INTRODUCTION
Dramatic reductions in the incidence of mumps have occurred wherever the mumps vaccine was made a routine component of paediatric healthcare. In the WHO European region declines in incidence have ranged from 88% to 99% [Reference Galazka, Robertson and Kraigher1]. Nevertheless, after a series of recent mumps outbreaks that involved large numbers of previously vaccinated individuals, questions have arisen about the adequacy of existing mumps vaccination programmes in Western countries.
Israel's experience with mumps has paralleled that of other highly vaccinated societies. In the pre-vaccination era mumps incidence had averaged over 4500 cases annually and ranged from 20 to 162 cases/100 000 population. In 1989 routine immunization with the MMR vaccine was introduced at the age of 15 months and in 1994 a second MMR dose was instituted for 6-year-olds. As a result, by the 1995–1998 period the average number of mumps cases had fallen to 367 annually and the number of cases/100 000 population had fallen to between 0 and 3 [Reference Slater, Anis and Leventhal2].
However, in September 2009 and continuing for 12 months Israel experienced its largest mumps outbreak since 1988, with over 5000 reported cases and a monthly incidence rate that peaked at 13·3 cases/100 000 population. In this mumps outbreak, as had also been the case in Israel's 2007–2008 measles outbreak [Reference Anis3], lower vaccination rates in some population subgroups played an important role. However, in marked contrast with the measles outbreak, in which over half the patients were aged <10 years and in which only 4·6% had been fully vaccinated for their age, the mumps outbreak had a greater impact on older age cohorts and on individuals who had been fully vaccinated – epidemiological features similar to those in recent mumps outbreaks in Europe and North America [Reference Dayan and Rubin4–Reference Savage8].
This study discusses the epidemiology of the 2009–2010 mumps outbreak in Israel in the context of recent outbreaks elsewhere; reviews the major approaches to the effectiveness issues that have emerged regarding the mumps component of the MMR vaccine; and considers the implications for public health policy.
BACKGROUND AND METHODS
Mumps has been notifiable by law in Israel since 1977. Cases are reported to the Health Ministry's district offices by physicians and medical laboratories, and national data are processed and analysed by the Ministry's Division of Epidemiology. Individual case-notification reports indicate age, gender, nationality, address, date of onset, laboratory diagnostic test results, and the patient's prior vaccination history. The reports are based on epidemiological investigations conducted by district health office staff. The data generated from these case reports are used to track the course of infectious disease outbreaks and to monitor key epidemiological indicators. All quantitative data regarding the 2009–2010 mumps outbreak in Israel are based on this information system. Other epidemiological assessments, such as the extent of infection in population subgroups or the relative vaccination coverage of these groups, derive from a familiarity with the demographic characteristics of the localities from which the case notifications are received, and from ongoing communication with professional staff in local health clinics and district health offices.
The clinical case definition used to detect cases for reporting purposes describes mumps as a self-limiting acute illness characterized by the swelling of a parotid or other salivary gland lasting ⩾2 days, with epidemiological linkage to other cases. Laboratory confirmation is not required for routine reporting purposes; during the 2009–2010 outbreak 704 cases were laboratory-confirmed (13% of total reported cases). Serological tests for specific IgG and IgM antibodies, and virus detection using RT–PCR, are conducted for verification purposes. Nucleic acid sequencing and genotyping of the virus strain are performed in selected cases. As is common in passive surveillance systems the extent of underdiagnosis and underreporting is unknown. Since notification methods have remained unchanged over the years, incidence data are considered indicative of actual trends.
The mumps vaccine that has always been used in Israel is the attenuated Jeryl Lynn strain, which is included in the MMR vaccine (M-M-R II, Merck, and Priorix, SKB) [Reference Slater, Anis and Leventhal2]. Vaccination coverage estimates for the first MMR dose are based on a representative sample of children born in each health district and registered in Mother and Child Health Services clinics, which provide care to pre-school children. Vaccination coverage data for the second dose, which is provided to 6-year-olds in the first grade, are submitted to the Health Ministry by the School Health Services. Since 1991 the first-dose coverage rate has ranged from 94% to 96%. Coverage data for the second dose are available for school years 2002–2003 to 2009–2010 and have ranged from 90% to 97%, averaging 94% over the period. Although these summary coverage rates might appear high enough to limit the spread of mumps once it had been introduced, Israel has always been plagued by relatively poor immunization compliance by certain subgroups within its ultra-orthodox Jewish community, a sector that comprises about 10% of the total population. Accurate compliance data for this subpopulation are unavailable, but it is estimated to be 5–15% lower than the national average for the first MMR dose. School health service records for the ultra-orthodox population are incomplete, and it can be assumed that fewer 6-year-olds in this sector receive the second MMR dose than do those in the general population.
RESULTS
Course of the outbreak
According to an epidemiological investigation the 2009–2010 mumps outbreak in Israel had its origin in a visit to Jerusalem by students from a New York yeshiva (religious boarding school). The students had been infected during a New York-area outbreak that originated at an orthodox Jewish summer camp; the virus had been imported there from England, where a mumps outbreak had begun earlier that same year [Reference Stein-Zamir9–11]. During the first 10 weeks of the Israeli outbreak 173 cases of mumps were reported in the Jerusalem area, half of which were among male yeshiva students. In the initial stages of the outbreak three-fifths of case patients were aged between 10 and 19 years; the median age was 15 years. Two thirds of these patients reported having received two doses of the MMR vaccine. [12] The Central Virology Laboratory of the Ministry's Public Health Services identified the virus in circulation as genotype G5, the same genotype identified in the 2009 outbreak in England [Reference Conly and Johnston5].
The populous Jerusalem yeshiva community continued to be the centre of mumps infection as the scope of the Israeli outbreak grew. Over time, however, the disease gradually spread outward into the community at large. As the outbreak moved from the older, male yeshiva population into the local community, a gradual shift to the left occurred in age-group incidence, along with a reduction in the male-to-female case ratio. From autumn 2009 to spring and summer 2010 the percentage of case patients aged ⩾10 years fell from 77% to 59%, and the percentage of male patients fell from 78% to 59% (see Table 1).
Table 1. Age and sex of case patients by stage of outbreak

Values are percentages.
Stage 1: 1 September 2009–31 December 2009.
Stage 2: 1 January 2010–31 March 2010.
Stage 3: 1 April 2010–31 August 2010.
Source: Individual case-notification reports.
After rising steadily during autumn and early winter of 2009–2010, monthly incidence peaked in February at just under 1000 new cases, and by summer had declined significantly (see Fig. 1). From September 2009 to August 2010 the number of mumps cases throughout Israel totalled 5239, half of which occurred in the Jerusalem health district. The outbreak was limited almost entirely to the Jewish population (over 98% of case patients were Jewish) and remained highest in the ultraorthodox community, primarily among male yeshiva students. Over the 12-month period two thirds of case patients were aged >10 years, nearly half were between 10 and 19, and nearly 40% were aged ⩾15 years (see Table 2). Sixty-four per cent of case patients were male. Of the 64% of patients whose vaccination status was known 78% had been fully vaccinated for their age. Sixty-six patients were hospitalized, and there were no fatalities. Reported complications included 14 cases of orchitis, four cases of epididymo-orchitis, five cases of meningitis, four cases of meningoencephalitis, two cases of hearing loss in one ear, two abortions, one case of pancreatitis, one case of labyrinthitis, and one oedema of the sternum.
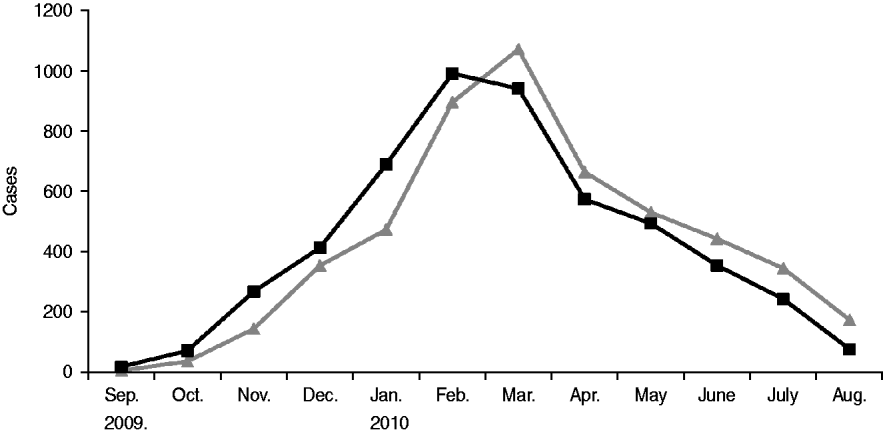
Fig. 1. Epidemiological curve of the 2009–2010 mumps outbreak in Israel. ![]() , Reported date; –▪–, date of onset.
, Reported date; –▪–, date of onset.
Table 2. Age group incidence distribution by stage of outbreak
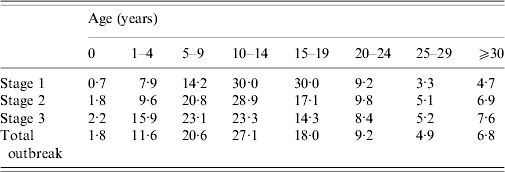
Values are percentages.
Stage 1: 1 September 2009–31 December 2009.
Stage 2: 1 January 2010–31 March 2010.
Stage 3: 1 April 2010–31 August 2010.
Source: Individual case-notification reports.
Control measures
The Ministry of Health instructed community professional staff to continue implementing standing orders regarding mumps prevention: to maintain vaccination coverage at high levels; to ensure the timeliness of vaccination, particularly for 12-month-old infants and first-grade pupils; to target those with incomplete immunization histories, particularly children and healthcare workers; to alert district office staff to the possibility of new mumps cases and to confirm and report incident cases. Vaccination catch-up operations were implemented wherever mumps was occurring and a second dose was offered in kindergartens, schools, religious academies, and in families to persons who had not previously received two doses of mumps-containing vaccine. A third dose of vaccine, or the measuring of antibody levels of those previously vaccinated, was not recommended.
Additional recommendations included exclusion from school and child care for 5 days from the onset of parotid gland swelling, and droplet precautions in hospitalized patients until 5 days after the onset of parotid gland swelling. Immunization of all contacts with one dose of mumps-containing vaccine, regardless of vaccination status, was considered but not implemented due to a temporary vaccine shortage. In all, a few thousand extra vaccine doses were given in defined outbreak settings. After the outbreak peaked in February and fewer new cases were observed each month, it appeared that the minority of young adults with low immunity had acquired mumps naturally or, in some cases, by immunization.
DISCUSSION
Based on long experience working with the ultra-orthodox community in Israel we hypothesize that the high incidence of mumps among yeshiva students can be linked to lower levels of immunization coverage among some ultra-orthodox sectors, and to the high-density congregate living environment, featuring long hours of face-to-face study in crowded study halls, that characterizes yeshiva life. While all yeshiva students are male and reside in dormitories or communal apartments, there were ample opportunities for the disease to eventually spread outwards into the community at large. Students with mumps are generally sent home to recover, where they can infect their younger siblings, i.e. boys and girls who attend community schools. Moreover, while the yeshiva academic year is 12 months long, there are three vacations of 3 weeks' duration, corresponding to major religious holidays, when students incubating mumps can infect other family members and friends in their home neighbourhoods.
Two epidemiological features of the 2009–2010 mumps outbreak in Israel are of special note: the relatively high incidence among older age cohorts during much of the outbreak, and the high rate of vaccination coverage among case patients. Prior to the vaccine era those most susceptible to mumps were children between the ages of 2 and 12 years [Reference Shanley13], and today in countries that do not vaccinate against mumps incidence is highest in children aged from 5 to 9 years [14, 15]. In the recent mumps outbreak in Israel, however, the virus infected a comparatively higher proportion of adolescents and young adults, and the most salient feature of the outbreak was the high rate (78%) of mumps patients who had been fully vaccinated for their age.
The shift to the left in age-group incidence, which continued as the outbreak progressed, was probably the consequence of the undervaccination of children in some sectors of the Jewish ultra-orthodox community. As with the measles outbreak in Israel 2 years earlier [Reference Anis3] this problem demonstrated how the clustering of individuals with relatively low immunization coverage can undermine the benefits of herd immunity in a highly vaccinated society [Reference McBryde16]. Mumps seropositivity rates were also found to be lower in this community just prior to the 2009–2010 outbreak. Rates averaged 51·8% in the ultra-orthodox population in Jerusalem compared to 68·1% for the general population and, for those aged ⩾10 years, 46% compared to 70% (D. Cohen, Tel Aviv University, unpublished data). As with the 2007–2008 measles outbreak, the undervaccination problem facilitated the spread of the virus in certain subgroups. But what was different about the mumps outbreak was how age-group incidence and patients' vaccination histories diverged from earlier experiences with vaccine-preventable childhood diseases.
Similar epidemiological trends have been appearing in recent mumps outbreaks elsewhere. In a major outbreak in the UK in 2005 the majority affected were college and university students between the ages of 19 and 23 years. Close to one third of these patients had received mumps vaccinations, although many were either unvaccinated or had been immunized only once before the two-dose MMR regimen had become routine [Reference Cohen17–19]. During 2009 in England and Wales, mumps incidence was most highly concentrated in the 15–24 years age group, primarily in university students. Here too many case patients were undervaccinated because they were too old to have benefited from the two-dose MMR schedule [19]. During a 2006 outbreak in Austria the highest incidence rates occurred in those aged 18–30 years [Reference Schmid20]. In a 2009–2010 mumps outbreak in The Netherlands 65% of case patients were students, the median age was 21 years, and of those students whose vaccination status was known 79% had received two mumps vaccinations [Reference Whelan7]. At the epicentre of the 2006 mumps outbreak in the USA where 85% of the cases were clustered, incidence was highest in the 18–24 years age group; of patients in this age group whose vaccination histories were known the proportion of two-dose recipients was 84% [Reference Barskey, Glasser and LeBaron6, Reference Dayan21]. In an 8-month period during a 2009–2010 New York area outbreak, 97% of all cases patients were orthodox Jewish yeshiva students and 61% were aged between 7 and 18 years. Of those in this age group with known vaccination histories, 75% had received two doses of MMR vaccine [10].
Several features of the recent mumps resurgence highlight the transition from the pre-vaccine to the post-vaccine era. Members of certain age groups were too old to have benefited from the two-dose MMR regimen, but too young to have gained immunity to mumps through natural infection during childhood. Many of these individuals were of university-student age in the past decade and were especially vulnerable to the outbreaks that occurred; they have been described collectively as a ‘lost cohort’ [Reference Conly and Johnston5, Reference Savage8, Reference Watson-Creed22]. Some health professionals have failed to recognize mumps symptoms when presented with them, delaying the implementation of measures that might have helped prevent the virus from spreading [Reference Conly and Johnston5, Reference Kancherla and Hanson23]. With the recent mumps resurgence numerous medical practitioners have been reintroduced to the disease or are seeing it for the first time. Further, the diagnosis of mumps has been found to be more difficult in previously vaccinated patients than in naive individuals [Reference Anderson and Seward24].
Given the large number of two-dose MMR recipients who have been infected during recent outbreaks, the efficacy of the mumps vaccine has been called into question [Reference Dayan and Rubin4, Reference Briss25, Reference Peltola26]. There are several potential causes for vaccine failure [Reference Kancherla and Hanson23] and their relevance to the epidemiological features of recent mumps outbreaks varies. While problems such as primary vaccine failure, or errors in the handling and storage of vaccines have always explained why some vaccinees remain susceptible to mumps, they fail to address the timing issue: Why has there been a resurgence of mumps outbreaks in recent years?
A potential explanation that has been suggested is that of genotype mismatch – the Jeryl Lynn strain, which is used in the USA, Canada, and widely in Europe, belongs to mumps virus genotype A, and the major outbreaks of recent years have been caused by virus genotype G [Reference Shanley13, Reference Kaaijk27]. It seems unlikely, however, that genotype mismatch is a major factor in these outbreaks. The Jeryl Lynn strain has been found to be both protective of the general population and reasonably effective under epidemic conditions [Reference Dayan21, Reference Cortese28]. In Israel's recent outbreak, for instance, the genotype G virus did not spread to the general population, which is protected by the Jeryl Lynn strain. In a recent serological study, the Jeryl Lynn strain effectively neutralized genotype G virus samples that had been taken from patients in the 2006 outbreak in the USA [Reference Rubin29]. Moreover, genotype mismatch cannot explain why suddenly, after years of experience to the contrary, incidence should be lower in children than in adolescents and young adults.
One feature of the mumps vaccine that could account for recently changing patterns in both age-group incidence and patient vaccination history is the waning immunity factor. Some studies had identified primary vaccine failure as the key reason for mumps infection during the vaccine era [Reference Briss25], but when mumps outbreaks began affecting large numbers of fully vaccinated adolescents and young adults, the waning immunity issue began receiving more attention [Reference Conly and Johnston5, Reference Peltola26, Reference Kaaijk27]. Several studies conducted in the USA found that the risk of contracting mumps was correlated with the number of years since last having received a dose of vaccine [Reference Dayan and Rubin4]. In one university that had been at the epicentre of the 2006 outbreak, and at which two-dose MMR coverage had exceeded 95%, the odds of having mumps were found to have increased in students aged 18–19 years who had been vaccinated ⩾10 years earlier [Reference Cortese28]. In England, waning immunity was found to have occurred in previously vaccinated children who were infected during the 2004–2005 outbreak in that country [19].
Aside from the recent epidemiological record, laboratory tests have shown a decline in seropositivity rates and antibody titres following vaccination, indicating a degree of waning immunity that could place older vaccinees at risk during an outbreak [Reference Choi30]. Antibody decay was discovered in tests among university students and staff between the ages of 19 and 30 years who had received two MMR doses [Reference Date31]. Further, a recent study of mumps seroprevalence in population groups in the USA raised similar concerns about borderline herd immunity levels [Reference Kutty32]. As Dayan et al. observed, even at high (95%) vaccination coverage and effectiveness levels, the resulting 90% protection rate comes precariously close to the estimated herd immunity threshold [Reference Dayan21].
High-density environments increase the risk of contagion, and the clustering of susceptible individuals has brought mumps prominently to the campus. In societies where MMR vaccination had become routine, mumps incidence has shifted from children in primary school to adolescents and young adults – particularly those in colleges and universities. These institutions bring together large numbers of individuals who, by virtue of their age, may be more susceptible to the virus, and mumps incidence in such settings has become more widespread as a result [Reference Barskey, Glasser and LeBaron6, Reference Dayan21, Reference Cortese28].
The changing profile of the typical mumps patient suggests that public health officials need to consider new strategies for combating the virus.
Given the increased risk of mumps infection in older age cohorts and the related risk of outbreaks in high-density environments, analysts have recommended that the clustering of susceptible adolescents and young adults be avoided. Since persons in these age groups tend to enter universities and the military in large numbers, the provision of booster vaccinations to all susceptible individuals entering these congregate living environments – including both the unvaccinated and those whose immunity may have weakened since their last vaccination – could prove a highly effective outbreak prevention measure [Reference Kaaijk27, Reference Date31, Reference Park33].
A large-scale (albeit unintentional) clinical trial may have bolstered the argument for just such a booster vaccination programme. During 2006 American soldiers should have been as susceptible to that year's US mumps outbreak as were their university counterparts. Military personnel live in high-density environments; recruits belong to the university-student age cohort that was central to the 2006 outbreak; and large numbers of soldiers are stationed on bases in the midwestern region of the country where the outbreak was concentrated. Military policy, however, was that all recruits without documentation of prior vaccination or other evidence of immunity to measles and rubella, receive a dose of MMR vaccine. Consequently booster vaccinations (and in an unknown number of cases a third dose of MMR vaccine) were provided to a large population of potentially susceptible individuals. The result was that, of a total population of nearly 1·4 million personnel, only 53 cases of mumps were reported in the US military during 2006 – a level that fell within the military's 10-year range of aggregate mumps incidence [Reference Barskey, Glasser and LeBaron6].
Today, most children and most adults are protected against mumps – the former group because they have been vaccinated fairly recently, the latter group because they are old enough to have gained natural immunity from exposure to the virus in the pre-vaccine era. But a new class of susceptibles has emerged: adolescents and young adults with no natural mumps immunity and whose vaccine-based immunity is disappearing. That this same cohort populates a multitude of congregate living environments greatly heightens the risk of future, large mumps outbreaks. Booster vaccinations for those at high risk of infection during mumps outbreaks, such as yeshiva and university students, merit priority consideration.
ACKNOWLEDGEMENTS
We acknowledge with thanks the Ministry of Health Advisory Committee on Immunization and Infectious Diseases; the National Center for Mumps, Measles and Rubella at the Central Virology Laboratory of the Ministry's Public Health Services; the staff of the Jerusalem Health District Office and other health district offices for their assistance in controlling the outbreak; and Mr Ruslan Gosinov for his assistance with data management and his work on the graphics.
DECLARATION OF INTEREST
None.





