INTRODUCTION
Wheeze leads to frequent presentations to pediatric emergency departments (PEDs).Reference Nelson and Zorc 1 Salbutamol, a beta-agonist often used to treat wheeze, was traditionally administered by nebulization (NEB). However, evidence shows that salbutamol administered by metered-dose inhalers with valved spacers (MDI-s) offers advantages to pediatric patients.Reference Cates, Crilly and Rowe 2 Salbutamol administered by MDI-s is associated with less tachycardia and tremor, decreased PED length of stay (LOS), and decreased risk of admission.Reference Cates, Crilly and Rowe 2 MDI-s also result in increased child and parent treatment satisfaction and may reduce staff and caregiver risk during respiratory infection epidemics.Reference Cotterell, Gazarian and Henry 3 - Reference Tran, Cimon and Severn 5 Although PEDs in Canada are now accepting MDI-s as the route of choice for salbutamol inhalation,Reference Schuh, Zemek and Plint 6 one of the factors linked to resistance to switching salbutamol inhalation methods is the perception of increased costs.Reference Hurley, Sargeant and Duffy 7 , Reference Scott, Osmond and O’Leary 8
The cost-effectiveness of MDI-s versus NEB has been documented,Reference Leversha, Campanella and Aickin 4 , Reference Dhuper, Chandra and Ahmed 9 - Reference Staggs, Peek and Southard 12 but no published Canadian study has used local clinical and administrative data in their analysis. One Canadian study estimated cost-savings of $155 per PED visit associated with salbutamol delivery via MDI-s.Reference Doan, Shefrin and Johnson 10 Doan and colleagues used hospital-level data for medication, labour and equipment costs, but drew on a systematic review of randomized controlled trials for patient outcomes.Reference Cates, Crilly and Rowe 2 While cross-regional extrapolation of epidemiological outcomes is commonly accepted in health economic evaluations, clinical outcomes such as LOS and hospitalization rates may be less generalizable because of jurisdictional variations in socioeconomic status, practice variability, and supply of health care resources.Reference Barbieri, Drummond and Rutten 13 - 15 Our objective was to conduct an economic evaluation of both salbutamol inhalation methods based on local, hospital-specific patient-level outcome and cost data.
METHODS
Approval was obtained from the Research Ethics Board of the IWK Health Centre (#1012427, September 28, 2012). Consolidated Health Economic Evaluation Reporting Standards (CHEERS) were used in the development of this paper.Reference Husereau, Drummond and Petrou 16
Data collection
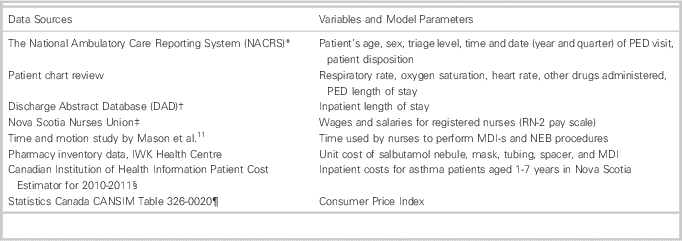
The setting was the IWK Health Centre, the tertiary care pediatric facility for Maritime Canada. The IWK PED sees approximately 28,000 children per annum, with approximately 2,000 (7.1%) presenting with an acute wheeze-related illness. Our cohort was established in a previous study.Reference Wing, Hill-Taylor and Sketris 17 A random sample of 1,376 children were chosen from the 4,140 patients diagnosed with asthma (J45), bronchiolitis (J21), other respiratory disorders (J98.8), or wheeze (R06.2) (International Statistical Classification of Disease and Related Health Problems, 10th Revision, Canada) between January 1, 2008–December 31, 2009. Multiple sources were used to obtain retrospective data and model parameters (see Table 1).
Table 1 Data Sources and Corresponding Variables and Model Parameters
PED=Pediatric Emergency Department; MDI=metered-dose inhaler; MDI-s=metered-dose inhaler with valved spacer; NEB=wet nebulization.
Data from the patient chart review were linked with data from the Discharge Abstract Database and National Ambulatory Care Reporting System to provide a patient-level data set. Each record represents a visit to the PED and captures age, sex, visit date and time, triage acuity, vitals, salbutamol inhalation method, total dosage, other medications given (ipratropium bromide and dexamethasone), PED and hospital LOS, and disposition. (Table 1)
We excluded patients whose data did not match between chart review, DAD and NACRS (n=191), patients who received both or neither methods of salbutamol inhalation (n=245) and those with missing or invalid data values (n=118). Our final sample numbered 822 visits.
Study perspective and discounting
Our analysis takes the hospital’s perspective. That is, our analysis did not include private or societal costs and benefits. Physician costs were not included, as PED physicians at the IWK are paid a rate per shift which is not dependent on patient volume or treatment choices. We did not perform present-value discounting, as all costs and clinically relevant outcomes occurred shortly after treatment.
Calculating cost of treatment
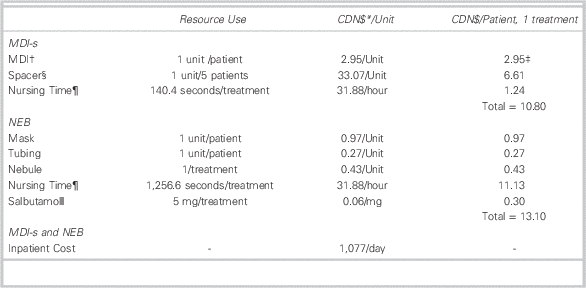
Patient-level costs were calculated using our hospital data on treatment method, dosage, and disposition, with local information on the cost of supplies, nursing time,Reference Mason, Roberts and Yard 11 and inpatient care. Table 2 describes the supplies and time required for each round of treatment, including the monetary cost per treatment. We used the patient’s recorded dosage of salbutamol and the IWK Health Centre’s Asthma Care Map (available on request) to derive the total number of treatments received by a patient while in the PED.
Table 2 Baseline Resource Use and Unit Cost Parameters Used to Calculate Cost per Treatment
MDI=metered-dose inhaler; MDI-s=metered-dose inhaler with valved spacer; NEB=wet nebulization.
* Monetary values are expressed in 2010 constant Canadian dollars.
† An MDI contains 10,000 mcg of salbutamol or 100 puffs. Since the dosage for each treatment is 10 puffs (or 5 puffs for under age 5), each MDI contains a maximum of 10 treatments (or 20 treatments for under age 5). Patients in the MDI-s group did not exceed the maximum number of treatments per MDI; therefore, each patient in the MDI-s group was allocated the full cost of an MDI. In this case, we allocate the value of $2.95 to each patient regardless of the number of treatments.
‡ Since each patient uses one MDI, costs for MDIs are given as costs per patient rather than costs per treatment (see note 2).
§ At the IWK, spacers are used up to five times across multiple patients, with sanitation occurring between uses.
¶ Nursing time parameters are extracted from a previous time and motion study conducted in the United Kingdom (Mason et al.Reference Mason, Roberts and Yard 11 ). Our baseline values are the midpoints of the interquartile ranges for total treatment time that were reported in this study. Total treatment time includes treatment setup and administration times. A nurse performs the setup and treatment administration in our setting.
ǁ The dosage for each NEB treatment is 5.0 mg (or 2.5 mg for under age 5).
All costs and prices were converted to 2010 constant Canadian dollars prior to calculating costs of treatment. Cost estimates were also converted into U.S. dollars. We also compared our cost estimates to others found in the literature. Where appropriate, citations of treatment costs from international jurisdictions were converted into Canadian dollars using foreign/Canadian exchange rates for the year of publication (http://fxtop.com), and then adjusted to 2010 constant dollars.
Analytic methods
We describe our data set using sample counts by selected patient groups. Means (95% confidence intervals) were calculated for the both patient groups. Regression models were used to estimate differences in outcomes and costs between groups. Because admissions and LOS in both the PED and hospital are significant drivers of treatment costs, all three clinical measures were used as dependent variables. Control variables included age, sex, triage acuity, patient vitals (e.g., respiratory rate, oxygen saturation, heart rate), date of PED visit, and the use of other medications. Baseline heart, respiratory rates, and oxygen saturation were converted to age-based Canadian Triage and Acuity Scale (CTAS) categorical scales prior to their inclusion in the regression models.Reference Warren, Jarvis and LeBlanc 18
Logistic regressions were used to estimate admission probabilities, while Poisson regressions were used for PED and inpatient LOS. Generalized linear models (GLMs) specified with a log-link and Gamma distribution were used to model inpatient and PED costs. Since 85% of visits to the PED resulted in a discharge and, therefore, zero inpatient costs, we adopted a two-part model for evaluating inpatient costs. In this case, we separately modeled the probability of inpatient admission and the level of inpatient costs among hospitalized patients. The estimates generated from these two models were combined to derive a single estimate of the difference in inpatient cost between the two treatment groups.
All analyses were conducted using Stata12.1 (www.stata.com).
Sensitivity analysis
We performed two sensitivity analyses to explore the sensitivity of our results to changes in the parameter values used to calculate the costs. First we used a scenario in which we assumed that baseline parameter values were biased in favour of MDI-s. Under an alternative scenario, we replaced baseline MDI-s parameters with higher prices and more intensive resource use values, whereas baseline NEB parameters were replaced with lower prices. The second sensitivity analysis used a multi-way (probabilistic) sensitivity approach. In this case, parameter values were selected at random from probability distributions and then used to re-calculate patient costs. Once these costs were produced, we re-estimated our regression models. This procedure was replicated 10,000 times to derive a simulated distribution of cost differences between groups.
RESULTS
Clinical and demographic characteristics
Of the 822 patients analyzed, 664 were in the MDI-s group and 158 in the NEB group. Patients in the NEB group were 1.1 years younger and presented to the PED with more severe clinical symptoms as measured by triage acuity (Table 3).
Table 3 Selected Clinical and Demographic Patient Grouping

MDI-s=metered-dose inhaler with spacer; NEB=wet nebulization; PED=Pediatric Emergency Department.
* Triage=Canadian Triage and Acuity Scale (CTAS).
Patient outcomes and costs
Our summary statistics (Table 4) show that MDI-s treatment is associated with lower costs and improved clinical outcomes. For example, only 11% of those in the MDI-s group were admitted after treatment in the PED, compared to one-third of those in the NEB group. Costs in the NEB group were $998 per patient-visit, compared to $173 for MDI-s. Inpatient care accounted for more than 93% of total patient costs (96% in NEB and 92% in MDI-s).
Table 4 Summary Statistics of Selected Clinical and Demographic Characteristics, Dependent Variables, and Costs
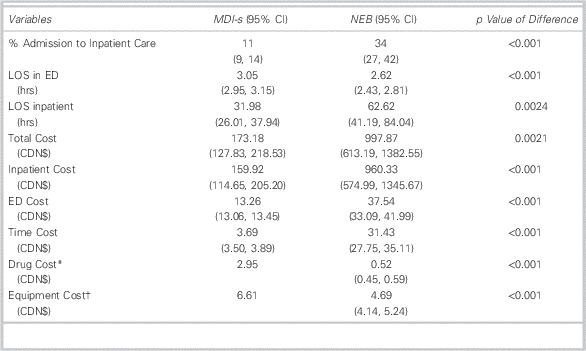
LOS=length of stay; MDI=metered-dose inhaler; MDI-s=metered-dose inhaler with valved spacer; NEB=wet nebulization.
* Since the dosage for each treatment is 10 puffs (or 5 puffs for under age 5), each MDI contains a maximum of 10 treatments (or 20 treatments for under age 5). Patients in the MDI-s group did not exceed the maximum number of treatments per puffer; therefore each patient in the MDI-s group was allocated the full cost of an MDI. In this case, we allocate the value of $3.14 to each patient regardless of the number of treatments. Consequently, the cost of drugs does not vary across patients in the MDI-s group.
† For the MDI-s group, the only equipment cost is the cost of the spacer. Spacers are used for up to five patients. We therefore assigned one-fifth of the cost of a spacer to each patient in the MDI-s group. Like MDI-s, the cost of the spacer does not vary across patients in the MDI-s group.
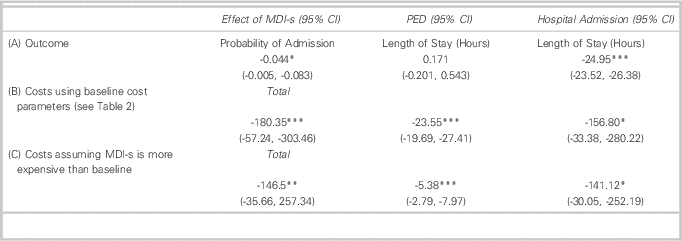
Tables 3 and 4 show summary statistics which do not control for confounding influences, including illness severity, additional medications and patient characteristics (e.g., CTAS, ipratropium, age). Table 5, however, shows regression-adjusted estimates that control for these baseline observed confounders. The addition of these controls reduces but does not eliminate group differences in patient costs and outcomes. For example, MDI-s is still associated with a 4.4% absolute reduction in probability of admission. Among admitted patients, those that received MDI-s in the PED spent 25 fewer hours in inpatient care compared to patients treated with NEB. Our results indicate that NEB and MDI-s patients had similar PED LOS.
Table 5 Regression Results: MDI-s Associated with Improved Clinical Outcomes and Lower Costs
MDI-s=metered-dose inhaler with spacer; PED=Pediatric Emergency Department.
*p<0.05, **p<0.01, ***p<0.001.
MDI-s is also associated with lower treatment costs. Treatment via MDI-s is associated with a $180 (US$175) reduction in total costs per patient-visit. While almost 90% ($156 (US$152)) of this reduction came in the form of lower inpatient costs, MDI-s was also associated with a $24 (US$23) reduction in PED costs.
Our scenario analysis (Table 5, Panel C) assumes greater resource intensity per treatment and higher equipment prices for MDI-s than at baseline. For example, under this scenario, it is assumed that spacers are used for three patients, rather than five as stated under our baseline assumptions. The analysis reduces the cost-savings of MDI-s from $180 to $146 (US$141). As before, cost-savings are driven by lower admission rates among patients treated with MDI-s. However, there is still PED cost-savings for MDI-s of $5 (US$4.85).
Figure 1 displays the results of the multi-way sensitivity analysis. Each point on the figure is an estimate of the cost-savings for MDI-s under a simulated set of input prices and resource use values for each treatment. Importantly, all of the simulated estimates indicate cost-savings for MDI-s. For example, the right tail of the distribution indicates that the use of MDI-s is associated with a minimum cost-savings of approximately $160 (US$155) per patient visit.
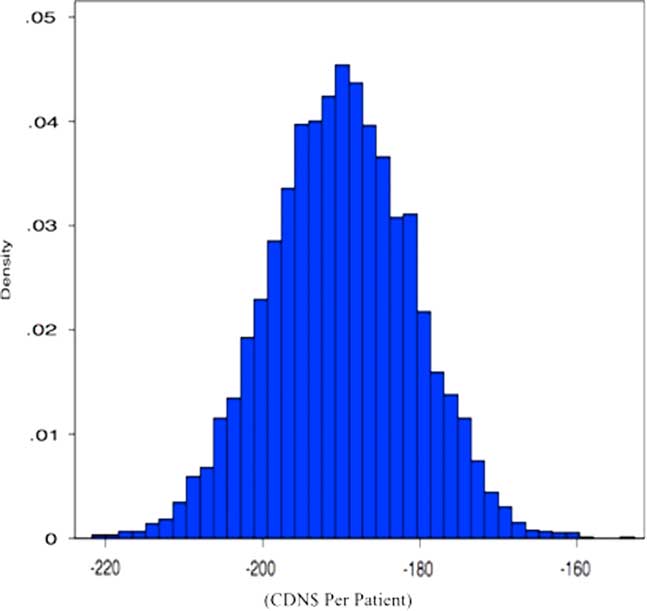
Figure 1 Simulated Distribution of Cost Savings Achieved by MDI-s.
Note: In the simulation, we allow prices and resource intensity values to range probabilistically according to different distributions. Here, distribution means are set according to the parameters given in Table 2. The simulations are specified as follows: wages, nebulization equipment (e.g. masks, tubing, nebules), salbutamol and inpatient costs vary within +/-10% of their means according to a normal distribution; nursing time for MDI-s varies from 92–188 seconds per treatment using a Gamma distribution; nursing time for nebulization varies from 806 seconds to 1725 seconds, also using a Gamma distribution; and spacers are used for 3–5 patients according to a uniform distribution.
DISCUSSION
Salbutamol inhalation by MDI-s is associated with lower admission rates and inpatient LOS, after controlling for observed differences between treatment groups in illness severity (e.g., CTAS, vitals, use of ipatropium) and patient characteristics (e.g., age, sex). This resulted in cost-savings of $180 (p<0.001) per patient, with most savings coming from lower admission rates. With approximately 2,000 patients presenting to our PED with an acute wheeze-related illness per year, even if only 75% are treated with salbutamol, the predominant use of MDI-s would realize total savings of $270,000 (US$262,000) per annum. Of these, $42,000 (US$41,000) per annum are direct savings to the PED.
While PED LOS was equivalent in both comparator groups, our model used the total treatment time as reported by the UK time and motion study,Reference Mason, Roberts and Yard 11 which was significantly higher for patients in the NEB group. The authors of the UK study measured administration and setup time separately and added the two to obtain total treatment time. At our PED, registered nurses perform setup and administration tasks, making total treatment time the most relevant model parameter. While PED cost differences are likely sensitive to setup time, PED costs make up only a small fraction of total patient costs at our institution.
Several studies have examined the economic impact of salbutamol inhalation procedures in emergency departments.Reference Dhuper, Chandra and Ahmed 9 , Reference Mason, Roberts and Yard 11 , Reference Staggs, Peek and Southard 12 In two studies, respiratory therapist or nursing costs for both procedures were assessed.Reference Dhuper, Chandra and Ahmed 9 , Reference Mason, Roberts and Yard 11 Both studies found similar costs of drug, delivery system, and staffing for both inhalation methods—savings of US$8.15 (CAN$9.87) associated with MDI-s were reported in one study,Reference Dhuper, Chandra and Ahmed 9 while a loss of US$7.33 (CAN$8.87) for a single treatment was noted by another.Reference Mason, Roberts and Yard 11 However, the authors of the latter study noted that savings had switched in favour of MDI-s by the fourth or fifth treatment. Another study examined differences in treatment time, reporting a 25-minute reduction in treatment time for MDI-s versus NEB. Based on historical ED charges, the authors estimate time-related cost-savings of US$216 (CAN$222) per patient-visit.Reference Staggs, Peek and Southard 12 The value of the assessment or education given in the PED when using MDI-s has yet to be assessed in comparative studies.
In addition, two studies examined cost-savings achieved through admission rate changes associated with MDI-s.Reference Leversha, Campanella and Aickin 4 , Reference Doan, Shefrin and Johnson 10 Leversha and colleagues documented significantly decreased costs in patients aged one to four years in a New Zealand PED through reduced hospital admissions in patients using MDI-s.Reference Leversha, Campanella and Aickin 4 Their savings, NZ$457 (CDN$547) per patient, are more than twice ours. A Canadian study, using non-local clinical data, arrived at estimated savings with MDI-s versus NEB that is remarkably similar to ours (CDN$155 versus $180).Reference Doan, Shefrin and Johnson 10
STRENGTHS AND LIMITATIONS
An important strength of our study is that it uses local patient-level clinical data, and local economic data from administrative sources. These data capture unique regional factors influencing treatment, admissions, and cost of treatment. Our results may not be generalizable to other health care systems because of work flow and practice culture. However, because we used data that are already collected in many jurisdictions, our methods can be used for cross-jurisdictional accounts of the cost-effectiveness of MDI-s.
An important methodological limitation of our study is sample size, hindering our ability to assess differences in costs and outcomes by patient sub-group. The data are derived from paper-based medical records. To validate the accuracy of the abstraction process, a randomly selected 10% of the medical record reviews were repeated by an independent researcher.Reference Wing, Hill-Taylor and Sketris 17 Kappa (κ) for the inter-rater reliability test was 0.97. Our data do not include patient co-morbidities or validated measures of asthma severity, and may be limited by other unmeasured confounding factors such as variations in physician choice of initial therapy in the PED and inpatient treatment.
We did not include administration costs involved in ordering drugs and supplies. Our time estimate for each visit is based on a UK time and motion study and may exclude the teaching time done in our PED to reinforce patient and caregiver MDI-s technique. Data limitations also precluded an assessment of patient relapse/readmission. Our study did not deal with the advantages offered by, or technical and physical differences of, using MDI-s instead of NEB for the delivery of salbutamol. These differences have been addressed elsewhere.Reference Dolovich, Ahrens and Anderson 19 , Reference Hill-Taylor, Hurley, Katrina and Sketris 20
CONCLUSIONS
We show that use of MDI-s for salbutamol inhalation in PEDs is associated with significant economic gains. Compared with NEB, MDI-s was associated with a 4.4% absolute reduction in admission to hospital and reduced costs by $180 per patient visit. These results suggest that broader adoption of MDI-s by other PEDs could result in substantial cost-savings.
Our study uses local data on patient outcomes, treatment protocols and input costs, thereby capturing unique regional factors influencing treatment, admissions, and costs. While these data are less transferable to other settings, we show, using sensitivity analyses, that our conclusions are not affected by changes in model parameters, such as treatment times or prices of medical supplies. Since most facilities collect the data used for this study, our methods could be adopted more widely for a cross-jurisdictional account of the cost effectiveness of MDI-s.
Acknowledgements
The authors would like to acknowledge the assistance of Dr. Kuan Xu (Dalhousie Economics) and Decision Support, IWK Health Centre.
Competing Interests: Funding was provided by a Category A Grant from the IWK Health Centre, and through Dr. I. Sketris’ Chair in Health Service Research funded by the Canadian Health Services Research Foundation/Canadian Institute of Health Research, and co-sponsored by the Nova Scotia Health Research Foundation. The opinions, views and major findings from this project are those of the primary author and do not necessarily represent the views of the IWK PED. The authors have no conflicts of interest.








