INTRODUCTION
Noroviruses cause about 500 000 episodes of gastroenteritis per year in the Dutch population [Reference de Wit1]. The onset of the disease is acute and is characterized by abdominal cramps, diarrhoea, nausea, and vomiting. In rare cases, severe dehydration can occur. Most patients recover within 12–60 h, but for some people, like the elderly, the duration of illness can be prolonged [Reference Koopmans and Duizer2] and long time shedding and recurring episodes have been described for immunocompromised patients [Reference Nilsson3, Reference Siebenga4]. Norovirus is highly contagious. Infections occur mostly by person-to-person transmission through faecal–oral contact, but they can also occur indirectly via contaminated surfaces or food. A second pathway is that of droplet transmission, especially when vomit is involved. In places where vulnerable individuals gather, e.g. nursing homes, noroviruses are easily spread and can cause outbreaks. During the period 1994–2005, 56% of 923 investigated suspected viral gastroenteritis outbreaks occurred in residential settings [Reference Svraka5]. Norovirus was found in 86% of these outbreaks in residential settings. In a 1-year study of all reported gastroenteritis outbreaks in The Netherlands, about 70% of the outbreaks in residential settings were caused by norovirus [Reference van Duynhoven6]. Norovirus outbreaks seriously disrupt the daily routine in nursing homes, due to relatively high attack rates in residents requiring more care, while some of the staff are absent because of sick leave. Moreover, extra costs incurred through the implementation of infection control measures and those related to staff, can turn such an event into a serious incident that should be prevented or eased wherever possible. The list of possible infection control measures is long, ranging from relatively cheap and simple interventions that are easy to implement to interventions that are difficult to implement and carry high costs. The control measures that are currently advised are mainly based upon experience from previous outbreaks and laboratory experiments on cleaning and disinfection [Reference Barker, Vipond and Bloomfield7–Reference Gehrke, Steinmann and Goroncy-Bermes9]. Field testing of different infection control protocols in nursing homes has not yet been done. In the current study, a more systematic approach was chosen to investigate the effectiveness of a number of infection control measures in daily practice.
METHODS
Design and setting
According to the Dutch Infectious Disease Act (1999), institutions have to report outbreaks of gastroenteritis to the relevant municipal public health service. This reporting system was used as foundation when setting up a collaboration between the National Institute for Public Health and the Environment (RIVM) and five municipal public health services in The Netherlands in 2005. All nursing homes and rest homes with a nursing ward within these regions were invited to participate in the study to evaluate infection control measures during norovirus outbreaks. In 2006, all participating homes were asked once again to participate in a second winter season. In addition, a sixth municipal public health service joined the collaboration and all eligible homes were contacted. All contacted homes were requested to complete a short baseline questionnaire to provide data about the size of the home (number of beds), type of wards (in particular psychogeriatric and somatic), whether they had experienced a gastroenteritis outbreak in the preceding year, and what measures are normally taken during such an event. A ward was defined as a number of residents that were considered as one group, mostly recognizable by a ward name and/or own group of staff members.
Infection control measures
At the start of the study, all participating homes were assigned to one of three infection control protocols. As far as possible, assignment was aimed at creating equally sized groups per protocol among participating homes. This was not done completely randomly as the public health services anticipated refusal of study participation when homes that usually responded actively during an outbreak were to be assigned to the basic protocol. Therefore, no opposite protocol was assigned in these instances. The protocols were composed from several existing protocols and were ordered according to the extensiveness of the interventions. The ‘basic’ protocol was based upon guidelines formulated by the Working group on Infection Prevention (WIP [10]). The protocol's main characteristics were cohorting of ill residents, immediate cessation of work when staff become ill, strict hand hygiene, and cleaning of toilets three times a day. The second protocol (‘generic’) was the intermediate protocol, with cleaning and disinfection measures, disinfection with 250 parts per million (ppm) chlorine, and recovered staff taking care of ill residents. The final protocol included an extensive set of measures (‘specific’), such as disinfection with 1000 ppm chlorine, preventing contact (i.e. staff exchange) between wards, ill staff staying at home until 48–72 h following recovery, and the use of face masks when in contact with vomit. The municipal public health services visited all participating homes in their region to explain the assigned protocol and the course of events in case of an outbreak.
Outbreaks
During both seasons (November–April 2005/2006 and November–April 2006/2007), all participating institutions were asked to report a (suspected) outbreak as soon as possible to the municipal public health service, send stool samples from 5 to 10 patients to the RIVM, and start the assigned infection control protocol. During an outbreak, staff were asked to complete an individual questionnaire for each gastroenteritis patient (residents as well as staff). This questionnaire included symptoms, duration of illness, and contact with other patients. At the end of an outbreak, all affected wards received an evaluation questionnaire about the total number of patients among residents and staff, and the implementation of the different measures during the outbreak. An outbreak was defined as two or more cases of gastroenteritis with onset within 3 days of one another on one ward within a home, and lasted up to the day the last case became ill on the involved ward. The duration of an outbreak at the home level was calculated by the time interval between date of onset of the first case and date of onset of the last case, independent of ward. The protocol had to be carried out until 7 days after recovery of the last case. A case of gastroenteritis was defined as (1) diarrhoea (⩾3 episodes in 24 h), and/or (2) vomiting (⩾3 episodes in 24 h), or (3) diarrhoea (<3 episodes) and two or more other symptoms (vomiting, stomach ache, abdominal cramps, nausea, fever, mucus in stool), or (4) vomiting (<3 episodes) and two or more other symptoms (diarrhoea, stomach ache, abdominal cramps, nausea, fever, mucus in stool). These case definitions were similar to those used in a previous Dutch population-based cohort study [Reference de Wit1].
Laboratory investigation of outbreaks
When a participating institution suspected an outbreak, stool samples were collected and sent by mail to the RIVM for testing for the presence of norovirus. The detection of norovirus was performed by reverse transcription–polymerase chain reaction (RT–PCR). At least one positive sample per outbreak was genotyped. Detection and typing were performed as described by Svraka et al. [Reference Svraka5]. The stool samples were not routinely tested for pathogens other than norovirus. If outbreak samples tested negative for norovirus, stool samples were later tested for rotavirus.
Analysis
Analyses were performed using SAS 9.1 for Windows (SAS Institute Inc. USA). Participation bias was studied by comparing participating nursing homes with those refusing. The risk of experiencing an outbreak during the study period was studied by comparing homes with an outbreak with homes without an outbreak. Variables included in both analyses were the size of the home (number of beds), percentage of psychogeriatric wards, occurrence of an outbreak in the previous year, whether staff worked on more than one ward (incidentally/frequently/standard by minority of the staff/standard by majority of the staff), and whether residents had a private room and/or toilet (none of the residents/some of the residents/all residents), all derived from the baseline questionnaire. The effects of the infection control measures were measured per ward by the duration of the outbreak per ward, attack rate of residents on the ward, and attack rate of staff on the ward. Attack rates were calculated by dividing the number of patients (residents or staff) on the ward by the number of residents or staff on the ward. Variables included in these analyses were all individual infection control measures (see Table 1) and the possible confounders: the affected ward being a psychogeriatric ward (yes/no), first ward within a home affected in the outbreak (yes/no), size of ward (⩽20 residents/21–30 residents/>30 residents), and genotype (GII.4/other). As a ward could experience more than one outbreak during the study period, and affected wards within one nursing home during an outbreak are likely to be related, hierarchical effects were tested using the null model likelihood ratio test. If this test indicated a significant improvement over the null model consisting of no random effects and a homogeneous residual error (used P value <0·05), multilevel analyses were performed using a random effect term relating all affected wards within a nursing home during one outbreak [Reference Hox11, Reference Singer12]. All analyses were done with a random effect, as hierarchical effects were found to be significant.
Table 1. Infection control measures analysed in the current study, with percentages of wards implementing these measures during a norovirus outbreak, restricted to wards implementing the measures within 3 days after illness onset of first patient (n=54)

Overall, all variables were first tested univariately using logistic regression (participation bias and risk of experiencing an outbreak), logistic-normal model with binomial data [Reference Condon13] (effect of infection control measures on both attack rates) or log-linear regression (effect of infection control measures on duration). Variables with P<0·20 were included in the multivariate analyses. Subsequently, a final model was determined by stepwise backward elimination of variables. For each step, the least significant variable was removed from the model, until all variables in the model had a P value <0·10 and the model was significant (P<0·05) compared to the null model, using the overall F statistic.
RESULTS
Participation
In the 2005/2006 season, the total participation rate was 52% (45/87); 36 nursing homes (51%) and nine rest homes with nursing wards (53%). For the second season, of the 45 homes from the first season and 15 nursing homes in the sixth health service region, 29 nursing homes (57%, four new homes) and six rest homes (67%) participated. Non-participation analyses showed an inverse relationship between participation of homes and the percentage of psychogeriatric wards: the odds ratio of participation was 0·82 [95% confidence interval (CI) 0·69–0·98] for an increase of 10%. Participating homes appeared to be larger and more often reported an outbreak during the last year, but these differences were not statistically significant (Table 2).
Table 2. Characteristics of participating and non-participating homes
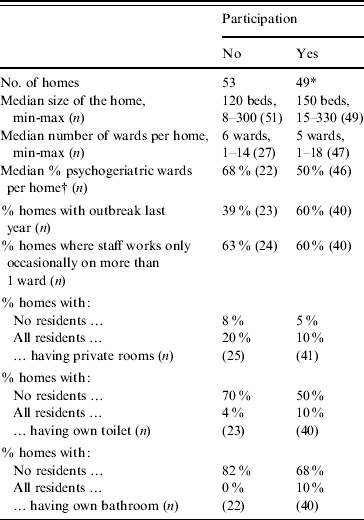
* Participation in one or both winter seasons.
† Difference is significant by Wilcoxon rank sum test, with P=0·02.
Outbreaks
A total of 48 outbreaks were registered over both seasons of which norovirus was detected in 37 outbreaks (77%) (Table 3). Norovirus of genogroup II was found in 33 outbreaks, of which 27 were genotype II.4. Genogroup I was detected in two outbreaks, and in the two remaining outbreaks the genotype could not be determined. Rotavirus was detected in four of the 11 remaining outbreaks. The risk for homes of experiencing a norovirus outbreak during the winter season was only related to the size of the home: an increase of 10 beds gave an odds ratio of 1·14 (95% CI 1·05–1·23). The percentage of psychogeriatric wards, residents having private rooms and/or toilets, staff working on more than one ward, and having had an outbreak in the previous year were not related to the risk of an outbreak in this study. No further information was available for six outbreaks (16%). Information was available for 75 wards involved in the remaining 31 outbreaks. The mean outbreak duration in a ward was 16 days (range 3–53 days), with mean attack rates of 35·0% (median 35·5%) in residents and 21·5% (median 17·9%) in staff.
Table 3. Outbreaks in nursing homes during November–April 2005/2006 and November–April 2006/2007, The Netherlands
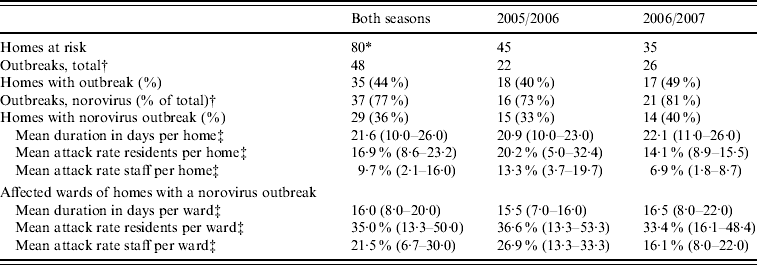
* Thirty-one homes both seasons, 14 homes only 2005/2006, four homes only 2006/2007.
† Some homes had multiple outbreaks.
‡ Values in parentheses are 25–75 percentiles.
Infection control measures
For 68 of the 75 affected wards, the date of start of the infection control measures was known. In 54 of these wards the measures were implemented within 3 days following disease onset of the first patient. Such immediate introduction of infection control measures decreased, independent of the assigned protocol, the attack rate in staff (mean attack rate 20·0% vs. 33·4%, P=0·019). The same applied for the duration of the outbreak (mean duration 15·9 vs. 18·5 days) and attack rate in residents (mean attack rate 35·9% vs. 39·3%), although not statistically significant. Therefore, analyses of the effect of the infection control measures were limited to these 54 wards with quick implementation of the measures. Comparison of the assigned protocols with the actual implemented measures revealed that compliance with the assigned protocol was poor. Sometimes more measures were applied than described in the assigned protocol, and sometimes assigned measures were not implemented. Only disinfection with 1000 ppm chlorine was not implemented in any of the nursing homes with the basic protocol. All other protocol specific measures were also sometimes taken by nursing homes not assigned to the particular protocol. Consequently, the analyses were done for the individual control measures. The measures studied are summarized in Table 1, using the percentages of affected wards that always implemented the measure. Three measures were implemented in at least 90% of the wards: immediate cleaning of a room when contaminated with vomit or stool, stringent hand washing with water and soap, and use of gloves when taking care of residents. Measures that were practised least often were recovered staff taking care of ill residents (18%) and cohorting of ill residents (20%).
Almost all measures showed a relationship with attack rates in residents in the univariate analyses with P<0·20 (Tables 4, 5), many measures decreased the attack rate, but several also increased it. Largest effects were seen for refusal of symptomatic visitors and exclusion of ill staff until 48–72 h following recovery. Removal of exposed food and disinfection with 1000 ppm chlorine were also strongly related to lower attack rates, whereas immediate disposal of incontinence material in closed plastic bags and use of new cleaning material for every room and toilet were significantly related to higher attack rates. All measures with P<0·20 were included in the multivariate model. The final model included only refusal of symptomatic visitors which was associated with a decrease of the attack rate of residents; Outbreaks of norovirus genotype II.4 appeared to increase the attack rates relative to outbreaks caused by other noroviruses. Ill staff remaining at home until at least 48 h following recovery was close to significance, but just dropped out of the final model.
Table 4. Median attack rates (25–75 percentiles) in residents and staff members, at ward level, for infection control measures and confounders with P<0·20 in the univariate model as obtained by logistic-normal regression
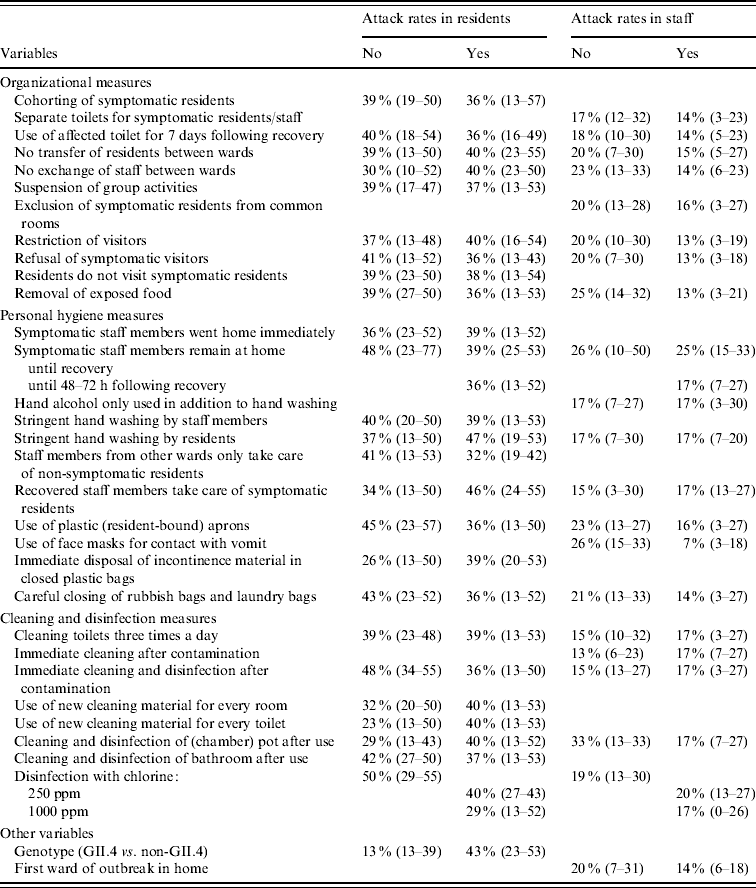
Table 5. Odds ratios (95% confidence interval), at ward-level, as obtained by logistic-normal regression for infection control measures and confounders with P<0·20 in the univariate model, separate for residents and staff
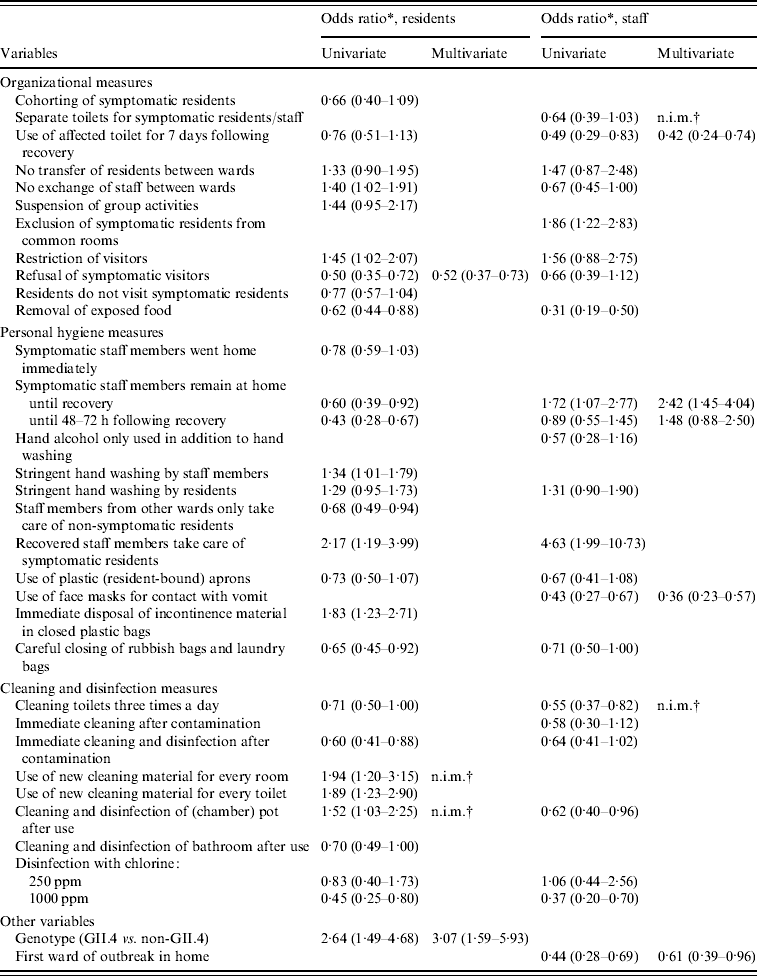
* Odds ratio compared to no implementation of control measure with 95% confidence interval.
† n.i.m., Not in multivariate model because of high correlation with one or more other measures.
Moreover, for attack rates in staff most infection control measures had P values <0·20. The three most effective measures were removal of exposed food, use of face masks when in contact with vomit and careful closing of trash bags and laundry bags, all lowering the attack rate (Tables 4, 5). Five other measures were also strongly related to the attack rate, of which use of the toilet for ill persons up to 7 days after their recovery, cleaning of toilets three times a day, and disinfection with 1000 ppm chlorine decreased the attack rate; Exclusion of symptomatic residents from common rooms and recovered staff taking care of ill residents appeared to increase the odds of staff becoming ill. In the multivariate model, use of the toilet for ill persons up to 7 days after they were recovered, use of face masks, and being the first ward affected in the outbreak within a home, significantly decreased attack rates in staff; Surprisingly, symptomatic staff remaining at home until recovery increased the attack rate.
Table 6 shows the effect of infection control measures on the duration of the outbreak. Except for the cohorting of ill residents, all other infection control measures mentioned seem to prolong an outbreak. When building the multivariate model, no significant final model was reached. Therefore, only disinfection with 1000 ppm chlorine was found statistically significant (P<0·05) to prolong an outbreak.
Table 6. Mean duration, and factors of change for duration of the norovirus outbreaks, at ward level, as obtained by log-linear regression for infection control measures with P<0·20 in the univariate model
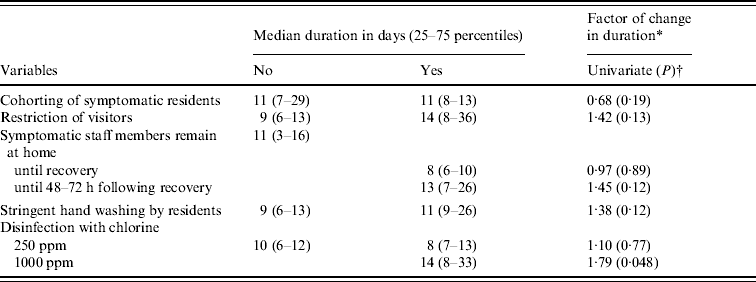
* Factor of change in duration compared to no implementation of control measure (with factor <1·0 is shorter duration when implementing the measure).
† Backwards elimination of variables did not yield any significant multivariate model.
DISCUSSION
The risk of experiencing a gastroenteritis outbreak was only dependent on the size of the nursing home; larger homes having a higher risk, possibly due to the greater chance of introduction of norovirus by visitors and staff. The nursing homes were assigned one of three protocols, partly taking previous routine during outbreaks into consideration. The evaluation questionnaire after an outbreak showed that the assigned protocol was not followed. The main reasons for omitting measures were time-consuming and practical problems. Therefore, the effect of individual infection control measures on attack rates of residents and staff, and the duration of the outbreak were investigated. First, a significant reduction in attack rates for staff and a non-significant reduction in attack rates for residents as well as reduction in duration of the outbreak were seen with implementation of control measures within 3 days. This finding is supported by two previous studies [Reference Ward14, Reference Navarro15] that showed infection control measures to be most effective when implemented before or at the moment that the first peak of symptomatic subjects is observed. Another study reported a reduction of the duration of outbreaks with a non-significant reduction in attack rates when the hospital units were closed within 3 days [Reference Lopman16]. In our study, the mean interval between the onset of illness in a patient and onset of illness in a resident who had contact with this patient was 3·4 days [Reference Heijne17], in good accord with the interval of 3·3 days reported for consecutive infections in children from a child centre and their secondary cases at home [Reference Götz18]. Thus, waiting >3 days before implementing control measures in a closed setting, means that more subjects are already infected, resulting in fewer susceptible subjects benefiting from the measures. Furthermore, such a delay in implementation can prolong the duration of an outbreak due to the continuation of secondary spread [Reference Marx19].
Person-to-person transmission plays a dominant role in spreading noroviruses [Reference de Wit, Koopmans and van Duynhoven20]. Reduction in attack rates of residents was most effectively achieved when symptomatic visitors were refused. Staff could also play a dominant role in the person-to-person spread of the virus to residents, as staff remaining at home until at least 48 h following recovery was found to be significantly related to lower attack rates in residents in the univariate analysis, and close to significance in the multivariate analysis. Nevertheless, the risk of illness in staff was higher when staff stayed at home until recovery compared to attending work earlier, even in the multivariate analysis. The mechanism behind this is unknown, especially as residents appear to benefit from this measure. Transmission between residents seems less important, probably because they have less physical contact and are less mobile. In an outbreak in a geriatric long-term care facility, debilitated residents who required frequent nursing contacts and nurses exposed to symptomatic residents had the highest attack rates, which supports our findings [Reference Marx19]. In staff, use of separate toilets for symptomatic residents/staff up to 7 days following recovery was effective for the odds of staff members becoming ill. Stringent contact precaution also appeared to be an effective way of decreasing the number of ill staff. Easy-to-implement measures that were found to be widely applied in nursing homes were stringent hand washing with water and soap, and use of gloves and plastic aprons when taking care of residents. Cohorting was often mentioned as being problematic, as in most nursing homes all beds are occupied. A similar problem was reported in a hospital outbreak [Reference Khanna21]. Moreover, moving residents from their own room to another room is considered undesirable from the perspective of the residents' welfare. An alternative could be closure of the entire ward. This should incorporate no exchange of staff between wards and extra precautions for staff working throughout the entire nursing home, e.g. physicians. In that way, spread of the infection to other wards can be prevented [Reference Lynn22]. Furthermore, closure of hospital units within 3 days of the date of onset of the primary case led to a faster containment of outbreaks [Reference Lopman16].
Norovirus can also be transmitted by aerosols. Three infection control measures dealt with this kind of transmission, and were found to reduce attack rates in staff in the univariate analyses, namely use of face masks when dealing with vomit, removal of exposed food, and careful closing of rubbish bags and laundry bags. Multivariately, only use of face masks remained significant. A possible explanation could be that this transmission route is less effective than the more efficient person-to-person spread and spread through contaminated surfaces. In an outbreak investigation, an inverse relationship between distance from the person vomiting and the risk of becoming ill was found [Reference Marks23], suggesting a short-term and location-restricted transmission in case of aerosols.
Experiments have shown that contaminated fingers could transfer norovirus to up to seven clean surfaces [Reference Barker, Vipond and Bloomfield7]. A feature of caliciviruses, encompassing norovirus, is the ability to survive on surfaces for up to 7–12 days [Reference D'Souza24, Reference Cheesbrough, Barkess-Jones and Brown25]. Furthermore, caliciviruses are resistant to high concentrations of chlorine and alcohol [Reference Duizer8, Reference Gehrke, Steinmann and Goroncy-Bermes9]. Immediate cleaning of a room when contaminated with vomit or stool prevents embedding of the virus and further spread. Although the effectiveness of disinfection with 1000 ppm chlorine could not be supported by a significant association in multivariate models, in univariate anlayses it decreased attack rates. However, as our study was of medium size, further large-scale studies are needed to draw conclusions on the effectiveness of disinfection with 1000 ppm chlorine. Furthermore, use of 1000 ppm chlorine may be incompatible with some surfaces, and requires correct safety measures to be taken by the cleaning staff, which can be unpractical [Reference Khanna21]. No differences were found between no use of chlorine or disinfection with 250 ppm chlorine.
A few measures appeared to be related to the duration of the outbreak. In a hospital setting in New Zealand, a similar phenomenon was observed; a faster and improved implementation of infection control measures led to lower staff attack rates, but did not change the outbreak duration and hospital attack rates [Reference Lynn22]. Only disinfection with 1000 ppm chlorine which is meant to stop transmission via contaminated surfaces, significantly prolonged the duration of an outbreak. This measure seems to reduce transmission of norovirus, but does not fully stop transmission. A decrease in the number of secondary cases caused by one patient after implementation of the measures compared to no implementation supports this hypothesis [Reference Heijne17]. If implementation leads to a mean decrease in secondary cases to <1 per patient, transmission is effectively stopped. A significant decrease in the number of secondary cases per patient after implementation compared to no implementation of the measure was found only for the use of new cleaning materials for every room [Reference Heijne17]. However, this measure was not found to be related to duration of the outbreak in the current analyses. The prolongation could also be caused by omission of measures occasionally. At the moment a measure is not applied new cases emerge, but with a longer interval between successive cases than when the measure had not been implemented at all.
The impact of a broad set of control measures has not been studied in practice previously, despite the great demand for evidence-based control measures for norovirus outbreaks. Nevertheless, our study suffers from some limitations. First, the confounding effect of co-infections during the outbreak was not taken into account. Faecal samples were initially tested for norovirus. When all samples from one outbreak were negative, the samples were tested additionally for rotavirus. Sixteen samples from 11 norovirus outbreaks (home level) of our study were tested for Clostridium, as part of another study where 350 stool samples of 56 norovirus outbreaks were tested [Reference Svraka26]. No C. difficile was detected, although in 11 of the 350 project samples other Clostridium spp. were found. One sample from one of the seven unexplained outbreaks of the current study was also tested as part of 120 stool samples of 34 unexplained outbreaks. Only that sample tested positive for C. difficile, with ribotype 001. Thus, co-infections of norovirus with C. difficile seem to be rare. Furthermore, in general the chance of a mixed outbreak seems to be small, as in only three out of 514 viral outbreaks investigated in the period 1994–2005 was more than one pathogen found [Reference Svraka5]. In two of these three outbreaks norovirus was involved: once combined with rotavirus and once with Clostridium (S. Svraka, personal communication).
Second, the homes did not implement all measures of the assigned protocol and sometimes implemented other measures. Therefore, the focus of the analyses was on the individual infection control measures, instead of on the three protocols, which would have allowed a more powerful analysis of the data. It also led to problems of relatively high correlations between the individual measures, which were dealt with in the multivariate analyses, although some measures could not be analysed multivariately because of collinearity. Furthermore, because of this departure from the original design, the results should be interpreted with caution, as other, non-controlled variables could have affected the results. This study can, therefore, indicate associations between infection control measures and the outcome variables, but it does not prove causality.
Third, the main analyses were done on the data of only 54 wards that implemented the measures within 3 days after onset in the first patient, which is a relatively small number. Finally, a substantial set of infection control measures was examined, increasing the chance of false-positive findings. Nevertheless, a method for correction of multiple comparisons does not provide an explanation of why a particular association was or was not found, and increases the chance of false-negative findings [Reference Rothman27]. Being the first study of its kind, false-negative results were considered especially undesirable. This study could, therefore, be best seen as the foundation laid for more evidence-based infection control measures against norovirus outbreaks. Further research with more rigorous efforts regarding compliance to the assigned protocol is needed in order to extend and refine the evidence.
In conclusion, the implementation of infection control measures reduced attack rates, but had little effect on the length of the outbreak. Overall, there are staff benefits from the implementation of infection control measures, as fewer staff members will become ill. Consequently, continuous care for residents is better guaranteed as well as fewer costs for sick leave and the substitution of staff. Furthermore, staff seem to play a predominant role in the spread of the virus to the residents. In cases of a closed setting, it is important to introduce the measures within 3 days after onset in the first patient. Measures reducing transmission between persons and via contaminated surfaces appear to be the most effective. Therefore, contact precautions, and immediate cleaning of contaminated surfaces are the most effective measures for the control of an outbreak.
ACKNOWLEDGEMENTS
We thank all participating nursing homes and rest homes with a nursing ward for their kind cooperation and efforts in the implementation of infection control measures and the data collection. Furthermore, we express our gratitude to the following persons from the participating municipal public health services for their kind assistance: Henk Klapwijk, Klazien Koma, Gera Clarkson, Karin Oudshoorn, Clementine Wijkmans, Sandy Oostveen, Mariëtte Waverijn, Petra van Nuenen-Hoffmans, Wineke Pronk, Larissa Helmhout, Anneke Jonkman, Jakob Keizer, Hans Frantzen. We thank Bas van der Veer for performing laboratory tests on the stool samples.
DECLARATION OF INTEREST
None.








