Introduction
Invasive predators are a primary threat to biodiversity at a global scale (Clavero and Garcia-Berthou Reference Clavero and García-Berthou2005, Doherty et al. Reference Doherty, Glen, Nimmo, Ritchie and Dickman2016). Native species often lack a shared evolutionary history with novel predators, and thus lack the requisite adaptations for coexisting with invaders (Sih et al. Reference Sih, Bolnick, Luttbeg, Orrock, Peacor, Pintor, Preisser, Rehage and Vonesh2010). Under these favourable conditions, introduced predators can achieve ecological release and exponential population growth in the presence of naïve prey (Sih et al. Reference Sih, Bolnick, Luttbeg, Orrock, Peacor, Pintor, Preisser, Rehage and Vonesh2010), with devastating ecological and economic effects (Savidge Reference Savidge1987, Clavero and Garcia-Berthou Reference Clavero and García-Berthou2005, Reaser et al. Reference Reaser, Meyerson, Cronk, De Poorter, Eldrege, Green, Kairo, Latasi, Mack, Mauremootoo, O’Dowd, Orapa, Sastroutomo, Saunders, Shine, Thrainsson and Vaiutu2007, Clavero et al. Reference Clavero, Brotons, Pons and Sol2009). Some of the most extreme impacts of invasive predators have been documented on oceanic islands (Medina et al. Reference Medina, Bonnaud, Vidal, Tershy, Zavaleta, Donlan, Keitt, Corre, Horwath and Nogales2011, Spatz et al. Reference Spatz, Zilliacus, Holmes, Butchart, Genovesi, Ceballos, Tershy and Croll2017), which are disproportionately vulnerable due to their isolation from continental terrestrial systems and high levels of endemism (Kier et al. Reference Kier, Kreft, Lee, Jetz, Ibisch, Nowicki, Mutke and Barthlott2009). For example, invasive predators have caused population declines, local extirpations, and extinctions of native bird species across island systems such as Hawai’i, New Zealand, and the Mascarenes (Atkinson Reference Atkinson1977, Clout Reference Clout2001, VanderWerf Reference VanderWerf2009, Cheke and Hume Reference Cheke and Hume2010, Doherty et al. Reference Doherty, Glen, Nimmo, Ritchie and Dickman2016).
Despite the wide-ranging and severe impacts of invasive predators on native island biota, population recovery has been documented in response to predator control. Eradications of invasive mammals on islands have already resulted in substantial conservation benefits to native species, such as positive demographic or distributional responses (Jones et al. Reference Jones, Holmes, Butchart, Tershy, Kappes, Corkery, Aguirre-Muñoz, Armstrong, Bonnaud, Burbidge and Campbell2016), and further gains are expected from future eradication projects (Holmes et al. Reference Holmes, Spatz, Oppel, Tershy, Croll, Keitt, Genovesi, Burfield, Will, Bond and Wegmann2019). For logistical reasons, eradication projects to date have occurred largely within fenced predator-proof exclosures (Tanentzap and Lloyd Reference Tanentzap A and Lloyd2017) and on relatively small, uninhabited islands, although larger, inhabited islands are increasingly being targeted (Glen et al. Reference Glen, Atkinson, Campbell, Hagen, Holmes, Keitt, Parkes, Saunders, Sawyer and Torres2013). Where successful eradication is not currently feasible, predator control can also substantially increase reproductive success and survival of island populations (Moorhouse et al. Reference Moorhouse, Greene, Dilks, Powlesland, Moran, Taylor, Jones, Knegtmans, Wills, Pryde and Fraser2003, Whitehead et al. Reference Whitehead, Edge, Smart, Hill and Willans2008, VanderWerf Reference VanderWerf2009). Adaptation of native species to introduced predators has also resulted in some examples of improved fitness and range recovery (Strauss et al. Reference Strauss, Lau and Carroll2006).
One location where recovery of native species has been particularly challenging is the Pacific island of Guam in the Mariana Archipelago, Micronesia. Following the introduction of the predatory brown treesnake Boiga irregularis to Guam after World War II, nine of the island’s 11 native forest bird species were extirpated in a matter of decades (Savidge Reference Savidge1987, Rodda et al. Reference Rodda, Fritts and Conry1992, Wiles et al. Reference Wiles, Bart, Beck and Aguon2003). During the peak of the irruption in the early 1990s, brown treesnake densities are estimated to have reached 50–100 individuals/hectare or higher, eventually declining to 25–50/hectare by the late 1990s (Rodda et al. Reference Rodda, Fritts, McCoid, Campbell, Rodda, Sawai, Chiszar and Tanaka1999), with an estimated island-wide population size of 1–2 million snakes (Rodda and Savidge Reference Rodda and Savidge2007). Nevertheless, some bird species have managed to persist in the presence of the brown treesnake (Wiles et al. Reference Wiles, Bart, Beck and Aguon2003), including the endangered Yåyaguak (Mariana Swiftlet) Aerodramus bartschi (Apodidae) which roosts in caves that may be relatively inaccessible to snakes, and the locally endangered Såli (Micronesian Starling) Aplonis opaca (Sturnidae). The Såli is a cavity-nesting omnivore and important seed disperser in the Marianas (Rehm et al. Reference Rehm, Chojnacki, Rogers and Savidge2017, Reference Rehm, Fricke, Bender, Savidge and Rogers2019, Pollock et al. Reference Pollock, Fricke, Rehm, Kastner, Suckow, Savidge and Rogers2020), with a broad geographic distribution across much of Micronesia (Craig and Feare Reference Craig, Feare, del Hoyo, Elliott, Sargatal, Christie and de Juana2018). Although historically common-to-abundant throughout Guam across all habitat types (Jenkins Reference Jenkins1983, Craig and Feare Reference Craig, Feare, del Hoyo, Elliott, Sargatal, Christie and de Juana2018), the Såli’s distribution and abundance on Guam declined precipitously along with the rest of the avifauna after the introduction of the brown treesnake (Savidge Reference Savidge1987, Wiles et al. Reference Wiles, Bart, Beck and Aguon2003). The last census in the early 1990s estimated the population at only 60–120 individuals, primarily restricted to Andersen Air Force Base (AAFB), a military installation in northern Guam (Wiles et al. Reference Wiles, Aguon, Davis and Grout1995).
The Såli population continues to persist on Guam, but there has been no formal assessment of its status since the early 1990s (Wiles et al. Reference Wiles, Aguon, Davis and Grout1995). Recent observations indicate that the population may be expanding, particularly at AAFB, where snake population control and containment aimed at protecting infrastructure and preventing spread to other islands has been ongoing since 1993 (reviewed in Clark et al. Reference Clark, Clark, Siers, Pitt, Beasley and Witmer2018). Although recent studies of radio-tagged Såli fledglings at AAFB have documented high post-fledging mortality due primarily to brown treesnake predation (Wagner et al. Reference Wagner, Tappe, Jaramillo, Kastner, Van Ee, Savidge and Pollock2018, Pollock et al. Reference Pollock, Savidge, Kastner, Seibert and Jones2019), regular sightings of Såli in urban areas in northern and central Guam not occupied since the 1980s suggest that its distribution may be expanding southward even without widespread snake control.
To assess the current status of Guam’s Såli population, we conducted an island-wide survey of their distribution and abundance. Our primary objectives were to obtain a current estimate of Såli population size and explore how distribution and abundance have changed over time. To do so, we leveraged multiple recent data sources (i.e. opportunistic sightings, transect surveys and standardised area searches) combined with a review of historical literature on the population on Guam. We discuss the potential reasons for a population increase and range expansion on Guam and describe possible management actions to facilitate Såli recolonization across the island.
Methods
Study site
Guam is the largest (541 km2) and most economically developed island in Micronesia with the region’s largest human population (~160,000 inhabitants as of 2010; Spies et al. Reference Spies, Mizerek, Reeves, Amidon, Miller, Goldstein and DellaSala2019). More than 20% (>11,000 ha) of the island’s area is developed (Spies et al. Reference Spies, Mizerek, Reeves, Amidon, Miller, Goldstein and DellaSala2019). The northern half of Guam is a limestone plateau that supports most of the island’s remaining intact karst forest, whereas the southern half is volcanic in origin, more mountainous, and composed largely of ravine forest and savanna habitat (Donnegan et al. Reference Donnegan, Butler, Grabowiecki, Hiserote and Limtiaco2004). Most of the island’s human population and developed habitats are concentrated in northern and central Guam, whereas southern Guam is less developed and more sparsely populated. Although Såli prefer forested areas (Rehm et al. Reference Rehm, Balsat, Lemoine and Savidge2018), they are generalists and historically were present in all available habitats on Guam, from roadside and urban areas to savanna and forest (Baker Reference Baker1947, Jenkins Reference Jenkins1983, Engbring and Ramsey Reference Engbring and Ramsey1984).
Literature review
To assess changes in Såli population size and distribution over time and contextualize our current survey results, we gathered all available published and grey literature that referred to Såli abundance and distribution on Guam. To do so, we searched Web of Science and Scopus in November 2020 using the search terms “Såli” AND “Aplonis opaca” AND “Micronesian Starling” AND “Guam” AND “population” AND “abundance” AND “distribution”. We also supplemented this literature with unindexed reports familiar to the authors.
Population size and age structure at AAFB
To estimate the size and age structure of the Såli population at AAFB, we conducted three consecutive week-long area searches of the base’s main developed area (its administrative and housing areas) in September-October 2018 (Figure 1). We also sampled areas to the south and west of the base perimeter once each week (Figure S4 in the online supplementary material) to ensure that we were not omitting appreciable numbers of birds off-base during our surveys. The extent of our sampling area was smaller than the Såli surveys in the 1980s and 1990s, which encompassed the flight line and large swaths of forest throughout northern Guam (Engbring and Ramsey Reference Engbring and Ramsey1984, Wiles et al. Reference Wiles, Aguon, Davis and Grout1995). For example, Engbring and Ramsey (Reference Engbring and Ramsey1984) conducted point-counts at 178 stations on or in proximity to AAFB, all within forest habitat. The primary reason we limited our survey to the main developed area of the base was to encompass the core Såli roosting habitat, where virtually all individuals appear to currently roost. Extensive radiotelemetry has demonstrated that Såli range widely throughout the forested areas along the eastern and southern perimeter of AAFB during the day (H. S. Pollock and H. S. Rogers pers. obs.). However, because of high snake predation in forested areas (Pollock et al. Reference Pollock, Savidge, Kastner, Seibert and Jones2019), birds of all age classes (>99% of n = 44 individuals, n = 444 roosting observations) return to the developed area in the afternoon (around 15h00) prior to roosting, where they are relatively sedentary and easier to count (H. S. Pollock and M. Kastner pers. obs.). More than 350 individuals in the AAFB population were colour-banded in 2017–2018 as part of a larger project on Såli demography (see Wagner et al. Reference Wagner, Tappe, Jaramillo, Kastner, Van Ee, Savidge and Pollock2018, Pollock et al. Reference Pollock, Savidge, Kastner, Seibert and Jones2019) and our method for estimating population size relies on resights of these colour-banded individuals (see Statistical analysis below). We assumed a closed population with no births, deaths, emigration, or immigration occurring between the successive counts. We are confident that emigration and immigration were minimal based on the aforementioned tracking of radio-tagged individuals, all of which used forest extensively and travelled off-base but returned to the core roosting area at night (H. S. Pollock and H. S. Rogers pers. obs.). By repeating intensive area searches each week for three consecutive weeks, we obtained three replicates while minimizing the confounding effect of mortality on population size estimates (Kendall Reference Kendall1999).
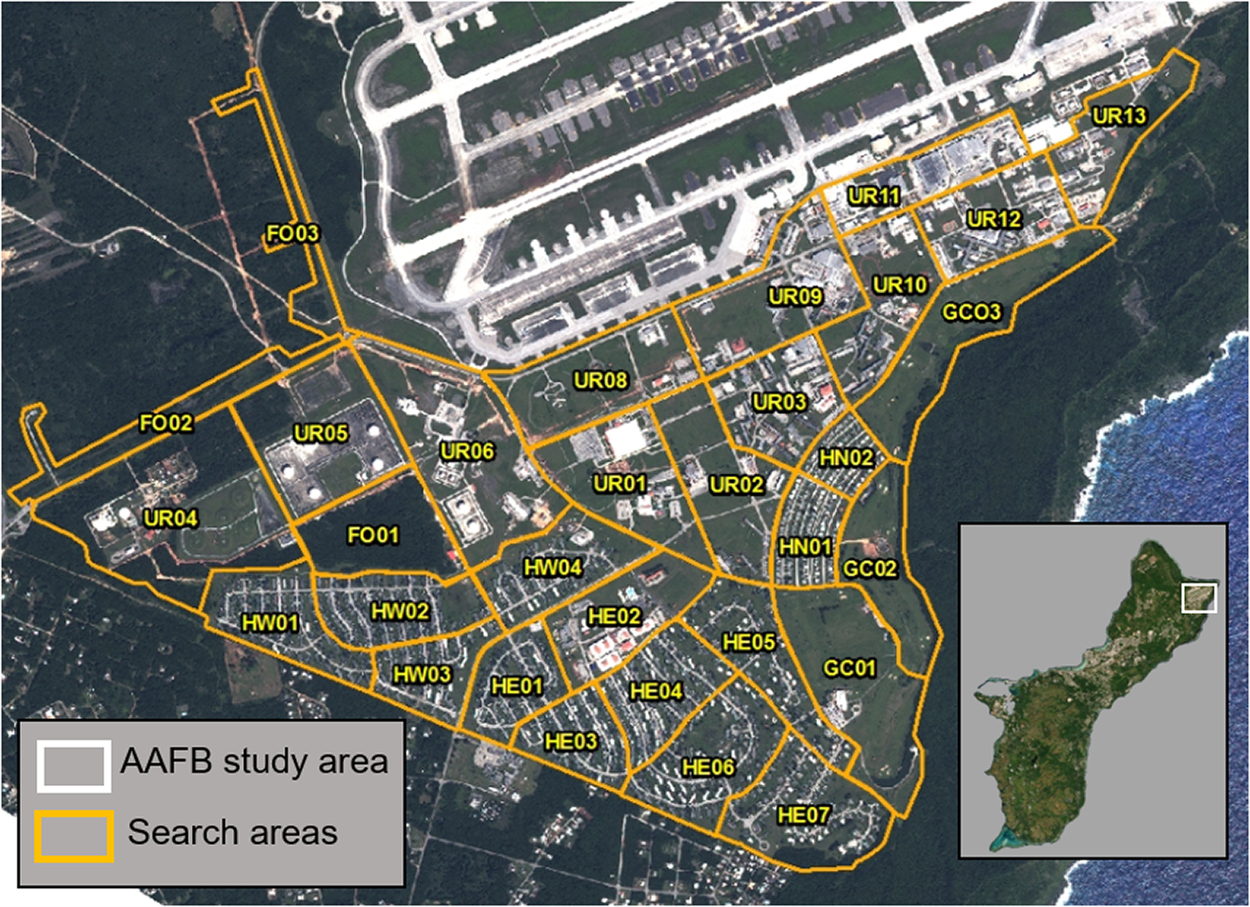
Figure 1. The study area on AAFB and the search areas used for the standardised area searches. The developed areas of AAFB were divided into 28 search areas, comprising three types of habitats (forest search areas FO01, FO02, and FO03 were included in the closest adjacent search area): urban (UR), residential housing (HW, HE, HN), and golf course (GC). Inset depicts the island of Guam, with the study area indicated by the white rectangle.
To count Såli, we divided the main developed area of AAFB into 28 search areas of roughly similar size, comprising three habitat types: urban (UR), residential housing (HW, HE, HN), and golf course (GC; Figure 1). Each day, we randomly selected four search areas (thus allowing all 28 search areas to be surveyed per week) and assigned groups of two observers to survey two search areas each. Observers traversed a given search area together, which increased overall detection probability and the accuracy of colour-band identifications. To minimise the risk of double-counting individuals, observers never searched adjacent search areas in a given day, remained in constant contact during surveys, and communicated movements of any birds throughout a given search area. Surveys lasted until the entire extent of the search area had been covered (50.4 ± 14.7 minutes; range: 27–91 minutes) and were allocated to one of two time blocks: ‘early’ (15h00–16h30) and ‘late’ (16h30–18h00). We changed observer teams and alternated the order of search areas on a weekly basis to control for the potential influences of observer bias and time of day on Såli counts (e.g. if we sampled UR01 in the ‘early’ time block in week 1, then we sampled UR01 in the ‘late’ time block in week 2, and then again in the ‘early’ time block in week 3). We randomly assigned half of the search areas to start during the early time block (with the other half assigned to the late time block by default). In each search area, observers counted all individuals detected by sight and sound and collected the following data whenever Såli were detected: GPS location, number of individuals of each age class (fledgling, juvenile, subadult, adult) in the group, number of colour-banded birds and their colour combinations, and time of the observation. Due to the open configuration of the landscape (i.e. no forest cover, sparsely populated with trees), we were able to approach and visually confirm age classes of all birds initially detected by sound. We assigned age class based on plumage development of known-age individuals tracked longitudinally on AAFB during 2017–2018 (H. S. Pollock and M. Kastner pers. obs.; see Figure S1 for examples). Any bird detected along the forest edge outside of the core study area was assigned to the closest adjacent search area.
Distribution and abundance outside of AAFB
To estimate the distribution and abundance of Såli outside the developed area at AAFB, we combined two data sources – opportunistic sightings and transect surveys. We excluded a small population (~200 individuals) of Såli that has remained stable on nearby Cocos Island, a small islet 2.4 km south of Guam (Engbring and Ramsey Reference Engbring and Ramsey1984, Engbring and Fritts Reference Engbring and Fritts1988, Wiles et al. Reference Wiles, Bart, Beck and Aguon2003; L. Barnhart Dueñas pers. obs.). First, we collated opportunistic sightings of Såli from three complementary sources of information: (1) eBird records (eBird 2019) from 2009 to 2018 (n = 9 observations; Table S1), (2) a database of Såli sightings from 2005 to 2018 (n = 39 observations; Table S1) maintained by the Guam Division of Aquatic and Wildlife Resources (DAWR), and (3) a database of Såli sightings from 2009 to 2019 maintained by MK (n = 16 observations; Table S1). For eBird data, we took a conservative approach and excluded sightings that did not include a detailed description of the bird or the specific location and date of the sighting. All sightings included in the DAWR database were independently verified by DAWR biologists through detailed discussions with observers who reported sightings as well as site visits after each report. All sightings collected in MK’s database were recorded by biologists familiar with the species. For each sighting, we recorded the village and specific location, the observer, the number of Såli, GPS coordinates, and year of the sighting.
We also surveyed 46 transects distributed across the island once each in April–May 2018 to aid in determining the island-wide distribution. First, we surveyed 19 spring bird count (‘SBC’) transects situated along trails or roads that were previously established by DAWR in 1985. Ten SBC transects (two northern, three central, and five southern) were located in rural areas with little development and nine (five northern and four central) were located in suburban areas near residential homes within 1 km of forest habitat. We sampled birds at 10 points along each transect. Average transect length was 5,189 ± 1,496 m (average distance between points: mean ± SD = 605 ± 210 m) and varied in length due to differences in landscape configuration and accessibility. At each point, experienced observers conducted 10-minute unlimited distance point-counts (sensu Matsuoka et al. Reference Matsuoka, Mahon, Handel, Sólymos, Bayne, Fontaine and Ralph2014) and recorded all individuals that were seen or heard. Second, we used the opportunistic sightings compiled by DAWR to inform placement of 27 additional ‘Såli’ transects located where Såli had recently been observed. Såli transects were ~500 m in length, did not overlap with SBC transects, and all except one were located within 500 m of forested habitat (either scrub forest or ravine forest; sensu Taboroši Reference Taboroši2013). The number of Såli transects per village depended on the number of opportunistic sightings in that area – we placed more transects in areas with more prior sightings to increase detectability given that Såli were often present in low numbers. Transect surveys began at dawn, lasted approximately 75 minutes, and were conducted by the same experienced observers as the SBC transects. We conducted 10-minute unlimited-distance counts at six points along each transect, all equally spaced 100 m apart, and recorded all individual Såli seen or heard at each point.
Statistical analysis
All analyses were conducted in R version 3.3.3 (R Core Team 2017). To estimate Såli population size on AAFB, we followed six steps. (1) We used unique resights of colour-banded individuals across the duration of the study period (21 days) to generate accumulation curves using function specaccum in the ‘vegan’ package (Oksanen et al. Reference Oksanen, Blanchet, Kindt, Legendre, Minchin, O’Hara, Simpson, Solymos, Stevens, Wagner and Oksanen2013). (2) We used the function specpool, which uses three non-parametric estimators (Chao’s estimator and two separate jackknife estimators) and a bootstrap estimator, to extrapolate these accumulation curves and estimate the total number of banded individuals present at the study site during the study period. Standard errors were lowest for the bootstrap estimator (Figure S2), so we opted for this approach. (3) We calculated the weekly detection probability of colour-banded birds for each week of the survey, estimated as the number of banded individuals detected in a given week divided by the total number of individuals counted that week. (4) We divided the extrapolated estimate of the total number of banded individuals present at the time of the counts in step (2) by the weekly detection probability in step (3) to generate a weekly population size estimate. (5) To quantify uncertainty in each weekly population size estimate, we used the standard errors from the bootstrap estimator in step (2) to create a range for each weekly estimate, bounded on the lower end by mean–SE and on the upper end by mean + SE. (6) We averaged the three weekly mean estimates generated in step (4) to create a total mean estimate of AAFB population size.
Because opportunistic sightings were not collected in a standardised way and transects were surveyed only once each, we were unable to provide a quantitative estimate of Såli abundance outside of AAFB. Therefore, we used our cumulative expertise and anecdotal repeat sightings to provide a semi-quantitative estimate for each region of Guam (northern, central, southern). We then summed this semi-quantitative estimate with the AAFB estimate to provide a rough island-wide estimate of abundance.
Results
Literature review
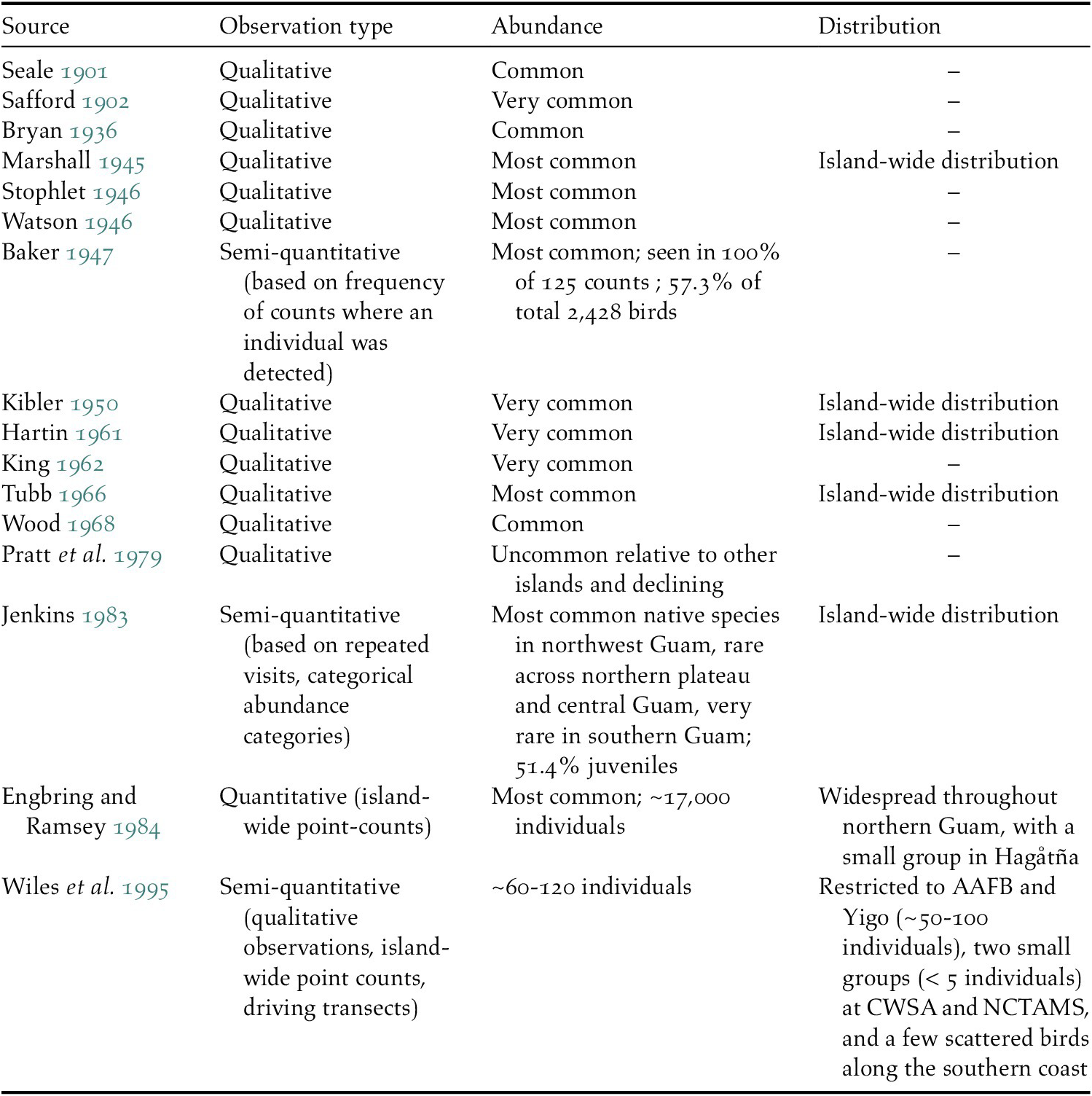
We found 16 papers in the literature from 1901 to 1995 that mentioned either the abundance or distribution of Såli on Guam (Table 1). Eleven of the 12 studies published prior to 1970 provided qualitative estimates only, describing Såli as ‘common’, ‘very common’, or the ‘most common’ bird species on the island. Baker (Reference Baker1947) detected Såli on 100% of his surveys (n = 125) and found that the species comprised nearly 60% of all birds counted. By 1978–1979, the species had become rare across southern Guam and was uncommon over most of northern and central Guam (Jenkins Reference Jenkins1983).
Table 1. List of 16 previous studies describing Såli population abundance and distribution on the island of Guam since 1900, including authors and year of publication and the type of observation (qualitative or quantitative). – indicates no information available.
The first quantitative estimate of Såli population size on Guam was made in 1981 by Engbring and Ramsey (Reference Engbring and Ramsey1984). Excluding the small Cocos Island population, they counted 1,667 individuals during island-wide point-counts and used distance-sampling accounting for imperfect detection to estimate an overall population size of 15,132–18,602 (mean estimate: 16,776 individuals). At this time, the range had contracted substantially relative to the study by Jenkins (Reference Jenkins1983) conducted only a few years prior in 1978–1979, and Såli were completely absent from southern and central Guam except for a small group of birds in the village of Hagåtña (Figure 2). Population size at and around AAFB’s airfield, administrative and housing areas, and adjoining plateau forest was estimated at only 231 individuals (Engbring and Ramsey Reference Engbring and Ramsey1984).

Figure 2. Såli distribution on the island of Guam during the last three population surveys. Panel a) indicates results from the 15 search areas surveyed in 1981 (Engbring and Ramsey Reference Engbring and Ramsey1984). Panel b) indicates results from the island-wide population assessment conducted between 1992-1994 (Wiles et al. Reference Wiles, Aguon, Davis and Grout1995; incidental sightings along southern coast not depicted). Panel c) indicates the current distribution on the island as derived from opportunistic sightings and the Andersen Air Force Base area search in 2018 (this study).
The overall population continued to decline as snakes reached higher densities across northern Guam, with almost no birds detected on any island-wide long-term survey routes after 1985 (Wiles et al. Reference Wiles, Bart, Beck and Aguon2003). By the early 1990s, Wiles et al. (Reference Wiles, Aguon, Davis and Grout1995) estimated an island-wide population (excluding Cocos Island) of only about 60–120 birds, including 50–100 Såli in the developed portion of AAFB and nearby areas of Mt. Santa Rosa and Gayinero, Yigo; two much smaller groups of birds numbering no more than five individuals each at the Conventional Weapons Storage Area (CWSA, now called ‘Munitions Storage Area’) on AAFB and at Naval Computer and Telecommunications Area Master Station (NCTAMS, now called ‘Naval Base Guam Telecommunications Site’) in Dededo; and a scattering of solitary birds along the southern coast.
Distribution and abundance at AAFB
In three successive week-long surveys of the AAFB Såli population, we counted 683, 609, and 844 birds, respectively (Table 2). We counted only 3–6 birds each week in the forests along the southern and eastern peripheries of the base (Figure S4), confirming that we were not omitting large numbers of Såli from our weekly counts. Birds were concentrated towards the centre of the base’s main developed area, with more Såli detected in interior search areas (n = 16 search areas) than in peripheral search areas adjacent to forest edge (n = 12 search areas; Table S2). The majority of birds were unbanded, with <5% banded birds counted each week (mean = 3.6%, range = 3.0–4.3%; Table 2). We registered 25 colour-banded individuals in week 1, 26 in week 2, and 24 in week 3, for a cumulative total of 42 unique individuals (Figure S5). Thirteen (46.4%) of the colour-banded birds detected in week 2 were unique resights, compared to only four (16.7%) in week 3. Extrapolation of the resight accumulation curve using the bootstrap metric estimated 50 unique colour-banded individuals on AAFB (mean ± SE: 49.9 ± 3.4 individuals; Figure S2), suggesting that our sampling approach was reasonably thorough (i.e. we detected 42/50 = 84% of projected banded birds present on AAFB).
Table 2. Summary statistics of weekly area searches conducted in September–October 2018 at Andersen Air Force Base (AAFB), Guam, including the number and percentage of colour-banded Såli detected each week, the number of birds detected per week in each age class, age ratio (i.e. % of overall total comprised by each age class), and total number of individuals counted per week.
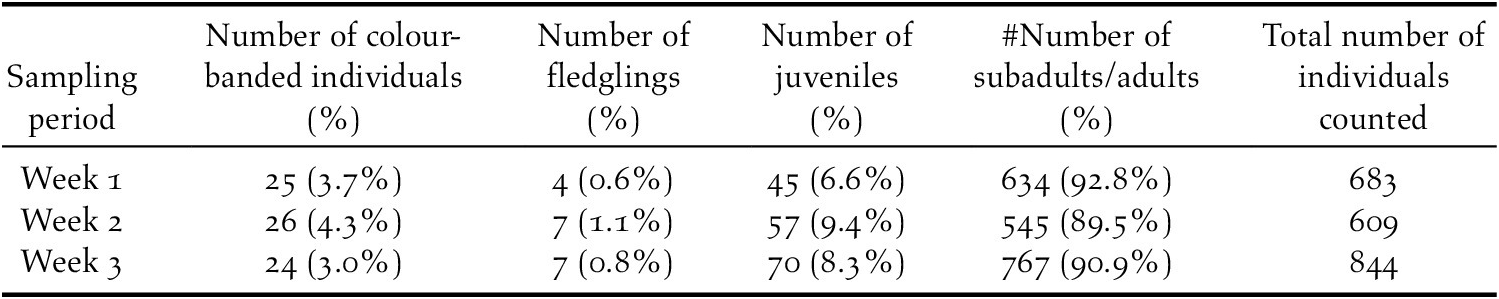
Our weekly estimates of the AAFB population size were as follows: 1,351 (range: 1,257–1,441) individuals for week 1; 1,160 (range: 1,081–1,240) individuals for week 2; and 1,663 (range: 1,550–1,777) individuals for week 3. By averaging the proportions of banded birds across weeks, our mean estimate of population size was 1,391 (range: 1,245–1,538) individuals. The age structure of the population was heavily skewed, with adults and subadults comprising a mean of 91.1% of the population across the three-week survey period (Table 2).
Distribution and abundance outside of AAFB
We compiled opportunistic sightings of Såli at 64 unique locations based on eBird data, Guam DAWR records, and MK’s records between 2005 and 2019 (Table S1). Sightings extended across nearly half of the island, but were largely concentrated in villages of northern and central Guam (excluding AAFB), with a few sightings in southernmost Guam (Figures 2, 3). Overall, we tallied 156 birds in 64 sightings in 12 of the island’s 19 villages, as follows: Tamuning-Tumon-Harmon (21), Hagåtña (11), Yigo (10), Dededo (6), Merizo (4), Santa Rita (3), Mongmong-Toto-Maite (2), Inarajan (2), Barrigada (2), Piti (1), Umatac (1), and Asan-Maina (1). Numbers of individuals per sighting averaged 2.4 ± 2.2 birds (range: 1–12 birds), with 69% (44/64) of sightings involving ≥2 birds. Observations occurred primarily in urbanised areas, including the island’s main business districts (particularly at large malls and shopping centres), residential areas, and city parks. Sightings were frequently made along main roads, streets, or trails, and none were more than 2 km from a built-up area or major arterial road. Birds were most often observed perched on power lines, power poles, buildings, and trees. Approximately 10 nest sites were recorded, all within lamp posts or power poles in Yigo, Hagåtña, Tamuning-Tumon-Harmon, and Dededo. No opportunistic sightings were recorded from the villages of Agana Heights, Chalan Pago-Ordot, Sinajana, Mangilao, Yona, Agat, and Talofofo. Regions without sightings included the developed east-central side of the island and nearly all of southern Guam (Figures 2, 3).

Figure 3. Satellite imagery of the island of Guam showing the locations of both SBC (white) and Såli (orange) transect surveys [panel a)] and the island’s 19 villages [panel b)]. Panel b) lists the villages where Såli were detected (pink polygons) or not (red polygons) during transect surveys.
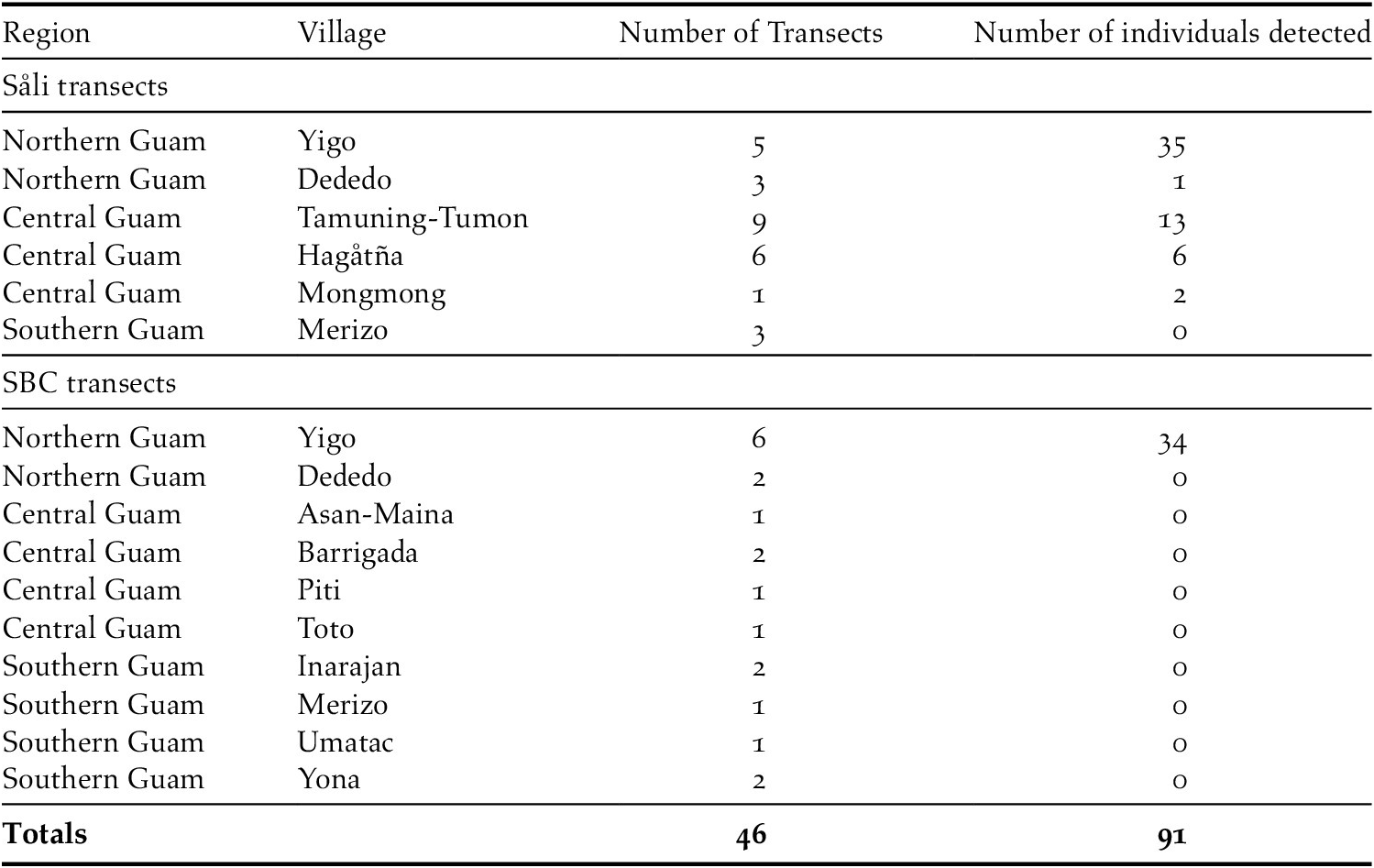
During the 19 SBC and 27 Såli transect surveys combined, we registered 91 unique Såli sightings on 20 of 46 (43%) transects surveyed (Figure 3, Table 3). On SBC transects, Såli were only present in Yigo and were not detected in any of the other eight villages. In contrast, on Såli transects, we detected Såli in five of six villages, except Merizo. All sightings occurred in northern and central Guam, with the highest concentration occurring in Yigo and along the periphery of AAFB (Figure 3). No Såli were detected in the southern villages of Merizo or Umatac (Table 3), despite the presence of the nearby Cocos Island population (~200 birds) and the opportunistic sightings noted above. We observed nesting behaviour (i.e. the presence of an active nest, birds transporting nesting material) and/or juvenile birds on four transects – two in Yigo (Figure S3) and one each in Hagåtña and Tamuning-Tumon-Harmon.
Table 3. Summary of Division of Aquatic and Wildlife Resources (DAWR) transect survey sampling effort in April–May 2018 (i.e. 27 newly established Såli transects and 19 spring bird count [SBC] historical transects) across the island of Guam. Included are the region and village where transects were located, number of transects per village, and the total number of Såli detected across all transects in each village.
Combining the opportunistic sightings and transect surveys, we estimated the population in urbanised areas of northern Guam (Yigo and Dededo) at 30–40 individuals, central Guam at 30–50 individuals (20–30 in Hagåtña, 10–20 in Tamuning-Tumon-Harmon), and up to 10 individuals scattered outside those areas. Thus, we estimated that 60–100 Såli were present outside of AAFB. Adding these 60–100 birds to the estimate for AAFB produces an overall island-wide population size estimate of 1,450–1,490 individuals (1,650–1,690 individuals if the population of ~200 Såli on Cocos Island is included).
Discussion
Using a combination of area searches, opportunistic sightings and transect surveys, we provide the first update on the distribution and abundance of Såli on Guam in more than 25 years. We found a 15-fold increase in population size (~100 vs. ~1,500 birds) since the last survey in the early 1990s (Wiles et al. Reference Wiles, Aguon, Davis and Grout1995), with an estimated 93–96% of the population concentrated in the main developed area of AAFB. Såli are also in the process of recolonising urbanised areas elsewhere in northern and central Guam, where the species has been absent since the expansion of brown tree snakes in the 1970s and early 1980s (Figure 2). As noted by Wiles et al. (Reference Wiles, Aguon, Davis and Grout1995), a few birds also continue to occur along the coast of southern Guam (Figure 2), but their status is unclear and most likely represent temporary residents originating from the small separate population on nearby Cocos Island or individuals regularly commuting from that island.
Outside of AAFB, Såli observations were largely limited to Guam’s main urban areas (Figure 2, Table 3), with most sightings taking place in four of the island’s most heavily developed and populated villages (Tamuning-Tumon-Harmon, Hagåtña, Yigo, and Dededo; Table 3, Table S1). In particular, Yigo’s proximity to AAFB likely accounts for its large number of sightings. Whether or not the birds in these areas form an established self-sustaining population is unknown. Most sightings in these areas involved pairs or small groups of birds, suggesting ample potential for breeding, yet we documented relatively few nests or juveniles. Additionally, birds were only reliably present at large shopping malls except in Hagåtña, and sightings from other locations may have been transient individuals, especially given the high mobility of the species. For these reasons, we cannot rule out the possibility that Såli presence in this region of the island remains strongly dependent on birds dispersing from the AAFB population, which likely functions as a source population for areas farther south.
Avoidance of brown treesnakes is critical to the survival of all birds on Guam (Savidge Reference Savidge1987, Wiles et al. Reference Wiles, Bart, Beck and Aguon2003). To that end, our findings suggest that two factors, ongoing snake control measures (reviewed in Vice Reference Vice2011, Clark et al. Reference Clark, Clark, Siers, Pitt, Beasley and Witmer2018, Engeman et al. Reference Engeman, Shiels and Clark2018) and the Såli’s adaptation to urban habitats (Wiles et al. Reference Wiles, Bart, Beck and Aguon2003), are likely responsible for the species’ partial population recovery on the island. AAFB’s main developed area, which covers about 17 km2, has been a focal point of snake interdiction efforts on Guam since 1993 (Vice Reference Vice2011), with thousands of individuals captured and removed annually (USDA APHIS Wildlife Services, pers. comm.). Control measures include mouse-baited traps installed on fencing and other structures along the base’s perimeter, airfield, and electrical infrastructure, and in the base’s cargo storage, administration, and housing areas; plastic tube bait stations containing dead mice implanted with acetaminophen placed on vegetation and structures along forest roads and the eastern forest edge; and nocturnal spotlight searches along fencing (Vice Reference Vice2011, Engeman et al. Reference Engeman, Shiels and Clark2018, USDA APHIS Wildlife Services pers. comm.). These efforts are likely reducing snake abundance in the main developed area of AAFB, especially in the centre, where we recorded the highest numbers of roosting and nesting Såli. Similar snake control efforts are also conducted at other military installations and port facilities on the island (Vice Reference Vice2011, Engeman et al. Reference Engeman, Shiels and Clark2018), but all of these operations except the one at the Guam International Airport cover considerably smaller geographic units and none have thus far enabled the establishment of resident Såli as on AAFB.
Developed areas on Guam appear to be serving as refugia from snake predation by providing safe roosting and nesting locations for birds, as hypothesised by Wiles et al. (Reference Wiles, Bart, Beck and Aguon2003). Indeed, brown treesnakes tend to avoid roads (Siers et al. Reference Siers, Savidge and Reed2014), highly lit areas (Campbell et al. Reference Campbell, Mackessy and Clarke2008), and open expanses such as grass lawns and parking lots, all of which typify developed areas. AAFB’s main developed area – the core area for Såli nesting and nighttime roosting (H. S. Pollock and H. S. Rogers pers. obs.) – is characterised by such habitat features including asphalt roads, runways, taxiways, and parking areas; expansive mowed lawns with isolated ornamental trees; and numerous buildings. Nesting sites on the base typically include solitary trees, building cavities, lamp posts and artificial nest boxes (Savidge et al. Reference Savidge, Kastner and Seibert2018).
A number of key differences exist between the main developed area of AAFB and Guam’s off-base urban areas, which likely explain the lower abundance of Såli outside of AAFB. In contrast to AAFB, off-base developed areas receive almost no intensive large-scale snake interdiction (the exception being at the Guam International Airport), contain scattered pockets of secondary vegetation and remnant forest that provide habitat for snakes, and possess far fewer areas of large, mowed lawns. Indeed, the limited available data indicate that snakes still occur in fairly high densities and that the largest-sized individuals tend to be found in developed areas, likely due to the increased availability of avian and mammalian prey (Siers et al. Reference Siers, Savidge and Reed2017, Wagner et al. Reference Wagner, Tappe, Jaramillo, Kastner, Van Ee, Savidge and Pollock2018). Nevertheless, Guam’s off-base urban areas possess other features absent from AAFB that inhibit snake presence and movement, and thus are probably beneficial to Såli. These include the presence of major roads with heavier traffic volumes, large parking lots with isolated trees (e.g., at large malls and commercial shopping centres), higher densities of larger buildings, and the presence of artificial nesting structures such as power poles.
Despite the growth and expansion of Guam’s Såli population since the early 1990s, several lines of evidence clearly indicate that current brown treesnake control measures (primarily intended to prevent off-island spread and damage to electrical infrastructure) and the island’s existing urban environment are insufficient to neutralise the continuing impacts of snakes on the population. First, snake capture rates along the perimeter of AAFB (USDA APHIS Wildlife Services pers. comm.) have remained relatively constant since the mid-1990s, rather than declining in number. While this is certainly causing a localised reduction in snake abundance (Siers et al. Reference Siers, Shiels, Payne, Chlarson, Clark and Mosher2019a), it has not translated to an overall population suppression. Second, recent studies of Såli at AAFB have found very low fledgling survival (~26%), primarily due to predation by brown treesnakes (56% of mortality) but also by feral or domestic cats (19% of mortality; Pollock et al. Reference Pollock, Savidge, Kastner, Seibert and Jones2019). Third, the age ratio of the Såli population has shifted drastically, from immatures forming an apparent majority of the population in the mid-1940s (Baker Reference Baker1951) and 51.4% of birds counted in late 1970s (n = 138 observations; Jenkins Reference Jenkins1983) to a ratio of only 8.9% juveniles in 2018 on AAFB. This shift is consistent with the low fledgling survival rate (Pollock et al. Reference Pollock, Savidge, Kastner, Seibert and Jones2019) and suggests exceedingly limited recruitment into the population. Fourth, Såli have failed to expand into a large, suburbanised area in east-central Guam, where few or no sightings have yet occurred. This area, composed of the villages of Barrigada, Mangilao, Chalan Pago-Ordot, and Yona, features high human populations, but less of the heavily urbanised setting found in Tamuning-Tumon-Harmon, Hagåtña, Yigo, and Dededo. Såli have also not yet recolonised the interior of southern Guam, which is largely covered in forest and grassland and has only minor development. Taken together, these factors suggest that brown treesnakes still pose a considerable threat to Guam’s remaining bird populations, including Såli. These findings support the generally shared presumption that there can be no island-wide recovery of native forest species without effective snake suppression on Guam. Application of novel control methods such as the automated aerial bait delivery system (Siers et al. Reference Siers, Shiels, Payne, Chlarson, Clark and Mosher2019a, Reference Siers, Pitt, Eisemann, Clark, Shiels, Clark, Gosnell, Messaros, Veitch, Clout, Martin, Russell and Westb, Reference Siers, Shiels and Barnhart2020), which deploys dead mice implanted with acetaminophen across the landscape, will likely be required to suppress the snake population to levels sufficient for making Guam habitable again for extirpated native birds. Our results provide a reference point for future studies of the Såli population and its expansion and inform conservation projects focused on reintroducing birds to Guam.
One possible additional factor aiding the population on AAFB has been the deployment of nest boxes for use by the cavity-nesting Såli. Nest boxes can boost reproductive success of cavity-nesting birds by increasing the availability of nest sites, providing adequate shelter from the elements and protection from predators. Small numbers were placed in the base’s main developed area in the late 1990s and early 2000s (D. Lujan, U.S. Navy Joint Region Marianas pers. comm.), and an expanded installation program of more than 50 predator-resistant boxes has been ongoing since 2015 (Savidge et al. Reference Savidge, Kastner and Seibert2018). These have improved the nesting success of Såli relative to that of unprotected nests and allowed over 800 nestlings to fledge successfully (J. Savidge and H. S. Rogers pers. obs.). The overall benefit to the population, however, has probably been marginal to date due to the high levels of snake and cat predation on fledglings (Wagner et al. Reference Wagner, Tappe, Jaramillo, Kastner, Van Ee, Savidge and Pollock2018, Pollock et al. Reference Pollock, Savidge, Kastner, Seibert and Jones2019). To date, given a population of ~1,250 breeding birds on AAFB and only 50–70 nest boxes, the majority of juveniles entering the population likely still originate from natural nests in cavities of buildings, power poles and trees (M. Kastner, personal observations).
The population size and distribution of Såli are crucial factors for ecosystem functioning on Guam. Såli have a very broad diet and are the only native frugivorous bird species remaining on the island (Pollock et al. Reference Pollock, Fricke, Rehm, Kastner, Suckow, Savidge and Rogers2020). An important consequence of their constrained distribution is the limitation of ecosystem services related to seed dispersal and consequent forest regeneration (Rehm et al. Reference Rehm, Balsat, Lemoine and Savidge2018, Reference Rehm, Fricke, Bender, Savidge and Rogers2019, Kastner et al. Reference Kastner, Pollock, Savidge, Fricke and Rogers2021), which are currently geographically restricted on Guam. Although the persistence of native wildlife in urban refugia may be beneficial from a species conservation perspective (Shaffer Reference Shaffer2018), significant range expansion into historical forest habitats is necessary to fulfil a broader vision of rewilding on Guam (Thierry and Rogers Reference Thierry and Rogers2020). Cohesive integration of technical advances in predator control (e.g. Siers et al. Reference Siers, Shiels, Payne, Chlarson, Clark and Mosher2019a, Reference Siers, Pitt, Eisemann, Clark, Shiels, Clark, Gosnell, Messaros, Veitch, Clout, Martin, Russell and Westb) with appropriate economic and social policy (Peltzer et al. Reference Peltzer, Bellingham, Dickie, Houliston, Hulme, Lyver, McGlone, Richardson and Wood2019) are necessary to achieve the successful implementation of species reintroduction on Guam.
Supplementary Materials
To view supplementary material for this article, please visit http://doi.org/10.1017/S0959270920000726.
Acknowledgements
We first and foremost thank Ovidio Jaramillo and Megan Pendred, who spent three consecutive weeks conducting area searches for Såli on AAFB. We also thank Julie Savidge, Chris Wagner, Bridget Strejc, and Carolin Tappe for their help in troubleshooting protocols during preliminary surveys in 2017; Rick Camp, Shane Siers and two anonymous reviewers for helpful comments that improved the manuscript; Aaron Collins for sharing data and insight into brown tree snake abundances on Guam; and Jim Watkins and Shermaine Garcia for assistance obtaining permits to conduct research on AAFB. This material is based upon work supported by the Strategic Environmental Research and Development Program and the US Army Corps of Engineers under Contract No. W912HQ16C0013 (Project RC-2441). This study was funded in part by the U.S. Fish and Wildlife Service through the Federal Aid in Sport Fish and Wildlife Restoration Program grant number F17AF00915. All research was approved under Guam Division of Aquatic and Wildlife Resources permit #RES-17-001 and Colorado State University Animal Care and Use Committee protocol #17-7176A. The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional guides on the care and use of animals. Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the U.S. Government.








