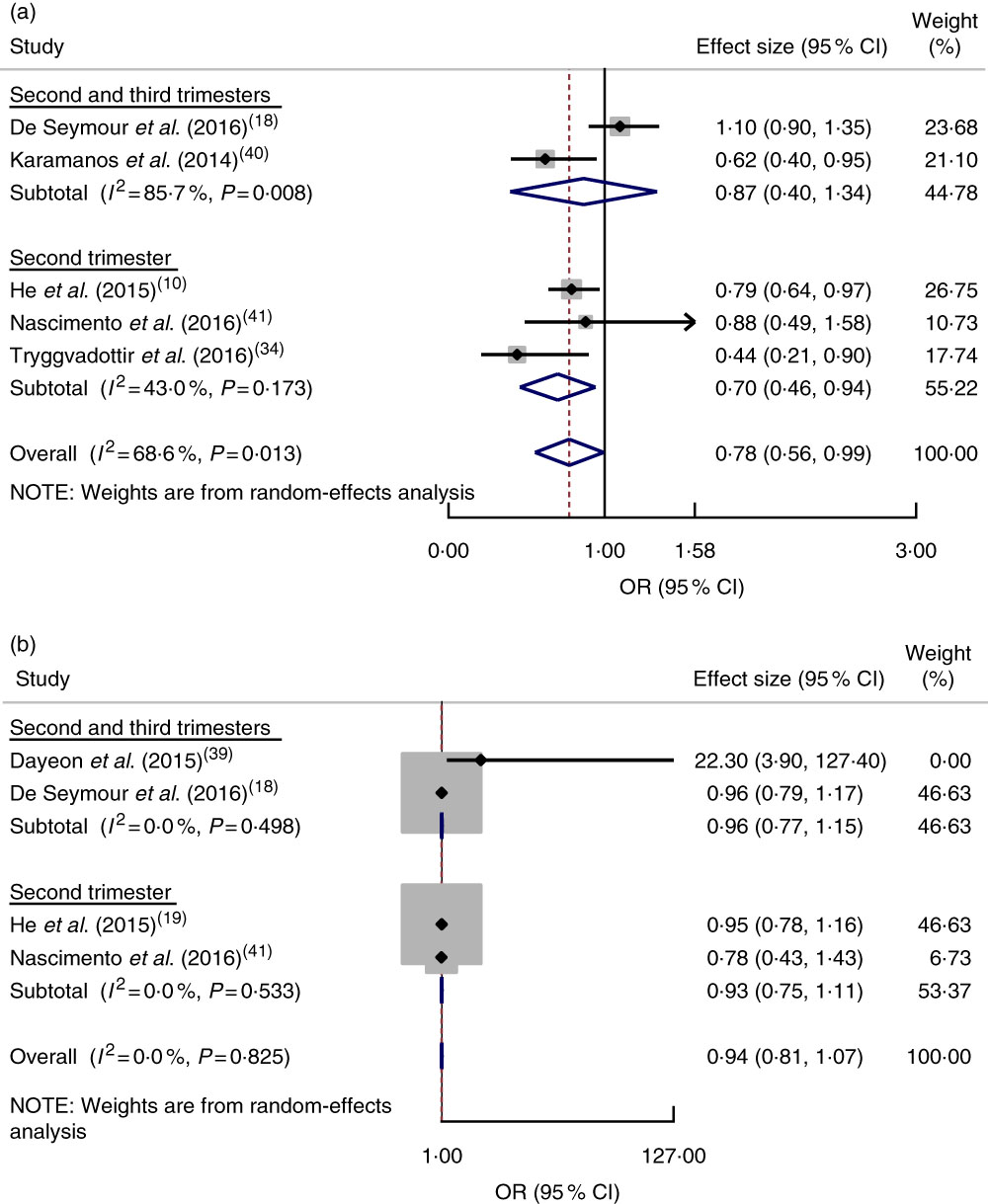
Fig. 3 (colour online) Forest plot for the pooled OR of the association between gestational diabetes mellitus (GDM) and different dietary patterns, with subgroup analysis regarding period of dietary assessment (second trimester v. both second and third trimesters): (a) association between GDM and healthy dietary pattern; (b) association between GDM and Western dietary pattern. The study-specific OR and 95 % CI are represented by the black diamond and the horizontal line, respectively; the area of the grey square is proportional to the specific-study weight to the overall meta-analysis. The centre of the blue open diamond and the red dashed vertical line represent the pooled OR; and the width of the blue open diamond represents the pooled 95 % CI
Western dietary pattern. Four studies( Reference De Seymour, Chia and Colega 18 , Reference He, Yuan and Chen 19 , Reference Dayeon, Kyung Won and Won 39 , Reference Nascimento, Alves and Fonseca 41 ) were combined, showing no relationship between adherence to a Western dietary pattern and odds of GDM (OR=0·94; 95 % CI 0·81, 1·07) and no heterogeneity between studies (I 2=0·0 %, P=0·825; Fig. 3(b)).
A cross-sectional survey in the USA( Reference Dayeon, Kyung Won and Won 39 ) and a prospective cohort study in China( Reference He, Yuan and Chen 19 ) reported that adherence to dietary patterns of refined grains (OR=4·9; 95 % CI 1·4, 17·0), high nuts, seeds, fat and soyabeans, low milk (OR=7·5; 95 % CI 1·8, 32·3), and sweets and seafood (RR=1·23; 95 % CI 1·02, 1·49) during pregnancy was associated with an increased likelihood of GDM.
The association between dietary patterns and adverse birth outcomes (preterm birth and low birth weight)
Dietary pattern and preterm birth
Based on a meta-analysis of nine studies( Reference Englund-Ogge, Brantsaeter and Sengpiel 9 , Reference Haugen, Meltzer and Brantsaeter 20 , Reference Rasmussen, Maslova and Halldorsson 21 , Reference Hillesund, Overby and Engel 36 , Reference Chia, de Seymour and Colega 42 – Reference Zerfu, Umeta and Baye 46 ), women who had good adherence to a healthy dietary pattern were shown to have reduced odds of PTB (OR=0·75; 95 % CI 0·57, 0·93), although significant heterogeneity was observed (I 2=89·6 %, P=0·0001; Fig. 4(a)). Further subgroup analysis indicated a difference in relation to dietary pattern assessment method (MDS, DDS or PCA; P=0·001). There was also a significant subgroup difference regarding dietary assessment period (second trimester or both second and third trimesters; P=0·001; Fig. 4(b)).
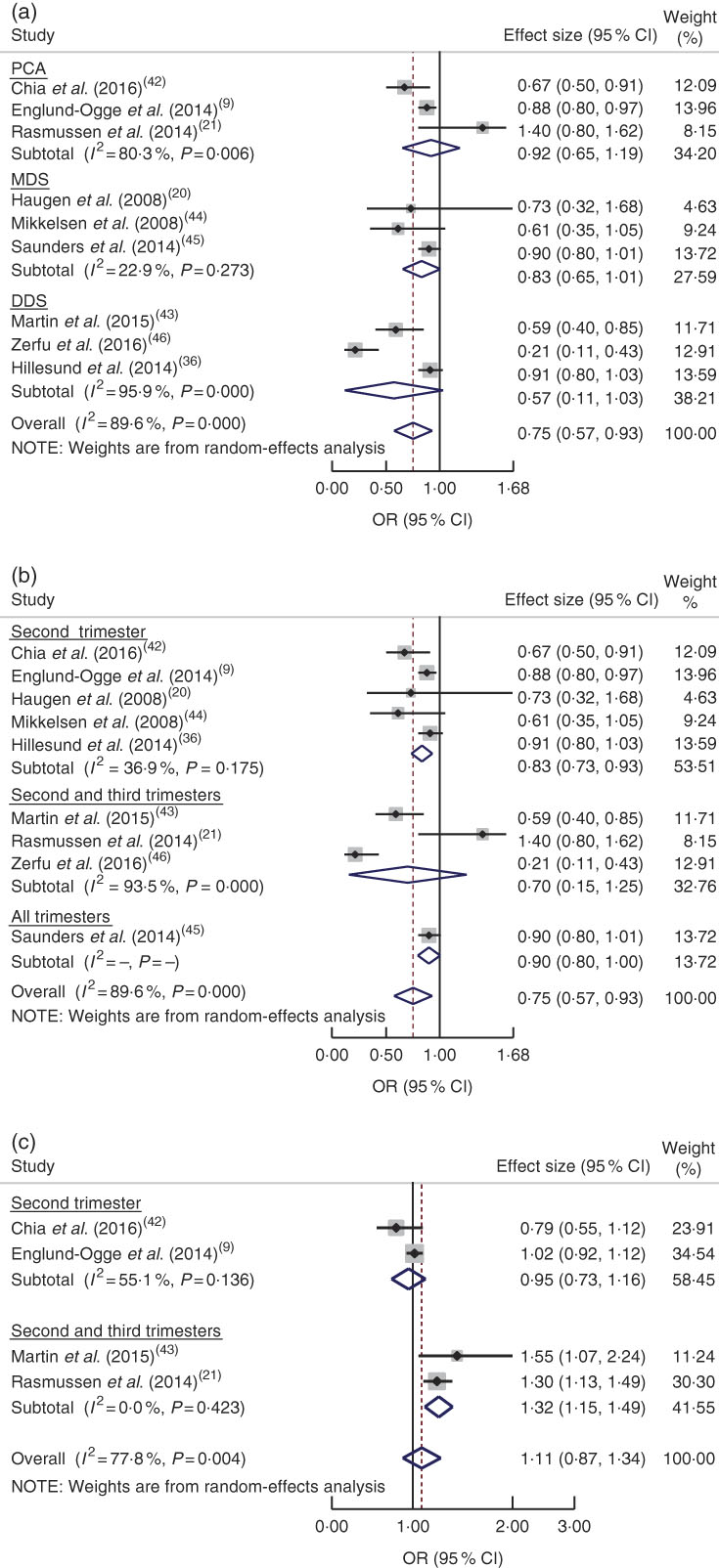
Fig. 4 (colour online) Forest plot for the pooled OR of the association between preterm birth (PTB) and different dietary patterns: (a) association between healthy dietary pattern and PTB, with subgroup analysis in relation to dietary pattern assessment methods (Mediterranean diet score (MDS) v. dietary diversity score (DDS) v. principal component analysis (PCA)); (b) association between healthy dietary pattern and PTB, with subgroup analysis regarding period of dietary assessment (second trimester v. both second and third trimesters v. all trimesters); and (c) association between Western pattern and PTB, with subgroup analysis regarding period of dietary assessment (second trimester v. both second and third trimesters). The study-specific OR and 95 % CI are represented by the black diamond and the horizontal line, respectively; the area of the grey square is proportional to the specific-study weight to the overall meta-analysis. The centre of the blue open diamond and the red dashed vertical line represent the pooled OR; and the width of the blue open diamond represents the pooled 95 % CI
On the other hand, the pooled estimate of four studies( Reference Englund-Ogge, Brantsaeter and Sengpiel 9 , Reference Rasmussen, Maslova and Halldorsson 21 , Reference Chia, de Seymour and Colega 42 , Reference Martin, Sotres-Alvarez and Siega-Riz 43 ) showed that a Western dietary pattern did not increase the odds of PTB (OR=1·11; 95 % CI 0·87, 1·34; I 2=77·8 %, P=0·004; Fig. 4(c)). There were subgroup differences between assessing diet in the second trimester and in both the second and third trimesters with respect to risk of PTB (P=0·001). We did not undertake a subgroup analysis regarding study design, as all studies had the same design (cohort).
Dietary patterns and low birth weight
Two studies assessed the effect of dietary pattern during gestation on LBW. A study in Ghana( Reference Abubakari and Jahn 47 ) reported that a ‘health conscious’ dietary pattern with a high intake of corn, rice, cassava, yam, fruits, vegetables (carrots, tomatoes, dark green leafy vegetables, cabbage, salad, cucumber), meat and eggs reduced the odds of LBW (OR=0·23; 95 % CI 0·12, 0·45). Similarly, that study reported that women who had a higher DDS were less likely to deliver an LBW baby v. those who had a lower DDS (OR=0·10; 95 % CI 0·04, 0·13). However, a high consumption of sweetened beverages, ice cream, chocolate, energy drinks, milk and local soft drinks, which was labelled the ‘non-health conscious’ dietary pattern, was not significantly associated with LBW (OR=1·04; 95 % CI 0·65, 1·67). Another study in Ethiopia( Reference Zerfu, Umeta and Baye 46 ) showed that women who had an adequate DDS were less likely to deliver an LBW baby (OR=0·49; 95 % CI 0·24, 0·97).
Discussion
The current systematic review and meta-analysis summarizes evidence focusing on the effects of different dietary patterns during pregnancy on adverse pregnancy (HDP and GDM) and birth (PTB and LBW) outcomes. Globally, adverse pregnancy outcomes and nutritional insufficiencies still remain public health problems( Reference Gernand, Schulze and Stewart 48 ). Sufficient consumption of energy, protein and micronutrients continues to be essential throughout pregnancy( Reference Wakimoto, Akabike and King 49 ).
Hypertensive disorders of pregnancy
The meta-analysis of four studies assessing the healthy diet pattern resulted in pooled estimates suggesting decreased odds of pre-eclampsia. However, other studies reported inconsistent findings on the association between adherence to a healthy dietary pattern and the likelihood of HDP occurrence. A cohort study in the Netherlands( Reference Timmermans, Steegers-Theunissen and Vujkovic 25 ) revealed that adherence to a Mediterranean diet pattern (vegetables, vegetable oils, pasta, fish, legumes and rice) or a traditional pattern (meat and potatoes) was not associated with gestational hypertension. A cohort study in Brazil( Reference Eshriqui, Vilela and Rebelo 35 ) revealed that adherence to healthy dietary patterns with high intakes of dairy products, fruit, green vegetables, legumes, fish, cakes, cookies–crackers and tea was not associated with a change in systolic or diastolic blood pressure. On the contrary, a cross-sectional study in Tanzania( Reference Mwanri, Kinabo and Ramaiya 37 ) reported that, compared with a lower score, having a high and medium DDS were associated with increased odds of gestational hypertension.
These inconsistencies might be due to the differences in method and population characteristics. The Tanzanian study was cross-sectional( Reference Mwanri, Kinabo and Ramaiya 37 ) and conducted in a resource-limited setting; however, the other studies were cohort studies conducted in well-resourced settings, except the Brazilian study( Reference Eshriqui, Vilela and Rebelo 35 ). These studies also assessed dietary intake using a different number of food items and methods. The Tanzanian study applied a 24 h recall method using sixteen food groups, while the studies from Brazil( Reference Eshriqui, Vilela and Rebelo 35 ) and the Netherlands( Reference Timmermans, Steegers-Theunissen and Vujkovic 25 ) assessed dietary intake using an eighty-two-item and a 293-item FFQ, respectively.
The healthy diet pattern is in line with dietary guidelines, which suggest consumption of whole grains, vegetables, fruits, potatoes, pasta, cereals, beans, lentils and fish( 50 ). Similarly, the beneficiary influence of diets high in fibre, K, fruits, vegetables, cereals, dark bread and low-fat dairy products was reported as decreasing the odds of pre-eclampsia( Reference Frederick, Williams and Dashow 51 ). It was also reported that a lower likelihood of pregnancy-induced hypertension or pre-eclampsia is observed with a diet comprising intake of plant-derived foodstuffs and vegetables( Reference Pistollato, Sumalla Cano and Elio 52 ). The risk of pregnancy complications like pre-eclampsia and LBW has been linked with maternal oxidative stress in the middle of pregnancy( Reference Hsieh, Chen and Lo 53 ). Evidence indicates that oxidative stress during pregnancy could be reduced by antioxidant compounds from fruit and vegetables( Reference Kim, Hwang and Ha 54 ). The findings of a multicentre study indicate that oxidative stress could be reduced by sufficient intakes of fruit, vegetables and vitamin C( Reference Kim, Hwang and Ha 54 ). A combination of vitamin C and E might lower the risk of pre-eclampsia( Reference Raijmakers, Dechend and Poston 55 ) through removal of free radicals which may cause oxidative stress in pregnancy( Reference Siddiqui, Jaleel and Tamimi 56 ). Therefore, it could be the cumulative effect of nutrients and their biochemical properties that influence pre-eclampsia risk.
Gestational diabetes mellitus
The meta-analyses of five studies assessing the healthy diet pattern resulted in pooled estimates that indicated reduced odds of GDM, but this was not statistically significant, most likely due to insufficient power, since few articles were included. Additionally, there were inconsistent findings among included studies for meta-analysis regarding the healthy dietary patterns and GDM; three studies showed decreased odds of GDM while the remainder reported no association. This might result from unmeasured factors due to the majority of studies not controlling for all possible confounding factors. For instance, He et al.( Reference He, Yuan and Chen 19 ) could not control for parity, energy intake, blood pressure and family history of type 2 diabetes mellitus. Likewise, parity, energy intake and blood pressure were not adjusted for in the other two studies( Reference Karamanos, Thanopoulou and Anastasiou 40 , Reference Nascimento, Alves and Fonseca 41 ). There was also a difference in assessing dietary intake across these studies, with four studies( Reference De Seymour, Chia and Colega 18 , Reference He, Yuan and Chen 19 , Reference Karamanos, Thanopoulou and Anastasiou 40 , Reference Nascimento, Alves and Fonseca 41 ) using a validated FFQ and the other an unvalidated FFQ( Reference Tryggvadottir, Medek and Birgisdottir 34 ). The dietary intake was assessed at different trimesters of pregnancy, even though there was no significant difference in subgroup analysis based on dietary intake assessment period. This could be a possible explanation for the variations across different studies.
Evidence indicates that pre-pregnancy adherence to a Mediterranean pattern style, with intake of fruit, vegetables, legumes, nuts, fish and cereals, and to the Dietary Approaches to Stop Hypertension (DASH) diet decreases the odds of GDM( Reference Tobias, Cuilin and Chavarro 57 , Reference Schoenaker, Soedamah-Muthu and Mishra 58 ). Similarly, a clinical trial reported that adhering to the DASH diet, which is high in fruits, vegetables, whole grains and low-fat dairy products, with low amounts of saturated fats, cholesterol and refined grains, reduced the need for insulin treatment( Reference Asemi, Samimi and Tabassi 59 ). Intake of fibre, fruits and cereals reduced the odds of GDM( Reference Zhang, Liu and Solomon 60 ).
A cohort study reported that higher odds of GDM was observed with adherence to a Western dietary pattern, which contained higher intake of refined-grain products, processed meat, red meat, French fries and pizza, sweets and desserts( Reference Zhang, Schulze and Solomon 26 ). However, our pooled estimate of four studies did not show a significant relationship between the Western pattern and GDM occurrence. The possible explanation may be the difference in the dietary pattern investigation methods (two studies used FFQ( Reference He, Yuan and Chen 19 , Reference Nascimento, Alves and Fonseca 41 ) and two studies used 24 h recall methods( Reference De Seymour, Chia and Colega 18 , Reference Dayeon, Kyung Won and Won 39 )) and population (one study was conducted in a Western population( Reference Dayeon, Kyung Won and Won 39 ) and three studies were done in an Asian population( Reference De Seymour, Chia and Colega 18 , Reference He, Yuan and Chen 19 , Reference Nascimento, Alves and Fonseca 41 )).
Preterm birth
In the current systematic review, a pooled estimate of nine studies indicated that compared with low adherence, higher adherence to a healthy dietary pattern significantly decreased the odds of PTB. Likewise, the pooled estimates of four studies on vegetable pattern and three studies on the Mediterranean diet indicated decreased odds of PTB, but this was not statistically significant. However, the meta-analysis result of four studies assessing the Western pattern and PTB showed that adherence to the Western pattern was not significantly associated with PTB. There were significant differences in subgroup analysis based on dietary intake assessment period. In two articles, the dietary intake was assessed in the second (13–27 weeks) and third (28–40 weeks) trimesters and reported that the Western dietary pattern significantly increased the odds of PTB. Nevertheless, the other two studies assessed the dietary intake in the second trimester (13–27 weeks) and the Western dietary pattern did not significantly increase the odds of PTB. A previous systematic review of randomized controlled trials revealed that macronutrient dietary interventions have reduced PTB( Reference Gresham, Bisquera and Byles 61 ).
Low birth weight
Two articles assessed the effect of dietary patterns on LBW. A dietary pattern labelled as ‘health conscious’, characterized by intake of local dishes made from corn flour, vegetables (carrot, tomatoes, dark green leafy vegetables, cabbage, salad, cucumber), rice, meat, a mixture of corn and cassava dough, yam, fruits, water and eggs, was associated with reduced odds of LBW( Reference Abubakari and Jahn 47 ). Similarly, women who had a higher DDS were less likely to deliver an LBW baby( Reference Zerfu, Umeta and Baye 46 , Reference Abubakari and Jahn 47 ). However, high consumption of sweetened beverages, ice cream, chocolate, energy drinks, milk and local soft drinks, which was labelled as a ‘non-health conscious’ dietary pattern, showed a significant effect on risk of LBW( Reference Abubakari and Jahn 47 ). This is in line with the evidence that suggests the occurrence of LBW has decreased through the consumption of foods and fortified foodstuffs( Reference Gresham, Byles and Bisquera 62 ).
It is suggested that pregnant women should be advised to eat a diet rich in fruits and vegetables, whole grains, beans, lean meats and fish/seafood, and low in added sugar, red meat and processed foods( Reference King 63 ). Intake of vegetables, fruits and legumes improves micronutrient and antioxidant intakes, which could improve pregnancy and birth outcomes( Reference King 63 ), particularly at the second trimester since oxidative stress has been shown to reach high levels mid-pregnancy( Reference Casanueva and Viteri 64 ). Pregnancy complications and adverse outcomes like pre-eclampsia and PTB have been related to oxidative stress and associated inflammation( Reference Hsieh, Chen and Lo 53 ). Antioxidant vitamins (C and E) and essential trace elements (Cu and Zn) through dietary intake of legumes and fruits, which are rich in these nutrients, could decrease this risk( Reference Mistry and Williams 65 – Reference Zhang, Zhou and Perkins 67 ). Oxidative stress-linked adverse pregnancy outcomes could be reduced by antioxidants through an intake of vegetables and fruits( Reference Asemi, Samimi and Tabassi 68 ).
Study limitations
The limitations of the present systematic review must be acknowledged. To acquire complete dietary data, most of the articles reviewed applied FFQ followed by diet scores. Nevertheless, there are unavoidable dietary intake misclassifications, which probably bias the degree of detecting real effects. Furthermore, problems of recall bias are also unavoidable because dietary information is dependent on memory. Including articles written only in the English language is another shortcoming of the systematic review. Due to the nature of nutritional research, it is difficult to make all dietary exposures similar for all study subjects. Heterogeneity among studies is a further issue; however, meta-analysis permits the inconsistent findings among studies to be evaluated, even with heterogeneity( Reference Higgins, Thompson and Deeks 69 ). As all included studies were observational epidemiological studies, the effect of confounders may be another limitation of the current review, despite controlling for some possible confounding factors. Publication bias is always a concern in any review. Reviewed studies that had negative results might not have been submitted for publication, and thus are less likely to have been published.
Conclusion
The evidence presented in the current systematic review indicates the inconsistent associations between different dietary patterns and pregnancy and birth outcomes. Some results in the systemic review show the importance of healthy dietary intake during gestation to improve pregnancy and birth outcomes for the mother and infant, even though inconsistencies have been observed among studies. Essentially, the review suggests that dietary patterns with higher intake of whole grains, vegetables/fruits, legumes and fish are associated with lower likelihood of adverse pregnancy and birth outcomes. However, as the evidence presented herein is inconsistent regarding the association between dietary intake and pregnancy and birth outcomes, caution should be given during advising pregnant women about diet. Since most of the articles included in the review were conducted in resource-rich settings, additional studies need to be done in resource-limited settings to elucidate the impact of limited resources on dietary intake and adverse pregnancy and birth outcomes.
Acknowledgements
Acknowledgements: The authors would like to thank Debbie Booth for her help with developing the literature search strategy and use of Endnote. The authors would also like to thank Dr Ryan O’Neill for his unreserved help in editing the English language. Financial support: This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors. Conflict of interest: The authors declare that they have no conflicting interests. Authorship: K.T.K., C.C., D.L. and E.G. formulated the research questions. K.T.K., C.C., D.L. and E.G. designed the study. K.T.K., C.C., D.L., E.G. and T.K.T. carried out the analysis. K.T.K., C.C., D.L., E.G. and T.K.T. analysed the data and wrote the manuscript. Ethics of human subject participation: Not applicable.