INTRODUCTION
For the past two decades, Salmonella enterica serovar Typhimurium definitive phage type (DT) 104 has become more common, especially in Western Europe and North America [Reference Helms, Ethelberg and Molbak1, Reference Glynn2]. Of human non-typhoidal salmonella isolates, 10–30% are usually found to belong to the serotype Typhimurium, and of these 30–50% are DT104 in many countries [Reference Helms, Ethelberg and Molbak1, Reference Glynn2]. The DT104 lineage is strongly associated with multidrug resistance (MDR) and its emergence has been attributed to antibiotic use in agriculture [Reference Helms, Ethelberg and Molbak1–Reference Talbot, Gagnon and Greenblatt5]. Possible sources of human infection with S. Typhimurium DT104 range from consumption of beef [Reference White4–Reference Isakbaeva8], dairy products [Reference Cody9, Reference Villar10], pork [Reference Helms, Ethelberg and Molbak1, Reference Molbak3], poultry [Reference Helms, Ethelberg and Molbak1] and mutton [Reference Helms, Ethelberg and Molbak1] to non-animal foodstuffs [Reference Andersson11, Reference Horby12]. Exposure to cattle and sandboxes has also been described as risk factors for DT104 infection [Reference Doorduyn13, Reference Wall14]. Salmonella infection can cause severe illness and although most infections are self-limiting, antimicrobial resistance can limit treatment alternatives for severe cases [Reference Glynn2, Reference Molbak3, Reference Helms15, Reference Varma16]. MDR salmonella has also been associated with higher frequencies of hospitalization and death than susceptible strains [Reference Helms15, Reference Varma16]. Thus, the emergence of MDR S. Typhimurium DT104 is of public health concern.
In The Netherlands, about 50 000 cases of non-typhoidal salmonellosis occur annually [Reference de Wit17, Reference de Wit18]. S. Typhimurium DT104 has come to comprise up to 15% of all salmonella infections and was especially prevalent in 2001 [Reference van Pelt19–Reference van Duijkeren21]. Our laboratory-based salmonella surveillance covers about 64% of the population and relies on regional public health laboratories sending about 2000 human salmonella isolates per year to the National Institute for Public Health and the Environment (RIVM) [Reference van Pelt19]. The isolates are routinely serotyped and phage-typed, the latter using a national collection of phages. Between 19 September and 28 November 2005, 169 isolates were found to be S. Typhimurium phage type 506 [Reference Notermans22, Reference Kivi23], which translates to DT104 in the English typing scheme [Reference van Duijkeren21]. This number of cases corresponded to a peak of about ten-fold more cases than expected for the same time of year over the past 5 years [Reference van Duijkeren21, Reference Widdowson24]. This large nationwide outbreak prompted an investigation to find the source and prevent further cases.
METHODS
Epidemiological investigation
This outbreak investigation used the Dutch laboratory-based salmonella surveillance at the RIVM as a source of S. Typhimurium DT104 cases and basic descriptive statistics with regard to gender, age and place of residence [Reference van Pelt19]. Trawling interviews with 19 recent DT104 cases performed in the beginning of November covered consumption not only of different meats, dairy products, fish, vegetables, snacks and condiments, but also of food from various establishments and contacts with animals. These pilot interviews suggested consumption of contaminated beef products as a source of infection. A case-control study was designed to test the main hypothesis that cases were more likely than controls to have eaten a particular beef product.
In the case-control study, cases were defined as residents of The Netherlands who had sought medical care for gastrointestinal symptoms, had not travelled abroad 3 days before onset of symptoms and whose DT104 isolates were included in the Dutch surveillance system between 19 September and 28 November 2005. Gastrointestinal symptoms were defined as diarrhoea (⩾3 loose stools in 24 h) or ⩾2 other symptoms of vomiting, nausea, blood in stool or abdominal pain. Additionally, for logistical reasons, the laboratory records of cases had to contain the addresses of the treating physician and the patient. Population controls were obtained from an already existing population register sample with about 7000 random residents of The Netherlands. The sample had been generated in 2002 to provide controls in another study [Reference Doorduyn13]. Controls were matched to the present cases by selecting the four closest available with regard to age, geographical locality and degree of urbanization, the latter defined by a standard five-level scale [Reference de Wit17]. Approval to approach the cases was obtained from the corresponding regional laboratories and treating physicians, while each participant gave written informed consent.
Data collection
In the beginning of December, study participants received self-administered postal questionnaires about personal and household characteristics, gastrointestinal symptoms and exposures. Exposures essentially included all main items from the trawling questionnaire, but detailed questions about specific products and place of purchase were largely restricted to beef products. Exposures referred to the 3 days before onset of illness for cases and to 3 typical days in October for controls. The predominant categorization of exposures was ‘yes’, ‘no’ and ‘don't know’. The questionnaire data were double-entered by different persons to reduce data entry errors (Epi-Info version 3.3.2; CDC, Atlanta, GA, USA).
Statistical analyses
Associations between infection and exposures were estimated by odds ratios (OR) and 95% confidence intervals (CI) obtained by conditional logistic regression (SAS version 9.1.3; SAS Institute Inc., Cary, NC, USA). Two approaches were used to accommodate the matching in the analysis. First, matching strata consisted of the strata of each individual case and the corresponding controls. We excluded poorly matched controls, defined as differing from the corresponding case by >10 years age, 100 km in geographical locality or three urbanization levels. Second, to increase the power of the analyses, individual matching strata were merged within three-level categories of the three matching variables (Table 1). Bivariate analyses preceded multivariate analyses, the latter considering gender and factors exhibiting bivariate positive associations with a P value ⩽0·20 as putative confounders.
Table 1. Descriptive characteristics of participants in the case-control study
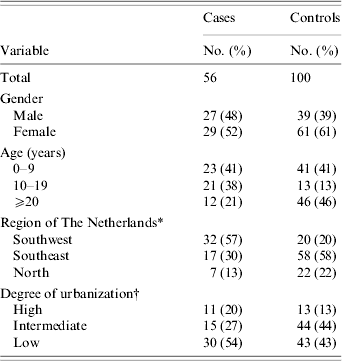
* Provinces categorized into Flevoland, Noord-Holland, Utrecht, Zeeland and Zuid-Holland (Southwest), Gelderland, Limburg and Noord-Brabant (Southeast) and Drenthe, Friesland, Groningen and Overijssel (North).
† Urbanization codes categorized into high (code 1 and 2), intermediate (code 3) and low (code 4 and 5) degree of urbanization [Reference de Wit17].
A sensitivity analysis excluded study participants who had been exposed to other persons with gastrointestinal complaints to take into account potential secondary transmission. Alternative case definitions excluded cases with early (1 October or before) or late (after 1 October) onset of symptoms, respectively, to identify potential changes in risk factors over time. Stratified analyses were performed by the categorized matching variables (Table 1) to assess effect measure modification.
Potential participation bias was assessed by comparing the distributions of age, gender, geographical locality and degree of urbanization in participants (Table 1) with the corresponding distributions in non-participants and overall. The comparisons were made separately in all 169 cases from the surveillance and all invited controls and differences between proportions were tested with two-sided Fisher's exact tests. To describe exposure patterns in the source population, the distribution of exposures in controls was similarly assessed using two-sided Fisher's exact tests.
Microbiological investigation
Upon detection of the outbreak, the molecular type of recent DT104 isolates was compared to historical and international DT104 isolates. Molecular typing techniques were pulsed-field gel electrophoresis (PFGE) [Reference Gatto25] and multiple-locus variable-number tandem repeats analysis (MLVA) [Reference Lindstedt26], the latter performed by the Norwegian Institute of Public Health. Standard antimicrobial susceptibility tests were performed using the microdilution test for 12 antimicrobials (amoxicillin, cefotaxime, imipenem, gentamicin, neomycin, tetracycline, sulphamethoxazole, trimethoprim, ciprofloxacin, nalidixic acid, chloramphenicol and florfenicol) [Reference Mevius, Pellicaan and van Pelt27]. Retrospectively, available DT104 isolates (195/243, 80%) in the laboratory-based salmonella surveillance during April–December 2005 were typed by PFGE to describe the emergence of outbreak cases and restrict epidemiological analyses to these.
Product tracing
Since 2005, the General Food Law of the European Union declares that food and feed should be possible to trace [28]. Serious food-related risks to human health should be notified to the Rapid Alert System for Food and Feed (RASFF) that informs the Member States, European Commission and European Food Safety Authority (EFSA) [28]. In The Netherlands, the Food and Consumer Product Safety Authority (VWA) is responsible for product tracing. At the time of investigation, traceability of meat was to be ensured by a commercial document accompanying the consignments, but this provision is no longer in force. All foodstuffs of animal origin must currently have an identification mark applied, specifying the country of origin and the approval number of the establishment of origin [29]. Furthermore, special labelling for beef should ensure, among other things, that there is a link between the food product and the animals concerned [30, 31]. Food business operators must have systems in place to identify from which establishments products have been received and to which other businesses products have been supplied [28]. A variety of establishments also voluntarily use uniquely numbered CMR (Convention Relative au Contrat de Transport International de Marchandises par la Route) documents to allow identification of merchandises during road transport.
Alerts about the present outbreak were distributed through Enter-Net, Eurosurveillance Weekly [Reference Kivi23], RASFF and a national medical journal [Reference Notermans22].
RESULTS
The 169 S. Typhimurium DT104 infections between 19 September and 28 November 2005 affected more cases aged 5–20 years (81/169, 48%) than in previous years (20–25% in 1996–2004). Males and females were equally represented. Cases were reported from throughout The Netherlands and there were no apparent clusters. As assessed in the case-control study, the onset of symptoms peaked during two consecutive weeks in September and October, about 2 weeks before the registration of cases in the laboratory-based surveillance peaked (Fig. 1).

Fig. 1. Distributions of all S. Typhimurium DT104 cases in the Dutch laboratory-based surveillance by week of registration (□) and of the 56 cases in the case-control study by week of onset of symptoms (■). The timings of key events that preceded the outbreak in The Netherlands are indicated.
In the case-control study, 62 out of 108 (57%) cases with available contact information of physician and patient contributed with questionnaire information. Compared to non-participating cases, those in the case-control study included more people aged 10–19 years (25/62, 40% and 21/107, 20%; P value 0·004), fewer aged ⩾20 years (14/62, 23% and 44/107, 41%; P value 0·02) and fewer from highly urbanized areas (12/62, 19% and 40/107, 37%; P value 0·02). None of these differences were statistically significant between participants and all 169 cases. Two cases who reported no symptoms were excluded from further analyses. Four additional cases were disregarded because they reported having been abroad during the time period for which exposures were assessed. Accordingly, subsequent analyses considered 56 cases (Table 1). Common symptoms among cases were diarrhoea (100%), stomach pain (96%), fatigue (89%), fever (86%), blood in stool (76%), nausea (70%) and vomiting (61%). Seventy-one per cent of cases reported having had gastrointestinal symptoms for a week or longer and 23% had visited a hospital in addition to the general practitioner, but no deaths were reported as a consequence of infection.
Of 411 controls, 110 (27%) completed the questionnaire. There was no evidence of participation bias among controls. Ten controls were excluded because they reported having been abroad during the time period for which exposures were assessed, leaving 100 controls for analysis (Table 1). In the first analysis approach, exclusion of 18 poorly matched controls improved the matching between cases and controls with regard to age (median difference 0 years, range 0–10 years), geographical distance (median difference 36 km, range 0–98 km) and degree of urbanization (median difference 1 level, range 0–3 levels).
The two conditional logistic regression approaches yielded comparable results, but the second approach with merged matching strata is presented here due to its higher precision (Table 2). Consumption of filet américain stood out as risk factor for infection in the bivariate analyses (OR 4·2, 95% CI 1·5–12·0). Filet américain, sometimes called steak tartare, contains raw minced beef as a main ingredient and in The Netherlands is typically eaten on bread. The cases had not purchased the product consistently from a single supermarket chain. Consuming food from a mobile caterer was also associated with infection (OR 4·9, 95% CI 1·1–22·1). More detailed information was not available for this exposure. None of the other variables exhibited positive associations with infection. Contrarily, several exposures showed negative associations with infection, occasionally reaching statistical significance (Table 2). The effects of consuming filet américain and food from a mobile caterer were not confounded by each other (OR 3·9, 95% CI 0·8–19·2 and OR 4·3, 95% CI 0·9–19·6, respectively) or by gender as assessed in multivariate models. Also, the results did not change after exclusion of participants (9/56 cases and 25/100 controls) who reported exposure to other persons with gastrointestinal complaints.
Table 2. Bivariate associations between food consumption and S. Typhimurium DT104 infection

n.a., Not applicable due to sparse data.
* Odds ratios (OR) and 95% confidence intervals (CI) were obtained by conditional logistic regression using matching strata defined by three-level categories of age, region of The Netherlands and degree of urbanization.
Stratified analyses by age, geographical location and degree of urbanization did not identify additional risk factors for infection. The strength of association between infection and consumption of filet américain appeared to decrease with increasing age (OR 6·3, 95% CI 1·7–23·6 in the 0–9 years age group and OR 1·0, 95% CI 0·1–10·1 in the ⩾20 years age group). Analyses restricted to 27 early cases suggested firmer associations with infection for consumption of both filet américain (OR 12·2, 95% CI 2·0–73·7) and food from a mobile caterer (OR 6·7, 95% CI 1·1–41·4), compared to analyses restricted to 29 late cases (OR 2·7, 95% CI 0·8–9·3 and OR 2·0, 95% CI 0·3–16·6, respectively). Among controls, filet américain appeared to have been consumed more frequently by older individuals (4/11, 36%, P value 0·24 for the 10–19 years age group and 19/37, 51%, P value 0·004 for the ⩾20 years age group, compared to 7/38, 18% in the 0–9 years age group).
The S. Typhimurium DT104 isolates from this outbreak yielded distinct PFGE and MLVA molecular types. The PFGE pattern differed from the typical DT104 type by a one-band difference and the MLVA type was 2-4-13-16-3. Of the antimicrobials tested, the outbreak strain was resistant to amoxicillin, chloramphenicol, sulphamethoxazole, tetracycline and florfenicol. The PFGE and MLVA types were indistinguishable from those observed in a DT104 outbreak in Denmark in August 2005 [Reference Ethelberg32]. That outbreak was caused by beef imported from Italy and served as carpaccio, which is a dish of raw, thin slices of beef with condiments.
The retrospective analysis of DT104 isolates from April–December 2005 did not identify the outbreak strain before late September, consistent with the time period in the case definition. For the 56 cases in the case-control study, 36 (64%) isolates were available for typing and four (11%) of them were not the outbreak-associated molecular type. These four cases did not report consumption of filet américain nor of food from a mobile caterer. The corresponding effect estimates were marginally influenced by restriction to the 32 cases that were verified as infected by DT104 of the outbreak-associated molecular type (OR 4·5, 95% CI 1·2–16·4 and OR 4·8, 95% CI 0·9–25·9, respectively).
Tracing of the incriminated Italian beef in The Netherlands was initiated in September 2005 after a RASFF alert about the DT104 outbreak in Denmark. RASFF informed the corresponding national authorities which approached the supplier. The supplier took action in its cutting plant and stated that several batches of beef, characterized by their production dates and cuts, were potentially cross-contaminated with S. Typhimurium. The batches were produced from mid-April to early May 2005 and had been distributed as deep frozen cuts to three companies in The Netherlands. Two of the companies were ship suppliers that had received two metric tonnes of beef and distributed it to dozens of ships of various types and nationalities (delivery dates unknown) (Fig. 2). This lead was not investigated further because there were no reports of gastroenteritis.

Fig. 2. Distribution routes uncovered by the product tracing, showing the shipment of incriminated beef from the European country of origin to The Netherlands and from there further internationally, May–November 2005. * Weight inconsistency of this recall is due to tare differences. † Weight inconsistency due to combination with other meat.
The third company in The Netherlands, a meat wholesaler with a wide distribution network, had received at least two separate deliveries of potentially contaminated beef. The investigation at the company was hampered by the circumstance that the RASFF information identified batches by production dates and cuts, while the company based its traceability on CMR documents. Consequently, the investigation paused until the original supplier provided the CMR numbers. This information was never received for two batches whose distribution remains elusive (809 kg, production date 3 May 2005, export date unknown) (Fig. 2).
However, one delivery of two batches could be traced in The Netherlands (985 kg rump cuts and 2006 kg topside, production date 12–14 April 2005, export to The Netherlands 11 May 2005) (Fig. 2). The first batch (985 kg rump cuts) was partly exported to a Member State (68 kg, export date unknown), while the remainder was combined with beef cuts from other sources and exported to another Member State (2584 kg, export date unknown). The second batch (2006 kg topside) was also combined with beef cuts from other sources and exported to a Member State together with the 68 kg from the first batch (3475 kg, export 3 June 2005). This exported beef was subsequently partly re-imported to The Netherlands (2607 kg, import 28 June 2005). Of this re-imported meat, a part was distributed to Denmark (1005 kg, import 23 July 2005), where it was implicated in the DT104 outbreak [Reference Ethelberg32]. Another part was sold to a Dutch butcher (21 kg, delivery date unknown), from where it was distributed as barbecue meat packages and to a restaurant. A remainder (7 kg) was available for sampling, which yielded DT104 of the outbreak-associated molecular type as determined by PFGE and MLVA. The rest of the re-imported incriminated meat was returned to the original cutting plant on a voluntary basis (1598 kg, inconsistent total weight is due to tare differences, export 2 November 2005).
DISCUSSION
This outbreak investigation pointed to imported contaminated beef as the source of S. Typhimurium DT104 infection. The combined approach of epidemiology, microbiology and product tracing yielded consistent results. The case-control study identified consumption of filet américain, a raw beef product, as a risk factor. Molecular typing of bacterial isolates from cases linked the outbreak with another beef-associated DT104 outbreak in Denmark [Reference Ethelberg32]. Part of the incriminated beef shipment, which had originated from a third European country, was traced in The Netherlands. Sampling of the beef yielded DT104 of the same molecular type as caused the outbreak.
The finding that raw beef caused this outbreak is consistent with carpaccio, another raw beef product, causing the outbreak in Denmark [Reference Ethelberg32]. Filet américain was also associated with the first national outbreak of Shiga toxin-producing Escherichia coli (STEC) O157 in The Netherlands during the same autumn of 2005 [Reference Doorduyn33]. In that investigation, cultures of 302 filet américain samples from one supermarket chain yielded three salmonella isolates, S. Meleagridis, S. Indiana and S. Typhimurium DT104. However, that DT104 strain was not related to the present outbreak as determined by MLVA. Human DT104 infection has repeatedly been linked to consumption of beef [Reference White4–Reference Isakbaeva8] and dairy products [Reference Cody9, Reference Villar10], in particular when the product was inadequately cooked.
Consumption of filet américain was more common in older controls, which may explain why cases in our outbreak had a different age distribution than expected from historical data. Furthermore, stratified analyses suggested that filet américain was particularly a risk factor in the 0–9 years age group, possibly reflecting greater susceptibility to salmonella infection in younger persons. Consuming food from a mobile caterer was also an independent risk factor for infection. This suggests an imperfectly cooked beef product sold by mobile food caterers or cross-contamination from raw beef offered at the same place. The importance of filet américain and food from mobile caterers increased when the analysis was restricted to early cases, indicating that these risk factors were more relevant during the early phase of the outbreak. However, small numbers suggest a need for caution in interpreting the subanalyses. That all cases were not explained by the two identified risk factors may partly be attributed to recall difficulties. The contaminated beef shipment may also have been prepared as several products with different consumption patterns and our study may not have had the power to identify all products that caused the outbreak. Moreover, we detected participation bias in our sample of cases, which suggests a possibility for additional unrecognized factors in cases that were less well represented in this study.
No additional exposures showed positive associations with infection, although some negative associations were identified, which can have several explanations. Information bias may result from controls reporting consumption of certain food items more frequently than cases. This may be because controls reported on any three typical days in October rather than on the three specific days before onset of illness that was asked of cases. Accordingly, better recollection of food consumption among controls is supported by the ‘don't know’ category tending to be associated with infection for several exposures (Table 2). Participation bias could also have played a role by selecting for controls who were more likely to report consumption of certain food items. However, we were not able to identify any participation bias among controls. Moreover, it is possible that frequent exposure to contaminated foods, for example meats, may have induced partial immunity to salmonella infection [Reference Doorduyn13]. Because of the probable spurious nature of the negative associations we refrained from adjusting for the corresponding data in the final multivariate models.
Population controls were identified through a previously generated population register sample. This enabled us to identify controls more rapidly and more cheaply than if a new sample had been requested. This approach has limitations when there is a long period between generation of the list and its application to identify controls. Notably, people may have relocated and stringent matching with regard to age may become impossible for the youngest cases. Nevertheless, these possible limitations were deemed to have marginal influence in this study setting.
The emergence of DT104 can largely be attributed to a clonal lineage, which can limit the discriminatory ability of molecular typing [Reference Gatto25, Reference Lindstedt26]. In our investigation, the atypical DT104 PFGE pattern made the discrimination of the outbreak isolates easier. The PFGE results were corroborated by MLVA which may prove to be a more discriminatory alternative to typing for DT104 [Reference Lindstedt26]. We were not able to consider molecular typing data in the case definition because many isolates were missing. Extrapolation of available PFGE data indicates that a minor proportion of cases (11%) was infected with DT104 of a molecular type not typical of the outbreak. The four such cases identified did not report consumption of filet américain nor of food from a mobile caterer. Accordingly, misclassification of cases as outbreak cases should make our effect estimates more conservative than if we had been able to exclude non-outbreak cases using the typing results.
Notwithstanding the complexity of the product tracing, the outbreak strain was isolated from a small sample of the incriminated beef shipment sold to a butcher in The Netherlands. However, it is likely that the bulk of untraceable beef (809 kg) caused most of the DT104 cases in The Netherlands although some additional unknown shipments could also have contributed. The first documented detection of contamination of the beef was in Denmark upon importation and that consignment was discarded [Reference Ethelberg32]. Testing for salmonella to prevent importation of contaminated food products is performed on a larger scale in Sweden and Finland, which have low rates of domestically acquired salmonella infection [29]. In The Netherlands, however, contamination of domestically produced meat is fairly common and no systematic testing is performed upon importation [Reference van Kreijl, Knaap and van Raaij34]. In light of the international distribution of the beef, the possibility that cases occurred outside The Netherlands and Denmark cannot be excluded. This outbreak shows the importance of multi-country collaboration when responding to outbreaks caused by products distributed internationally.
Could the outbreak in The Netherlands have been averted by the recognition of the outbreak in Denmark? Against this notion is the complexity of the product tracing which stretched the investigation into weeks, thus rendering a rapid intervention as a response to the RASFF alert impossible. The traceability of food products has been under new European regulations since 2006 [29]. When fully implemented, these should facilitate product tracing and timely intervention into distribution chains. However, even if the product tracing in this outbreak had been more straightforward, there were no legal means to prevent the beef from reaching the market. This is because Dutch legislation does not apply to products that are generally unsuitable for consumption without prior heating, as for meat [35].
There are efforts to reduce the occurrence of salmonella and other zoonotic agents in agriculture [Reference Talbot, Gagnon and Greenblatt5, 36], with some positive results [Reference Naugle37]. As of 2006, the new European regulation on microbiological criteria for foodstuffs strives to reduce the occurrence of salmonella and other pathogens [38] and a ban of non-therapeutic use of antibiotics in animals is in force to reduce the emergence of antibiotic resistance [39]. The former regulation requires slaughterhouses to introduce process hygiene criteria to randomly monitor the presence of salmonella on carcasses [38]. When salmonella is detected, corrective measures should be taken. However, the corrective measures are not specified other than that they must comprise improvements in slaughter hygiene and a review of process controls and origin of animals. Furthermore, the testing does not have to be real-time, so contaminated meat may be distributed before test results become available. Systematic real-time testing, although cumbersome to implement, could identify contaminated batches that should be either decontaminated or discarded. National and international regulatory and supervisory functions should be developed to attain more stringent control of foodborne infections. Optimally, suppliers should be able to guarantee that their products are free from pathogens of public health concern, especially if the meat may be consumed raw. The prerequisites for implementing such guarantees should be explored from political, food industry and consumer perspectives.
In conclusion, this investigation supports our working hypothesis by showing that consumption of raw imported beef was the primary cause of the large outbreak of S. Typhimurium DT104 in The Netherlands in September–November 2005. This finding points to the need for sustained public information about the risk of infection associated with raw meat products, including advice in safe food handling practices to prevent cross-contamination and to eliminate potential pathogens by proper cooking. Vulnerable groups such as the very young, elderly or immunocompromised should be discouraged from eating products that include microbiologically unsafe constituents, such as raw meat and unpasteurized milk. Furthermore, we recommend that consumers are informed about the presence of such constituents in pre-processed food items and the associated risk of disease. In the event of increasing trends and outbreaks of foodborne pathogens, the potential for international spread should be considered. Testing and traceability of food products in conjunction with international collaboration could facilitate rapid intervention in the distribution chains. Development of the associated national and international regulations and procedures should promote better utilization of these comprehensive means for prevention of human disease caused by foodborne pathogens.
ACKNOWLEDGEMENTS
We thank the physicians and microbiologists, whose collaboration made this investigation possible, Carolien de Jager and Anneke Westerhof for contributions to the epidemiological investigation, Henny Maas and Anjo Verbruggen for serotyping, Frans Bensink for phage typing, Dik Mevius for antimicrobial resistance testing, Enne de Boer and Teunis van Hoogdalem for contributions to the product tracing and Bjørn-Arne Lindstedt for MLVA analyses.
DECLARATION OF INTEREST
None.






