Introduction
Phytase is commonly supplemented in laying hen diets to improve phytate phosphorus (P) availability and retention and ultimately to reduce excretion (Ravindran et al., Reference Ravindran, Cabahug, Ravindren, Shelle and Bryden2000, Jalal and Scheideler, Reference Jalal and Scheideler2001, Gao et al., Reference Gao, Ji, Zhang, Zhao and Ma2013, Gosh et al., Reference Ghosh, Huynh, Sodhi, Sharma, Kim, Kim, Mongre, Park, Shin, Ko, Oh, Choi, Oh and Jeong2015). Phytate is the main phosphorus source in the plant-based feed ingredients, which not only has limited availability to laying hens, but can also reduce the availability of other nutrients. Phytate can complex with amino acids (Ravindran et al., Reference Ravindran, Cabahug, Ravindren, Shelle and Bryden2000, Rutherfurd et al., Reference Rutherfurd, Chung, Morel and Moughan2004), starch and fat (Ravindran et al., Reference Ravindran, Selle, Ravindran, Morel, Kies and Bryden2001, Newkirk and Classen, Reference Newkirk and Classen2001), thus reducing the absorption of these nutrients. In addition, phytate can interact with endogenous enzymes and result in a negative impact on Na and amino acid (AA) digestion and increase endogenous AA losses. Furthermore, in the intestine, phytate binds to Ca and other minerals, forming insoluble salts and rendering these minerals unavailable for absorption.
Phytase enzymes have the ability to hydrolyse phytate, releasing P which reduces the formation of phytate-mineral complexes, increasing the bioavailability of minerals (Kornegay et al., Reference Kornegay, Denbow, Yi and Ravindran1996, Jalal and Scheideler, Reference Jalal and Scheideler2001). In addition to the P and Ca release, inclusion of phytase in the diet may reduce the negative impact of phytate on AA and starch digestion, improving digestibility of dietary AA and energy. Phytase supplementation improves the availability of nutrients such as protein and amino acids (Ravindran et al., Reference Ravindran, Cabahug, Ravindren, Shelle and Bryden2000, Rutherfurd et al., Reference Rutherfurd, Chung, Morel and Moughan2004) and energy (Kornegay et al., Reference Kornegay, Denbow, Yi and Ravindran1996, Namkung and Leeson, Reference Namkung and Leeson1999, Ravindran et al., Reference Ravindran, Cabahug, Ravindren, Shelle and Bryden2000, Reference Ravindran, Selle, Ravindran, Morel, Kies and Bryden2001, Newkirk and Classen, Reference Newkirk and Classen2001, Hughes et al., Reference Hughes, Dahiya, Wyatt and Classen2009) in poultry.
Some nutritionists apply only P and Ca contribution values for phytase, others consider the energy, dig AA and Na contribution to fully benefit from the supplementation. These contributions are called ‘matrix values’ when used during least cost formulation, indicating the amount of nutrients (amino acids, ME, P, Ca, Na) contributed by the addition of phytase to the diet (Shelton et al., Reference Shelton, Southern, Gaston and Foster2004). The term ‘full matrix’ refers to applying dig AA, ME and Na contribution in addition to P and Ca contribution. When matrix values are used, diets can be formulated with a lower amount of Ca, P, crystalline amino acids and ME, subsequently reducing the cost of feed (Shelton et al., Reference Shelton, Southern, Gaston and Foster2004). Recent studies reported the benefit of increasing phytase dose in the diets for broilers and laying hens. Li et al. (Reference Li, Angel, Kim, Jiménez-Moreno, Proszkowiec-Weglar and Plumstead2015, Reference Li, Angel, Kim, Brady, Yu and Plumstead2016) observed that increasing Buttiauxella phytase dose from 500 to 1000 FTU/kg increased phytate degradation rate, ileal P and AA digestibility and improved performance of animals in broilers. Truong et al. (Reference Truong, Yu, Moss, Partridge, Liu and Selle2016) reported that increasing Buttiauxella phytase dose from 500 to 2000 FTU/kg further improved starch digestibility and body weight gain (BWG) in broilers. In laying hen diets phytase is traditionally supplemented at 300 FTU/kg although recent studies showed that increased phytase dose in laying hens diets can further increase phytate P degradation, ileal P digestibility and reduce P excretion (White et al., Reference White, Bold, Wealleans, Dersjant-Li and Kwakernaak2016).
The main objective of the study was to evaluate the productive performance and egg quality parameters in laying hens fed diets with reduced nutrient density based on the nutritional contribution of a new generation Buttiauxella phytase at doses of 300 or 600 FTU/kg in a commercial trial with laying hens from 21 to 57 weeks of age. In addition, the effect of phytase at a higher dose level of 1,200 FTU/kg was tested, using the nutrient contribution of 300 FTU/kg. Laying performance, egg quality and bone mineralisation are normally influenced by dietary P levels and are sensitive indicators of mineral adequacy in the diet and were used as the response criteria.
Materials and methods
The experimental protocol was approved by the Research Ethical Committee of the University of Murcia and was performed in accordance with the recommendations specified in the Guide for the Care and Use of Agricultural Animals in Research and Teaching (FASS, 2010; http://www.fass.org/).
Animals and Housing
At 18 weeks of age, a total of 480 Isa Brown laying hens were housed in laying cages under controlled climate conditions at the IMASDE Poultry Centre on the University of Murcia campus. Ten birds were placed in each cage (cage dimensions 63×121 cm with a height of 45 cm. The length of the light period was increased from 14L:10D to 16L:8D with a light intensity of 10 lux. The ambient temperature was maintained at a minimum of approximately 20°C throughout the trial. During the pre-experimental period (up to 21 weeks of age) a commercial laying hen diet was offered ad libitum, whereas from 21 weeks onwards, the hens were randomly assigned to one of the four dietary treatments (Table 1). Each dietary treatment was replicated 12 times with 10 hens per cage (replicate) in a randomised design.
Table 1. Description of experimental treatments
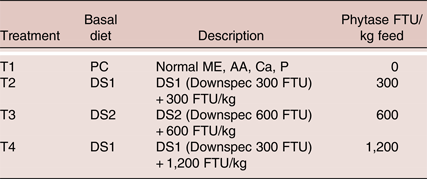
Experimental Diets
The ingredient and nutrient composition of the experimental diets is shown in Table 2. Three corn-wheat-soybean meal-based diets were formulated. The PC diet was formulated with adequate nutrients according to ISA breeder recommendation (2009) without phytase. The treatment diets DS1 and DS2 were formulated with reduction of available P, Ca, AME, CP, digestible amino acids and Na, according to the nutritional contribution of the phytase at dose 300 and 600 FTU/kg, respectively. Three levels of phytase were tested, a dose of 300 FTU/kg added to DS1, a dose of 600 FTU/kg added to DS2 or a dose of 1,200 FTU/kg added to DS1 (Table 3). No negative control treatments were included due to the duration of the study, as it was not deemed ethical to feed nutrient deficient diets for 9 months period. It was considered that the trial design would still yield useful results in terms of practical applications of phytase enzyme and down specification matrix values, as the PC diets were formulated to be representative to current practical commercial diets. The phytase was a 6-phytase derived from Buttiauxella sp. (Danisco Animal Nutrition, DuPont Industrial Biosciences, Marlborough, UK). Experimental diets were fed from 21 to 57 weeks of age in three phases based on the requirement of the specific age of the laying hens (Table 2).
Table 2. Ingredients and nutrient composition of the experimental diets (as is)
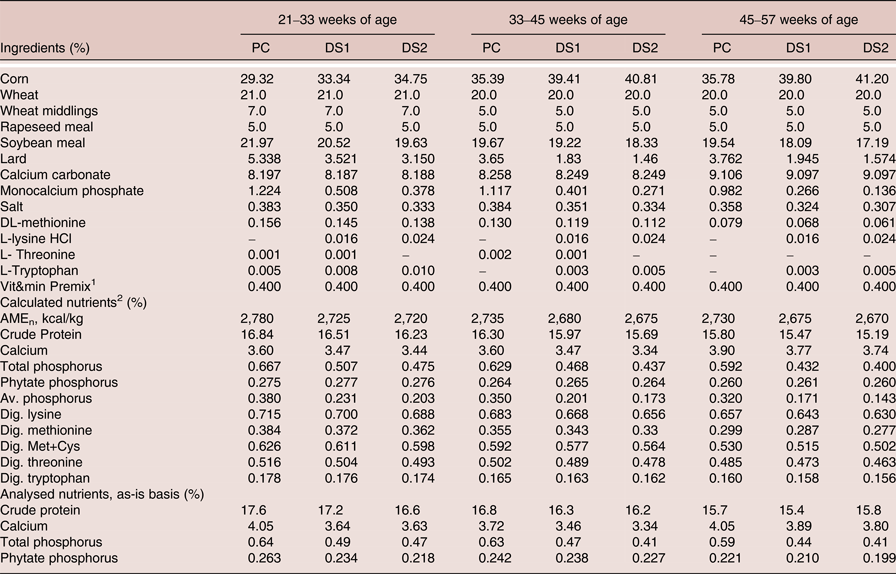
1 Provided per kg of diet: Vitamin A (E 672): 10,000 IU; Vitamin D3 (E 671): 2,500 IU; Vitamin E (α-tocopherol): 10.0 mg; Vitamin K3: 1.5 mg; Vitamin B1: 1.0 mg; Vitamin B2: 4.0 mg; Vitamin B6: 1.0 mg; Vitamin B12: 18.0 µg; Nicotinic acid: 20.0 mg; Pantothenic acid: 7.0 mg; Choline chloride: 240 mg; Cu (CuSO4·5H2O): 10.0 mg; Fe (FeSO4.H2O): 60.0 mg; I (IK): 3.0 mg; Mn (MnSO4.H2O): 140 mg; Se (Na2SeO3): 0.10 mg; Zn (ZnO): 100 mg.
2 Based on FEDNA (Reference de Blas, García and Mateos2010) values for feed ingredients.
Table 3. Phytase contribution used for the formulation of test diets
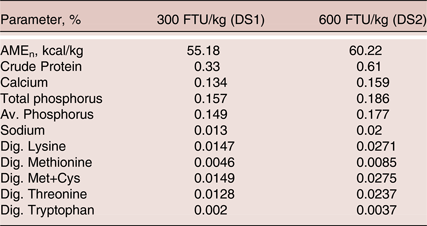
Birds had free access to feed and water throughout the experiment. Experimental diets were analysed for crude protein, calcium and total phosphorus according to AOAC (2000) procedures. Feed samples were analysed for phytase activity prior to the study start by Danisco Innovation Laboratories (Brabrand, Denmark), using the method described by Yu et al. (Reference Yu, Kvidtgaard, Isaksen and Dalsgaard2014). One phytase activity unit (FTU) is defined as the amount of enzyme that liberates one micromole of inorganic phosphate per minute from a sodium phytate substrate at pH 5.5 at 37°C.
Data Collection
Egg production was recorded on a replication basis daily. Eggs were classified as normal, broken, cracked and soft shelled. Classification was based on all the eggs collected daily. Egg weights were measured from all the eggs laid during one day every four weeks and calculated on a replication basis. Subsequently, the same eggs were used to assess shell percentage, yolk percentage, albumen percentage, yolk colour and eggshell breaking strength. Yolk colour was determined instrumentally by Minolta Chroma-meter (CR-300, Konica Minolta, Osaka, Japan) in the CIE L* a* b* space. The L* value indicates the lightness, representing dark to light (0–100). The a* (redness) value gives the degree of the red-green colour, with a higher positive a* value indicating a deeper red colour. The b* (yellowness) value indicates the degree of the yellow-blue colour, with a higher positive b* value indicating more yellow. Shell breaking strength was measured using an Instron Testing Machine, Model 5542 (Instron Ltd., High Wycombe, UK), equipped with a 500 N load cell. The eggs were compressed at a constant crosshead speed of 10 mm/min and the breaking strength was determined at the time of the eggshell fracture. Feed intake was determined on a replication basis by weighing back all feed every four weeks. Mortality was recorded daily on a replication basis. At the end of trial (57 weeks of age), the left tibia of one hen per replicate selected at random was removed, cleaned of adhering tissue, and frozen for determination of amount of tibia ash, Ca and P. The tibias were dried for 24 h at 105°C, weighed, and defatted for 24 h with ethyl ether. The tibias were then dry-ashed for 24 h in a muffle furnace at 600°C. Ash percentage of defatted left tibia and its relative Ca and P contents were determined after ashing at 600°C, according to the method of AOAC (2000).
Statistical Analyses
Statistical analyses of data were performed using the GLM procedure of IBM SPSS Statistics for Windows Version 19.0. (IBM Corp., Armonk, NY). Duncan's multiple range test was used to separate the means when ANOVA was significant. Differences were considered significant when P≤0.05.
Results and discussion
Feed analyses were within target values for crude protein, Ca and P for PC, DS1 and DS2 in each phase (Table 2). Phytase recovery analyses was variable among phases, but on average for the three phase diets values were within targets (309, 725 and 1,308 FTU/kg feed above PC diet for the treatments 300, 600 and 1,200 FTU/kg feed, respectively, Table 4).
Table 4. Effects of dietary treatments on productive performance, egg quality traits from 21 to 57 weeks of age and bone mineralisation of laying hens at 57 weeks of age
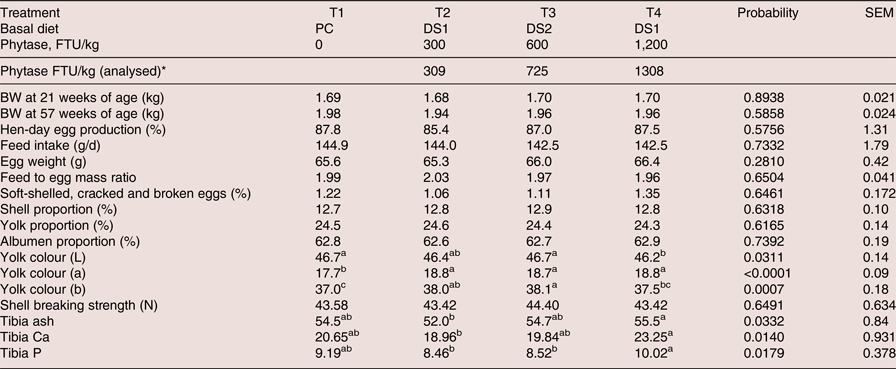
a-c Means within a row with different superscripts are significantly different (P < 0.05).
*Average of three phases and after subtract the basal phytase levels in PC
No significant differences were found for hen-day egg production, feed intake, egg weight, feed to egg mass ratio, shell, yolk or albumen proportion, unsaleable eggs or shell breaking strength in laying hens fed the PC diet or the DS diets with added phytase (Table 4).
Beneficial effects of dietary phytase supplementation on egg production, feed intake and egg weight of laying hens have been reported (Panda et al., Reference Panda, Rama Rao, Raju and Bhanja2005, Hughes et al., Reference Hughes, Dahiya, Wyatt and Classen2008, Kozłowski and Jeroch, Reference Kozłowski and Jeroch2011, Valaja et al., Reference Valaja, Tuunainen, Koivunen and Kühn2013). In these studies, phytase was added to negative control diets with reduction of only P, not with reduction of Ca, dig AA and energy. The nutrient contribution values for phytase used in the present experiment included Ca, available P, AME, CP, dig AA and Na. The availability of Ca (Jalal and Scheideler, Reference Jalal and Scheideler2001), P (Żyła et al., Reference Żyła, Mika, Świątkiewicz, Koreleski and Piironen2011, Gao et al., Reference Gao, Ji, Zhang, Zhao and Ma2013, Gosh et al., Reference Ghosh, Huynh, Sodhi, Sharma, Kim, Kim, Mongre, Park, Shin, Ko, Oh, Choi, Oh and Jeong2015), energy (Ravindran et al., Reference Ravindran, Cabahug, Ravindren, Shelle and Bryden2000, Reference Ravindran, Selle, Ravindran, Morel, Kies and Bryden2001, Newkirk and Classen, Reference Newkirk and Classen2001, Hughes et al., Reference Hughes, Dahiya, Wyatt and Classen2009) and AA (Jalal et al., Reference Jalal, Scheideler and Wyatt1999, Liebert et al., Reference Liebert, Htoo and Sunder2005, Ravindran et al., Reference Ravindran, Selle, Ravindran, Morel, Kies and Bryden2001, Reference Ravindran, Morel, Patridge, Hruby and Sands2006) has increased in laying hens when phytase is supplemented to the diets. The response observed in the present experiment suggested that the nutrients contribution values used for the tested phytase at given doses were applicable when laying hen performance and egg quality traits were used as response variables, using the current trial settings for a nine-month trial duration. These results indicated that phytase overcame the nutrient reduction in DS diets and maintained production performance comparable to PC.
Yolk colour, expressed as lightness (L*), redness (a*) and yellowness (b*), increased significantly in the treatment groups supplemented with phytase (Table 4). The effect in yolk colour was consistent, and the improvement was significant during each of the 28-d evaluated periods (data not shown). The inclusion of phytase in the diet of laying hens has been shown to improve the availability of phytate phosphorus and other minerals such as calcium and zinc (Jalal and Scheideler, Reference Jalal and Scheideler2001), the availability of energy (Ravindran et al., Reference Ravindran, Cabahug, Ravindren, Shelle and Bryden2000, Reference Ravindran, Selle, Ravindran, Morel, Kies and Bryden2001, Newkirk and Classen, Reference Newkirk and Classen2001, Hughes et al., Reference Hughes, Dahiya, Wyatt and Classen2009) and fat (Um and Paik, Reference Um and Paik1999). Thus, the improvement in yolk colour observed in the present study could indirectly indicate improved nutrient digestibility, mainly fat by phytase supplementation, although a negative control diet would be required to confirm this. In addition, this observation might be partially explained by the higher level of corn present in the DS1 and DS2 diets compared to that in the PC diet (about 4% more corn used in DS diets vs. PC), resulting in increased levels of lutein and zeaxanthin of the phytase supplemented diets. However, phytase treatment at 1,200 FTU/kg had the same feed formulation as 300 FTU/kg but showed significantly lower colour L vs. PC, which might indicate that the effect of phytase on egg colour was more related to energy balance than corn inclusion levels. Other studies did not find significant differences in albumen index, yolk index, yolk percent or yolk colour with phytase supplementation (Jalal and Scheideler, Reference Jalal and Scheideler2001, Park et al., Reference Park, Rhee, Um and Paik2009, Lucky et al., Reference Lucky, Howlider, Alam and Ahmed2014).
In the present study supplementing the DS1 diet with 300 FTU and the DS2 diet with 600 FTU resulted in non-significant differences in tibia ash, Ca and P, compared to the PC diet (Table 4). Birds were healthy and no sign of any illness and leg weakness occurred during the test period. Therefore, the response to these three parameters indicated that applying the AvP and Ca contribution values for 300 and 600 FTU, and supplementing with phytase at respective dosages, could reduce total dietary P levels in layer diets without compromising bone integrity. When supplementing the diet formulated by subtracting the matrix values for 300 FTU from the PC diet with a higher phytase dose of 1,200 FTU/kg, tibia ash, Ca and P increased significantly compared to the 300 FTU/kg supplementation. The extra-dose of phytase used was able to liberate more P and Ca than 300 FTU/kg, which resulted in a numerically higher tibia ash, Ca and P vs. PC. Although no significant differences were found between PC and the 300 FTU/kg phytase treatment, tibia ash was numerically lower in the 300 FTU/kg phytase treatment group. This may indicate that either the birds did not fully compensate for the Ca and P reduction or there is a Ca to P imbalance, as birds maintain the Ca to P ratio at approximately 2:1 in bones. Increasing phytase dose to 1,200 FTU would release more P and restore Ca: P balance, resulting in improved bone ash. It is worth mentioning that no leg weakness was observed in this study. Hughes et al. (Reference Hughes, Dahiya, Wyatt and Classen2009) reported significantly improved tibia bone ash with the addition of phytase at 200 or 400 FTU/kg in 61-week-old laying hens diets deficient in P. Gao et al. (Reference Gao, Ji, Zhang, Zhao and Ma2013) showed significantly improved tibia ash in 66-week-old laying hens supplemented with phytase at 500 or 5,000 FTU/kg to a P-deficient diet. However, no differences in tibia ash were found in several other studies (Gordon and Roland, Reference Gordon and Roland1997, Van der Klis et al., Reference Van der Klis, Versteegh, Simons and Kies1997, Boorman and Gunaratne, Reference Boorman and Gunaratne2001, Usayran et al., Reference Usayran, Farran, Awadallah, Al-Hawi, Asmar and Ashkarian2001, Sohail and Roland, Reference Sohail and Roland2002, Snow et al., Reference Snow, Rafacz, Utterback, Utterback, Leeper and Parsons2005, Meyer and Parsons, Reference Meyer and Parsons2011). The different responses observed in these studies may be explained by the differing levels of dietary P deficiency used and varying dietary Ca to P ratios.
Although overall results from the nine-month study showed no significant improvement in feed efficiency at higher phytase dose, the economic benefit, expressed as the feed costs per kg egg production of the groups supplemented with phytase were better than those fed the non-supplemented one (Table 5).
Table 5. Calculated feed costs per kg egg per treatment

1 Calculations done for the specified combination of raw materials, prices from February 2016, when the trial was done, including phytase price.
For comparison of phytase dose, overall results showed that phytase inclusion at 300 FTU/kg to DS1 diets maintained non-significant differences for egg production performance and egg quality parameters compared to PC, which indicated the nutritional values were met. Thus, no significant improvement on production parameters was seen with the extra phytase dose of 1,200 FTU/kg and no additional production benefit was seen with comparison of phytase at 1,200 FTU/kg vs 600 FTU/kg.
Data showed that laying hens fed diet supplemented with 600 FTU/kg resulted in the lowest feed costs per kg egg mass. Phytase at 300 FTU/kg with 300 FTU full matrix down spec reduced feed costs by 2.9%, phytase at 600 FTU/kg with 600 FTU full matrix down spec reduced feed costs by 8.3% while phytase at 1,200 FTU/kg using 300 FTU full matrix down spec showed 7.8% cost reduction compared to PC.
Better economic efficiency values with supplementation of phytase to laying hen diets have been previously reported (Ligeiro et al., Reference Ligeiro, Junqueira, da Silva Filardi, de Laurentiz, Ferreira Duarte and Andrade Marchizeli2009, El-Shikha et al., Reference El-Shikha, Attia, Soliman and Mahrose2013). Costa et al. (Reference Costa, Goulart, Figueiredo, Oliveria and Silva2008) suggested that adjusting the diet formulation by reducing nutrients and adding exogenous enzymes in order to restore nutritional value of the standard diet leads to a reduction in production costs.
On comparison of feed cost per kg egg mass for each phase, phytase at 600 FTU/kg added to DS2 diets showed consistently higher economic benefit compared to phytase at 300 FTU/kg or 1200 FTU/kg added to DS1. The economic benefit was higher in the second and third three months than first three months (data not shown), which might be explained by the difference in feed formulation. The diets for first three months had higher CP levels (about 2% higher) than the diets in the second and third trimester periods. This indicated that production benefits could be higher in a diet formulated with lower protein. This finding may imply that older birds benefit more from a higher dose of phytase supplementation (e.g. 600 FTU), due to reduced feed efficiency with increasing age.
Based on the cost benefit calculation, phytase at 600 FTU added to DS2 diets is the optimal dosing approach, which resulted in higher production benefit, compared to phytase at 300 FTU or 1,200 FTU added to DS1. Using phytase dose at 1,200 FTU/kg showed improved bone mineralisation vs 300 FTU, which was measured only at the end of the study where diets contained low P levels (total P content was 0.59, 0.44 and 0.41 respectively in PC, DS1 and DS2 diets). This may indicate that a high dose of phytase may be beneficial for mineralisation in older birds, however, more research should be carried out to test this hypothesis.
Based on the results of this study, it can be recommended that under current practice for commercial diets formulated based on ISA breeder recommendations, when supplementing phytase in the diets, the nutrients contribution of P, Ca, ME, dig AA and Na at corresponding phytase dose should be applied. Phytase dose at 600 FTU/kg added to diets with reduction of above mentioned nutrients can result in better production benefit than the traditional dose of 300 FTU/kg with corresponding nutrients reduction, or dosing at 1,200 FTU/kg with 300 FTU matrix values.
Conclusions
The data from this commercial feeding trial indicated that supplementation with Buttiaxella phytase at doses of 300 FTU/kg could replace 55 kcal/kg of AMEn, 0.325% CP, 0.134% Ca, 0.149% av.P, 0.0147% dig. Lys, 0.0149% dig. Met+Cys, 0.0128% dig Thr and 0.002% dig. Trp, and at dose of 600 FTU/kg could replace 60 kcal/kg of AMEn, 0.607% CP, 0.159% Ca, 0.177% av.P, 0.0271% dig. Lys, 0.0275% dig. Met+Cys, 0.0237% dig Thr and 0.0037% dig. Trp in diets formulated based on ISA breeder recommendations. Applying these nutrient contributions maintained the egg production, BW, egg quality parameters, but with an increased egg colour compared to PC. The supplementation of higher phytase dose of 1,200 FTU to the 300 FTU/kg matrix reduced diet did not outperform laying performance or egg quality, but improved tibia mineralisation. Supplementation of phytase resulted in cost benefit; the best economic efficiency value during the whole experimental period was recorded with phytase at 600 FTU/kg when full matrix values are used. Under current practice when commercial diets are formulated based on ISA breeder recommendations, lowering diet nutrient density while supplementing with phytase reduces the overall diet cost, which should contribute to the profitability of the production of eggs.







