The incidence and prevalence of type 2 diabetes mellitus (T2DM) have increased markedly worldwide, and its complications are the leading causes of morbidity and premature mortality(Reference Mokdad, Ford and Bowman1). The importance of diet in the prevention, treatment and control of T2DM has been recommended(2). It has been reported that nut consumption may improve glycaemic control(Reference Kendall, Esfahani and Truan3).
Peanut consumption may moderate appetite, food intake and glycaemic control, and has been negatively associated with type 2 diabetes risk(Reference Sabaté and Ang4). These beneficial effects may be due to their nutritional components. Besides being a low-glycaemic-index food, peanuts are energy dense, and a good source of fibre, protein, niacin, folate, Mg, Se and Mn. They also contain bioactive compounds that exert antioxidant and anti-inflammatory effects(Reference O'Neil, Keast and Fulgoni5). However, the mechanisms responsible for their health benefits have not been completely elucidated(Reference Allen6).
Processing whole nuts to butter form results in cell-wall rupture and greater fat and fat-soluble nutrient bioaccessibility(Reference Ellis, Kendall and Ren7). The higher availability of fat in the intestinal lumen may decrease the rate of carbohydrate absorption (by delaying gastric emptying), favouring a reduced glycaemic response, and stimulate the secretion of intestinal hormones that may curb appetite and food intake as well as stimulate insulin release(Reference Gentilcore, Chaikomin and Jones8). Therefore, the form in which peanuts are consumed (whole or butter) may lead to different metabolic responses(Reference Cassady, Hollis and Fulford9).
The aim of the present study was to assess the effects of peanut consumption (whole peanuts or peanut butter) on first- and second-meal glucose metabolism (blood glucose, insulin and NEFA) and selected gut satiety hormone responses (glucagon-like peptide-1 (GLP-1), cholecystokinin (CCK) and peptide YY (PYY)), subjective appetite ratings (visual analogue scale) and food intake in obese women with high T2DM risk.
Methods
Participants
Study participants were recruited through public advertisements. Eligibility criteria included the following: age 18–50 years; BMI 30–35 kg/m2; not taking medications known to affect glycaemia, fat metabolism or appetite; regular breakfast consumer ( ≥ 420 kJ ingested within 2 h of waking on ≥ 4 d/week); limited body weight fluctuation ( < 5 kg in the past 3 months); willingness to eat all test foods; no self-reported allergy to the foods provided in the study; no self-reported sleep disorders. In addition to the aforementioned criteria, participants had to meet one of the following conditions: waist circumference ≥ 88 cm; reported family history of T2DM in first-degree relatives; fasting capillary blood glucose 5·5–7·0 mmol/l; and/or 2 h blood glucose 7·8–11·1 mmol/l (impaired glucose tolerance). Participants presenting with T2DM, dyslipidaemia or high blood pressure were excluded.
A total of 141 individuals completed the first screening visit, of which sixty-eight were eligible for and completed the second screening visit. Finally, fifteen participants met all screening criteria and completed the full study protocol.
The protocol was approved by the Human Research Ethics Committee of the Federal University of Viçosa, Brazil (no. 004/2009). The present trial was registered at ClinicalTrials.gov (registration no. NCT01413126). All volunteers were informed about the objectives of the study and provided written informed consent. Power calculations indicated that thirteen individuals were necessary to detect a change in blood glucose of 0·35 mmol/l (α = 0·05; power = 0·80, sd 0·3)(Reference Wolever and Mehling10).
Study design
The present randomised cross-over clinical trial required participants to complete three experimental sessions where whole peanuts without skins (WP), peanut butter (PB) or no peanuts (control) were consumed with a breakfast meal separated by a washout period of at least 8 d. Participants were instructed not to consume alcohol or conduct any non-habitual physical activity 24 h before the sessions, and to consume a low-carbohydrate meal the night before the test sessions. Postprandial concentrations of blood glucose, insulin, NEFA, GLP-1, CCK, PYY, appetite sensations and food intake were assessed before and after breakfast treatments and a standard lunch (Fig. 1).

Fig. 1 Experimental study protocol. B, breakfast; L, lunch; LL, leave the laboratory; A, appetite; P, palatability; ![]() , glucose, insulin, NEFA, glucagon-like peptide-1 (GLP-1), cholecystokinin and peptide YY analyses;
, glucose, insulin, NEFA, glucagon-like peptide-1 (GLP-1), cholecystokinin and peptide YY analyses; ![]() , glucose, insulin, NEFA and GLP-1 analyses.
, glucose, insulin, NEFA and GLP-1 analyses.
For screening, participants arrived in the laboratory between 07.30 and 08.00 hours after a 12 h overnight fluid and feed deprivation for the 2 h oral glucose tolerance test, and for measurement of height, waist circumference, body weight, body composition, resting energy expenditure and blood pressure. Participants were also asked to answer questionnaires regarding stress, physical activity and eating and sleeping habits.
At each experimental session, body weight, capillary glucose level, the number of hours of sleep the night before and the time and composition of the last meal were assessed. Finger stick blood glucose was measured using a glucometer One Touch Ultra 2 (Johnson & Johnson Company) to ensure that the participants were feed-deprived (glucose < 5·5 mmol/l).
An indwelling catheter was placed in the participant's forearm and blood samples were drawn and appetite was rated at baseline ( − 10) and at 15, 45, 60, 90, 120, 180 and 240 min after test breakfast completion (first-meal responses). At 240 min, participants consumed a standard lunch. Afternoon blood sampling and appetite scoring occurred at 265, 295, 310, 340, 370, 430 and 490 min after consumption of the test breakfast (second-meal responses), resulting in a total of 8 h of biochemical assessment. After leaving the laboratory, participants recorded all food consumed and filled out the appetite ratings(Reference Flint, Raben and Blundell11) at 550, 610, 670 and 730 min.
Participants were not allowed to eat or drink anything (except water) besides the foods that were provided during the study sessions. They were also not allowed to watch any television show or talk about anything related to food, or anything that could affect the assessed parameters. They were allowed to read, listen to music, watch TV, use the computer and walk inside the laboratory.
Anthropometric and body composition measurements
Body weight was assessed using an electronic platform scale (Model 2096 PP, Toledo Brazil), with a capacity for 150 kg and precision of 50 g. Height was measured using a stadiometer (SECA model 206; Seca) fixed to the wall. BMI was computed based on weight (kg) and height (m2) (kg/m2), and classified according to the parameters of the WHO(12). Waist circumference was measured midway between the lowest rib and the iliac crest with a precision of 0·1 cm(13), and classified according to the parameters of the Third Report of the National Cholesterol Education Program Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults(14). Body fat percentage was measured by tetrapolar electrical bioimpedance (Biodynamics, Model 310, TBW) according to the protocol of Lukaski et al. (Reference Lukaski, Bolonchuk and Hall15). Participants were instructed not to use diuretics 7 d before the assessment, not to exercise during the preceding 12 h, not to drink alcohol for the preceding 48 h and to avoid drinking any beverage 12 h before the test.
Clinical assessments
Blood pressure was assessed by auscultation with a recently calibrated aneroid sphygmomanometer. The measurement was performed during the initial evaluation by a trained professional, and classification of the values obtained was done according to the recommendations by Pickering et al. (Reference Pickering, Hall and Appel16).
The level of physical activity was estimated using the Johansson & Westerterp(Reference Johansson and Westerterp17) questionnaire. Energy expenditure was estimated by multiplying the physical activity level obtained by the BMR calculated using the Mifflin et al. equation(Reference Mifflin, St Jeor and Hill18).
Participants were instructed to eat a hypoglycaemic meal for dinner on the night before. This meal was intended to contain only meat and vegetables. High-carbohydrate foods (i.e. breads, rice, pasta, potatoes, cassava, etc.) were not allowed. Once the participants arrived at the laboratory, their feed-deprived state was verified (One Touch Ultra 2, Johnson & Johnson Company). An oral glucose tolerance test was performed on screening day and the participants were classified according to the American Diabetes Association(19).
Homeostasis model assessment insulin resistance index was used to evaluate insulin resistance level. Pancreatic β-cell function was assessed by homeostasis model assessment pancreatic β-cell function according to the Matthews et al. equation(Reference Matthews, Hosker and Rudenski20). According to Geloneze et al. (Reference Geloneze, Repetto and Geloneze21), the cut-off point for insulin resistance diagnosis in Brazilian obese women aged approximately 40 years is 2·71.
Test meal
In accordance with the Food and Drug Administration(22)-qualified health claim regarding daily nut intake, 42·5 g of WP or PB were added to a breakfast meal in each test session. On each testing occasion, each participant consumed a test breakfast within 10 min that consisted of orange juice (250 ml) (Tropical Indústria de Alimentos S/A.) and cereal-containing treatment WP, PB or control (no peanuts) in randomised order. The cream of wheat was prepared by adding 56 g of instant cream of wheat (Original Instant Cream of Wheat, B&G Foods, Inc.) to brown sugar (P= 8·7 g; PB = 9·6 g; control = 9·9 g) (Lowçucar, LightSweet, Inc.), 1 g of aspartame sweetener (NutraSweet Company) and 300 ml of water, which was then heated for 1·5 min in the microwave. For the peanut treatments, 42·5 g of whole peanuts without skins (Nuts Online Company) (WP treatment) or creamy peanut butter (Arrowhead Mills, The Hain Celestial Group, Inc.) (PB treatment) were added. At minute 240, participants consumed a standard lunch within 10 min that consisted of white bread (50 g) (Seven Boys LTDA), strawberry jam (89·3 g) (Fugini Alimentos LTDA) and water (250 ml) containing 75 g of available carbohydrate (Table 1). Test breakfasts and lunch meals were matched on palatability, evaluated by appearance, smell, texture and intensity of taste (sweet, salty, bitter and sour)(Reference Flint, Raben and Blundell11).
Table 1 Test breakfast and lunch nutritional composition*
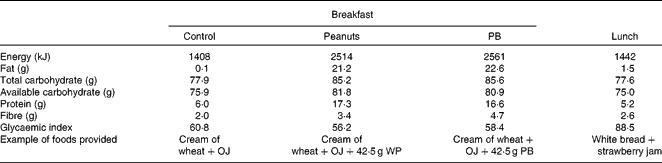
OJ, orange juice; WP, whole peanuts without skins; PB, peanut butter.
* Based on nutrition label information.
The breakfast meal's glycaemic index was estimated using the International Glycemic Index Table(Reference Atkinson, Foster-Powell and Brand-Miller23), according to the Wolever & Jenkins(Reference Wolever and Jenkins24) equation (see Supplementary information, available online).
Biochemical measurements
A measure of 3 ml of blood was collected in a red top vacutainer at each draw. After clotting and centrifugation, insulin, glucose and NEFA concentrations were measured. A measure of 4 ml of blood was collected in an ice-cooled EDTA-plasma vacutainer, and 40 μl of dipeptidyl peptidase-4 inhibiter (Millipore) was added for analysis of GLP-1, and 325 μl aprotinin (500 KIU/ml) (Sigma-Aldrich) was added for PYY and CCK analyses; all samples were handled according to the manufacturer's directions. Insulin and glucose were measured by electrochemiluminescence and the glucose oxidase method, respectively. Sensitivity of the insulin immunoassay was 1·39 pmol/l (within-run CV of 1·9 %). The glucose oxidase sensitivity was 0·12 mmol/l (within-run CV of 0·41 %). NEFA were analysed by an enzymatic colorimetric method on an automated analyser with a sensitivity of 0·14 μmol/l (within-run CV of 0·75 %). GLP-1, PYY and CCK were assessed by ELISA. Sensitivity of the GLP-1 assay was 2 pmol/L (within-run CV of 7·4 %) (Linco Research), PYY sensitivity was 1·4 pg/ml (within-run CV of 0·86 %) (Millipore) and CCK sensitivity was 3·86 ng/ml (within-run CV < 10 %) (Ray Biotech, Inc.).
Appetite profile
Appetite ratings – hunger, satiety, desire to eat and desire to consume specific food types (salty, sweet or greasy) – were scored at baseline and immediately after blood sample collection on a 100 mm visual analogue scale anchored with descriptors of ‘not at all’ and ‘extremely’(Reference Flint, Raben and Blundell11). The visual analogue scales were completed eight times throughout the test day at − 10, 15, 45, 60, 90, 120, 180 and 240 min after consumption of the test breakfast (first-meal responses) and seven times at 265, 295, 310, 340, 370, 430 and 490 min (second-meal responses). After leaving the laboratory, participants recorded all food consumed and filled out the appetite ratings at 550, 610, 670 and 730 min. Therefore, appetite ratings were assessed for a total of 12 h.
Food intake assessment
Before the beginning of the study, all participants were instructed to register their food intake on three non-consecutive days (two week days and one weekend day)(Reference Serra-Majem and Aracenta-Bartrina25) to describe their eating habits at baseline. In addition, after leaving the laboratory (490 min), participants were asked to keep a food record for the rest of the day. To ensure accuracy, participants received written guidelines and were trained to estimate the consumed food portions using household items. Participants received a standardised record form to register the type and amount of foods and beverages consumed before the beginning of the study (baseline) and after they left the laboratory on each test meal day. Each dietary record was reviewed in the presence of the volunteer in order to ensure its accuracy and completeness. Food portions were converted into grams and the subsequent meal energy intake, 24 h total post-meal energy intake, macronutrients and fibre consumption were analysed using the software DietPro 5.0i (A.S. System).
Statistical analyses and calculations
While the incremental AUC (IAUC) (glucose, insulin, GLP-1, PYY, CCK and appetite sensations) was calculated excluding the values below the baseline values, the incremental area above the curve (IAAC; NEFA) was obtained excluding any value above (IAAC) the baseline values(Reference Wolever26). IAUC and IAAC were computed using the trapezoidal method, using Slide Write software, version 7.0 (Advanced Graphics Software, Inc.). Data analyses were conducted considering the following periods of time: 0–490 min (defined as the whole study response), 0–240 min (defined as the first-meal response), 240–490 min (defined as the second-meal response) and 490–730 min (defined as the post-laboratory period).
Residual plots of data were examined to consider homogeneity of variance and the Shapiro–Wilk test was performed to determine data distribution normality, and a logarithmic transformation was used when required. Repeated-measures ANOVA was used to examine the effects of meal and time on the postprandial responses and appetite sensations. This was performed using PROC MIXED (SAS, version 9.1). Post hoc comparisons were made using Bonferroni adjustments for meal and for meal × time interactions when significant. ANOVA was used to examine the effect of meal on IAUC, IAAC and food intake, and, when appropriate, post hoc comparisons were made using the Tukey's test. PROC TTEST (SAS, version 9.1) was applied to compare morning v. afternoon IAUC/IAAC and peak values of each treatment on each variable. All statistical analyses were performed using the Statistical Analysis System software package, version 9.1 (SAS Institute, Inc.). The criterion for statistical significance was P< 0·05 (two-tailed). The results related to the characterisation of the sample and dietary intake are presented as mean values and standard deviations, and biochemistry and appetite responses are presented as mean values with their standard errors.
Results
Participant characteristics
Two participants did not return their visual analogue scale and diet records and data analyses were conducted on thirteen participants. Their baseline characteristics are shown in Table 2. There were no differences in body weight (P= 0·95), capillary glucose (P= 0·93) and number of hours of sleep (P= 0·39) before the beginning of each experimental session. None of the feed-deprived variables assessed in the study differed (P>0·78).
Table 2 Baseline characteristics of the participants (Mean values and standard deviations)
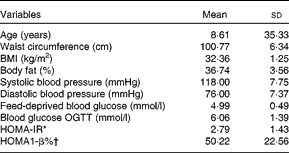
OGTT, oral glucose tolerance test; HOMA-IR, homeostasis model assessment insulin resistance index; HOMA1-β, homeostasis model assessment pancreatic β-cell function
* HOMA-IR = (fasting insulin (μU/ml) × fasting glucose (mmol/l))/22·5. (Insulin: 1 μU/ml = 6·945 pmol/l.)
† HOMA1-β = (20 × fasting insulin (μU/ml))/(fasting glucose (mmol/l)–3·5). (Insulin: 1 μU/ml = 6·945 pmol/l.)
Blood glucose
Although the first-meal glycaemic response IAUC did not differ between the breakfast treatments (P= 0·48), the ingestion of the PB meal resulted in significantly lower second-meal glycaemic response IAUC (P= 0·03), as compared with the control breakfast. The PB and the WP meals' glucose IAUC responses (0–490 min) were 18·7 and 14·4 % lower than the control meal (P= 0·48), respectively (Table 3). The inclusion of peanuts reduced the glycaemic index of the breakfast meals from 60·8 (control) to 56·2 (PB) and 58·4 units (WP) (Table 1).
Table 3 Area of the curve obtained at different time intervals for the biochemical parameters assessed after the ingestion of the study test meals (Mean values with their standard errors)

IAUC, incremental AUC; GLP-1, glucagon-like peptide-1; CCK, cholecystokinin; PYY, peptide YY.
a,bMean values within a row with unlike superscript letters were significantly different (P< 0·05).
* IAUC.
† Incremental area above the curve.
There was a time × meal interaction on the first-meal postprandial glycaemic response (P= 0·03). Post hoc comparisons indicated a lower glucose concentration at 15 and 310 min (both P= 0·04), and a tendency for lower glucose concentration at 45 min (P= 0·05) after the PB compared with the control breakfast (Fig. 2(a)). Mean first- (15 min) and second-(295 min) meal glucose peaks did not differ (P>0·26) according to the study treatment. The first-meal glycaemic IAUC responses for all treatments were significantly lower (P< 0·03) than the second-meal responses.

Fig. 2 Fasting and postprandial (a) glucose, (b) insulin and (c) NEFA responses to the breakfast meals containing peanuts (![]() ), peanut butter (
), peanut butter (![]() ) or control (
) or control (![]() , no peanuts). Values are means, with their standard errors represented by vertical bars. * Mean values were significantly different (P< 0·05). Insulin: 1 μU/ml = 6·945 pmol/l.
, no peanuts). Values are means, with their standard errors represented by vertical bars. * Mean values were significantly different (P< 0·05). Insulin: 1 μU/ml = 6·945 pmol/l.
Serum insulin
The WP and the PB meals' insulin IAUC responses (0–490 min) were 50·2 and 23·1 % higher than the response to the control meal (P= 0·53), respectively (Table 3). Post hoc comparisons indicated a higher insulin concentration for the WP meal at 45 min (P= 0·03) and for the PB meal at 120 (P= 0·04) and 370 min (P= 0·02) compared with the control breakfast (Fig. 2(b)). The first-meal insulinaemic response peaks (WP: 45 min; PB: 15 min; and control: 15 min) were higher (P< 0·04) compared with the second meal (WP: 310 min; PB: 370 min; and control: 295 min) for all the treatments. The WP and the PB first-meal insulinaemic responses (P< 0·002) were higher compared with the second-meal responses.
NEFA
The PB first-meal (0–240 min) NEFA IAUC was lower (P= 0·02) compared with the control breakfast. The WP and the PB NEFA IAUC responses (0–490 min) were 44·0 and 21·4 % lower than that obtained for the control meal (P= 0·06), respectively (Table 3). There was a time × meal interaction on the first-meal postprandial NEFA response (P= 0·002). Post hoc comparisons indicated a lower NEFA concentration at 90 (P= 0·02) and 120 min (P= 0·02), and a tendency for lower NEFA concentration at 180 min (P= 0·06) after the PB compared with the control breakfast (Fig. 2(c)). Peak NEFA did not differ between the first and the second meals according to the study treatment (P>0·44). There was no difference between the meal time period for NEFA IAUC responses among the study treatments.
Glucagon-like peptide-1
The WP and the PB GLP-1 IAUC responses (0–490 min) were markedly but not significantly higher (116·5 and 131·0 %, respectively) than the observed rise for the control meal (P= 0·46) (Table 3). GLP-1 concentrations did not vary significantly between the treatments (Fig. 3(a)). The PB first-meal GLP-1 response (P= 0·008) was higher compared with the second-meal response. Peak GLP-1 did not differ between the first and the second meals according to study treatment (P>0·32).

Fig. 3 Fasting and postprandial (a) glucagon-like peptide-1 (GLP-1), (b) peptide YY (PYY) and (c) cholecystokinin (CCK) responses to the breakfast meals containing peanuts (![]() ), peanut butter (
), peanut butter (![]() ) or control (
) or control (![]() , no peanuts). Values are means, with their standard errors represented by vertical bars. * Mean values were significantly different (P< 0·05).
, no peanuts). Values are means, with their standard errors represented by vertical bars. * Mean values were significantly different (P< 0·05).
Peptide YY
The PB and the WP first-meal (0–240 min) PYY IAUC were higher (P= 0·006) compared with the control breakfast (Table 3). There was also a time × meal interaction on the first-meal postprandial PYY response (P= 0·004). Post hoc comparisons indicated a higher PYY concentration at 120 (P= 0·04), 180 (P= 0·01) and 240 min (P= 0·01) after the WP compared with the control breakfast (Fig. 3(b)).
Cholecystokinin
The WP first-meal CCK IAUC responses (0–240 min) were three-fold higher, albeit not significantly, than the control meal (P= 0·65) (Table 3). CCK concentrations did not vary significantly between the treatments (Fig. 3(c)).
Appetite sensations
During the post-laboratory period (490–730 min), the WP and the PB IAUC desire to eat were lower (P= 0·04) compared with the control breakfast. However, self-reported fullness was significantly higher at 610 min after the control compared with the WP (P= 0·001) and the PB (P= 0·01) breakfast consumption. There was a lower desire to eat something fatty at 610 and 670 min after the control compared with the WP (P= 0·02) and the PB (P= 0·03) breakfast meals. There was a significant time × meal interaction for desire to eat something sweet (P= 0·03) after the participants left the laboratory (490–730 min). Post hoc comparisons revealed a higher (P= 0·02) desire to eat something sweet at 610 min for the PB compared with the WP breakfast meals. There was no treatment effect on first- and second-meal appetitive responses.
Food intake
Habitual intake and the WP breakfast fat consumption was higher (P= 0·003) than observed after the control breakfast. On the other hand, the control breakfast carbohydrate consumption was higher (P= 0·01) than the habitual intake (Table 4). There was no treatment effect on daily energy (P= 0·56), protein (P= 0·11) or fibre (P= 0·18) intake compared with the habitual intake. Daily food intake did not differ (P>0·34) according to the study treatment after the participants left the laboratory.
Table 4 Daily habitual intake and on each study session (Mean values and standard deviations)
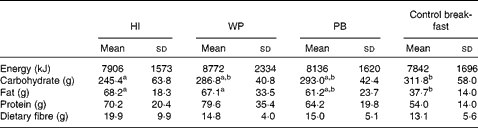
HI, habitual intake; WP, whole peanuts without skins; PB, peanut butter.
a,bMean values within a row with unlike superscript letters were significantly different (P< 0·05).
Discussion
The primary aim of the present work was to compare the effects of peanut consumption (WP or PB) on first- and second-meal responses. A secondary aim was to contrast responses to nut form, i.e. whole nut v. butter, on indices related to obesity and T2DM risk. All test meals (control, PB and WP) were equally palatable and had a medium glycaemic index (59·9–66·3).
Compared with the control meal, consumption of a breakfast meal containing 42·5 g of PB reduced the first-meal (0–240 min) NEFA concentrations and early glycaemic response. In addition, the consumption of WP and PB elicited a significant increase in the postprandial PYY concentration (180 and 240 min) as well as marked, but non-significant, GLP-1 and CCK elevations (0–240 min). A reduction on the second-meal (240–490 min) glycaemic response was also observed after PB consumption. It also reduced the desire to eat from 480 to 730 min after breakfast. Only preliminary evidence is available on potential mechanisms accounting for these outcomes. The observed effects may favour obesity and T2DM prevention and control, as discussed later.
Glucose metabolism
Although reduced glycaemic responses after peanut(Reference Reis, Bordalo and da Rocha27, Reference Johnston and Buller28) and almond(Reference Jenkins, Kendall and Josse29, Reference Josse, Kendall and Augustin30) consumption have been reported by other investigators, only one trial with almonds has examined the effect of nut consumption on the second-meal glycaemic response. It noted improved blood glucose control over successive meals when almonds or almond oil were added to high-glycaemic-index breakfast and lunch meals. In this trial, almond butter was not effective at moderating blood glucose excursions after the meals(Reference Mori, Considine and Mattes31). No explanation for this finding was apparent, as the likely mediator, unsaturated fat provided by the almonds, would have been more bioaccessible from the almond butter than whole almonds. The present trial examined the same effect with peanuts, allowing determination of the reliability and specificity of the responses. The results of the present study indicate that, in women at elevated risk for T2DM, the consumption of PB leads to a reduced glycaemic response acutely after breakfast and also 240–480 min later. In contrast to the findings with almonds, the whole nut responses were slightly weaker. Whether the difference between peanuts and almonds stems from greater bioaccessibility of lipids and lipophylic compounds from peanuts warrants further study. Greater fat availability may reduce the gastric emptying rate, decrease carbohydrate absorption rate and favour a reduction of glycaemic response(Reference Gentilcore, Chaikomin and Jones8). Thus, differences in the physical form of consumed peanuts can affect the postprandial glycaemic response. If this hypothesised mechanism holds, the finding with almond butter remains to be clarified.
It has been documented that the addition of fat and protein to meals leads to a reduction of glycaemic response. These effects have been attributed to delayed gastric emptying rate(Reference Feinle, O'Donovan and Doran32) and to increased insulin secretion mediated by intestinal hormones (glucose-dependent insulinotropic polypeptide and GLP-1)(Reference Collier, Greenberg and Wolever33). Although, in the present study, the fat and protein content of peanut and the PB breakfasts were very similar pre-ingestively, the incrementally greater reduction on the second-meal (240–490 min) glycaemic response observed after PB consumption suggests that the effect that these macronutrients exert on glycaemic response may also depend on food form.
According to the second-meal phenomenon, a reduction of the first-meal NEFA decreases the second-meal postprandial glycaemic response(Reference Jovanovic, Gerrard and Taylor34). This effect is probably due to improvement in insulin sensitivity, as an increase in fatty acid concentration impairs insulin signalling downstream to the insulin receptor, leading to insulin resistance(Reference Saini35). Therefore, the lower NEFA concentrations obtained after the consumption of the PB breakfast may be responsible for the lower glycaemic and insulinemic responses 240–480 min later. The extent of rise in postprandial glycaemia is considered a risk factor for CVD(Reference Cavalot, Petrelli and Traversa36). The results of the present study suggest that PB consumption might exert a protective effect against this disorder.
Peanuts contain high concentrations of arginine and protein, which are insulin secretagogues(Reference O'Neil, Keast and Fulgoni5). They also have a high Zn content that could stimulate the tyrosine kinase receptor, improving insulin sensitivity(Reference Jansen, Karges and Rink37). The high content of MUFA in PB might also increase insulin sensitivity and favour a reduction in the glycaemic response in insulin-resistant participants through increased GLP-1 secretion(Reference Paniagua, de la Sacristana and Sanchez38). This also may explain the second-meal glycaemic IAUC reduction observed in the present study.
Appetite control and food intake
In the present trial, there was a higher mean GLP-1 IAUC in the PB and the WP sessions compared with the control session. This is consistent with the elevated insulin concentrations for these treatments. Similar results were noted in another study where there was a significant increase in the GLP-1 concentration 240 min after the consumption of Korean pine nut oil (Pinus koraiensis) compared with the control(Reference Pasman, Heimerikx and Rubingh39). A trend in this direction has also been reported for almonds (whole and butter)(Reference Mori, Considine and Mattes31).
The high energy density, fibre and protein content of peanuts may augment their satiety property and promote body weight management(Reference Mattes and Dreher40). A reduction in hunger and in food intake has been reported in response to nut consumption (peanuts, PB, almonds and chestnuts) compared with foods with low energy density (e.g. rice cake and pickles) and a no-preload condition in healthy participants(Reference Kirkmeyer and Mattes41). In a recent study, higher fullness ratings were observed after almond meal (whole and butter) than control breakfast meals in glucose-intolerant participants(Reference Mori, Considine and Mattes31). In the present study, there was a lower desire to eat in response to peanut meals (whole and butter) compared with the lower-energy density meal (control meal).
Conclusion
These results indicate that PB can be added to breakfast meals to favour a reduction on the second-meal glycaemic and the first-meal NEFA responses, an increase on the first-meal PYY concentrations and a reduction on the desire to eat in obese women with high T2DM risk. Effects of WP were in a similar direction to the PB meal, but not as great. The present findings have practical implications, because peanuts and PB are often served in breakfast meals worldwide. Long-term feeding trials are now required to assess the feasibility and benefits associated with chronic peanut or tree nut consumption.
Supplementary material
To view supplementary material for this article, please visit http://dx.doi.org/10.1017/S0007114512004217
Acknowledgements
We are grateful to Leandro Licursi de Oliveira, Federal University of Viçosa, Brazil, for the assistance on GLP-1, CCK and PYY concentration determinations and to Alexandre Azevedo Novello, Federal University of Viçosa, Brazil, for the assistance on glucose, insulin and NEFA concentration determinations. We also thank the undergraduate project students (Millena Coutinho Tonázio and Ana Cecília Santos de Totaro) who provided technical assistance with the sample collection and data entry. The present project was supported by grants from the Brazilian government organisations (CAPES and FAPEMIG; 472297/2009-0), and the United States Agency for International Development through the Peanut Collaborative Research Support Program. N. M. B. C., J. B., R. C. G. A. and R. D. M. designed the research; C. E. G. R. and D. N. R. conducted the research; C. E. G. R., and R. C. G. A. analysed the data; C. E. G. R., D. N. R., N. M. B. C., J. B., R. C. G. A. and R. D. M. wrote the paper; and C. E. G. R. and R. C. G. A. had primary responsibility for the final content. All authors read and approved the final manuscript. The authors declare no conflict of interest.









