A stable and balanced gut microbiota is important for overall gastrointestinal health(Reference Simpson, Dogan and Rishniw1–Reference Suchodolski, Xenoulis and Paddock3). Indices of gastrointestinal health include fermentative end-product concentrations, faecal scores and gut microbial populations. The composition and activity of gut microbial populations can be manipulated by one's diet. As defined, prebiotics are non-digestible food ingredients that are (1) resistant to gastric acidity, hydrolysis by mammalian enzymes and gastrointestinal absorption; (2) fermented by intestinal microbiota; and (3) able to selectively stimulate the growth and/or activity of those intestinal bacteria that contribute to the host's health and well-being(Reference Gibson and Roberfroid4–Reference Gibson, Scott and Rastall7). Currently, there are three established classes of prebiotics (e.g. fructans, galacto-oligosaccharides and lactulose), but others may exist.
Prebiotics are widely used in human and pet nutrition products because of their many functional and nutritional properties. Fructans, for example, serve as a highly fermentable substrate in the hindgut, leading to decreased faecal pH, increased faecal SCFA concentrations, and increased Bifidobacterium spp. and Lactobacilli spp. in healthy adult dogs(Reference Willard, Simpson and Cohen8–Reference Swanson, Grieshop and Flickinger11). The majority of prebiotic research in dogs has focused on fructans, galacto-oligosaccharides and lactulose. More research, however, is needed to test the potential of other carbohydrate sources with prebiotic potential for use in pet nutrition.
Polydextrose is a polysaccharide synthesised by random polymerisation of glucose, sorbitol and a suitable acid catalyst at a high temperature and partial vacuum. It is composed of many glucosidic bonds, but the 1,6-glycosidic bond is predominant in this polymer(Reference Allingham12). Polydextrose has an average degree of polymerisation of 12 and an average molecular weight of 2000, ranging anywhere from 162 to 20 000(Reference Craig, McCleary and Prosky13). It is a water-soluble, low-energy bulking agent that is currently used in a variety of foods, including baked goods, functional beverages and diabetic products(Reference Jie, Bang-yao and Ming-jie14–Reference Mäkivuokko, Kettunen and Saarinen17). Because polydextrose is a randomly bonded polysaccharide, it is resistant to mammalian enzymes, which allows for most of the substrate to pass through the body unabsorbed(Reference Figdor and Bianchine18). Previous human research has shown polydextrose to be partially fermented in the large intestine, leading to increased faecal bulk, softening of the faeces, decreased faecal pH, increased faecal SCFA concentrations, increased faecal Lactobacillus and Bifidobacterium and decreased faecal Bacteroides (Reference Jie, Bang-yao and Ming-jie14). This study, which used traditional culture methods, is the only in vivo evidence that polydextrose possesses prebiotic activity in humans. Moreover, the prebiotic potential of polydextrose has not yet been tested for use in pet food.
The purpose of the present study was to evaluate the effects of graded concentrations of polydextrose on faecal characteristics, microbial populations and fermentative end products in healthy adult dogs. The ultimate aim of the present study was to test whether polydextrose has prebiotic potential in dogs when fed at doses that are practical in terms of cost and gastrointestinal tolerance. Increased inclusion of polydextrose was hypothesised to decrease faecal pH, increase faecal SCFA concentrations, and decrease faecal phenol and indole concentrations. Based on the human literature, the inclusion of polydextrose was also hypothesised to alter the gut microbial populations by increasing Lactobacillus spp. and Bifidobacterium spp., and decreasing Clostridium perfringens and Escherichia coli.
Experimental methods
Animals and diets
All animal care and study procedures were approved by the University of Illinois Institutional Animal Care and Use Committee before animal experimentation. A total of eight healthy adult intact female hound-mix dogs (3·5 (sem 0·5) years; 20 (sem 0·5) kg) were used. Dogs were housed individually in runs (2·4 × 1·2 m) in a temperature-controlled room (22°C; 23 % relative humidity) with a 16 h light–8 h dark cycle. Dogs were weighed and assessed for body condition score (nine-point scale) before the morning feeding on every Friday of the study.
A total of four diets were formulated to contain approximately 30 % protein and 20 % fat, with low-ash poultry by-product meal, brewer's rice, poultry fat and maize constituting the main ingredients of the dry, extruded kibble diets (Table 1). The diets were formulated to meet or exceed the National Research Council's(19) recommended allowances for adult dogs at maintenance. The diets were extruded at Kansas State University's Bioprocessing and Industrial Value-Added Program facility (Manhattan, KS, USA) under the supervision of Pet Food and Ingredient Technology, Inc. (Topeka, KS, USA). Each diet contained a specified concentration of polydextrose (0, 0·5, 1·0 or 1·5 %, Sta-Lite® Polydextrose; Tate and Lyle, Decatur, IL, USA), in place of cellulose (Solka-Floc; International Fiber Corporation, North Tonawanda, NY, USA). All polydextrose concentrations were incorporated into the diets before extrusion. All dogs were fed to maintain body weight throughout the duration of the study. Fresh water was offered ad libitum.
Table 1 Ingredient and chemical composition of canine diets containing varying levels of polydextrose

CP, crude protein; GE, gross energy; MEAAFCO, metabolisable energy by American Association of Feed Control Officials; MEC, metabolisable energy calculated.
* Provided per kg of diet: vitamin A, 5·28 mg; vitamin D3, 0·04 mg; vitamin E, 120 mg; vitamin K, 0·88 mg; thiamin, 4·40 mg; riboflavin, 5·72 mg; pantothenic acid, 22·00 mg; niacin, 39·60 mg; pyridoxine, 3·52 mg; biotin, 0·13 mg; folic acid, 0·44 mg; vitamin B12, 0·11 mg.
† Provided per kg of diet: Mn (as MnSO4), 66·00 mg; Fe (as FeSO4), 120 mg; Cu (as CuSO4), 18 mg; Co (as CoSO4), 1·20 mg; Zn (as ZnSO4), 240 mg; I (as KI), 1·8 mg; Se (as Na2SeO3), 0·24 mg.
‡ MEAAFCO = 35·564 kJ ME/g fat+14·644 kJ ME/g CP+14·644 kJ ME/g N-free extract.
§ MEC = (GE intake (kJ/d) − faecal GE (kJ/d) − ((CP intake/100) − (faecal CP/100)) × 1·25)/DM intake (g/d).
Sample collection
A replicated 4 × 4 Latin square design with 14 d periods was conducted. Each period consisted of a diet adaptation phase (days 0–10) and a total and fresh faecal collection phase (days 11–14). Total faeces excreted during the collection phase of each period were taken from the pen floor, weighed and frozen at − 20°C until further analyses. All faecal samples during the collection phase were subjected to a consistency score according to the following scale: 1 = hard, dry pellets, and small hard mass; 2 = hard, formed, dry stool, and remains firm and soft; 3 = soft, formed and moist stool, and retains shape; 4 = soft, unformed stool, and assumes the shape of the container; and 5 = watery, liquid that can be poured.
For each period, one fresh faecal sample was collected within 15 min of defecation on day 1 of the 4 d collection phase. Fresh faecal samples were prepared immediately to minimise the loss of volatile components. The samples were weighed and pH determined using a Denver Instrument AP10 pH meter (Denver Instrument, Bohemia, NY, USA) equipped with a Beckman electrode (Beckman Instruments, Inc., Fullerton, CA, USA). Fresh faecal DM was determined. For the analysis of phenols and indoles, aliquots were frozen at − 20°C immediately after collection. An aliquot (2 g) of faeces was mixed with 5 ml of 2 m-HCl for the determination of NH3, SCFA and branched-chain fatty acids and stored at − 20°C until analysis. Aliquots of fresh faeces were transferred to sterile cryogenic vials (Nalgene, Rochester, NY, USA) and frozen at − 80°C until DNA extraction for microbial analysis.
Chemical analyses
Diet samples were subsampled and ground through a 2 mm screen in a Wiley Mill (model 4; Thomas Scientific, Swedesboro, NJ, USA). Composited faecal samples (one per dog per period) were dried at 55°C for 1 week and ground through a 2 mm screen in the Wiley Mill. Diet and faecal samples were analysed for DM (105°C), organic matter and ash according to procedures by the Association of Official Analytical Chemists(20). Diet and faecal crude protein (CP) was calculated from Leco total N values (model FP-2000; Leco Corporation, St Joseph, MI, USA)(20). The total lipid content (acid-hydrolysed fat) of the diets and faeces was determined according to the methods of the American Association of Cereal Chemists(21) and Budde(Reference Budde22). Gross energy of the diet and faecal samples was measured using an oxygen bomb calorimeter (model 1261; Parr Instruments, Moline, IL, USA). Dietary fibre concentrations (total dietary fibre) were determined according to Prosky et al. (Reference Prosky, Asp and Furda23).
SCFA and branched-chain fatty acid concentrations were determined by GC according to Erwin et al. (Reference Erwin, March and Emery24) using a Hewlett-Packard 5890A series II gas chromatograph (Hewlett-Packard, Palo Alto, CA, USA) and a glass column (180 cm × 4 mm inner diameter) packed with 10 % SP-1200/1 % H3PO4 on 80/100+ mesh Chromosorb WAW (Supelco, Inc., Bellefonte, PA, USA). Phenol and indole concentrations were determined using GC according to the methods of Flickinger et al. (Reference Flickinger, Schreijen and Patil9). NH3 concentrations were determined according to the method of Chaney & Marbach(Reference Chaney and Marbach25).
Microbial analyses
Faecal microbial populations were analysed using methods described by Middelbos et al. (Reference Middelbos, Godoy and Fastinger26) with minor adaptations. Briefly, faecal DNA was extracted from freshly collected samples that had been stored at − 80°C until analysis, using the repeated bead beater method described by Yu & Morrison(Reference Yu and Morrison27) with a DNA extraction kit (QIAamp DNA Stool Mini Kit; Qiagen, Valencia, CA, USA), according to the manufacturer's instructions. Extracted DNA was quantified using a spectrophotometer (NanoDrop ND-1000; Nano-Drop Technologies, Wilmington, DE, USA). Quantitative PCR was performed using specific primers for Bifidobacterium spp.(Reference Matsuki, Watanabe and Fujimoto28), Lactobacillus spp.(Reference Collier, Smiricky-Tjardes and Albin29), E. coli (Reference Malinen, Kassinen and Rinttila30) and C. perfringens (Reference Wang, Cao and Franklin31). While Bifidobacterium and Lactobacillus are generally considered to be ‘beneficial’ microbes, E. coli and C. perfringens represent potential pathogens, and are commonly measured in prebiotic studies. Amplification was performed according to DePlancke et al. (Reference DePlancke, Vidal and Ganessunker32). Briefly, a 10 μl final volume contained 5 μl of 2 × SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA, USA), 15 pmol of the forward and reverse primers for the bacterium of interest, and 10 ng of the extracted faecal DNA. Standard curves were obtained by harvesting pure cultures of the bacterium of interest in the log growth phase in triplicate, followed by serial dilution. Bacterial DNA was extracted from each dilution using a DNA extraction kit (Qiagen) and amplified with the faecal DNA to create triplicate standard curves (ABI PRISM 7900HT Sequence Detection System; Applied Biosystems). Colony-forming units in each dilution were determined by plating on specific agars; lactobacilli MRS (Difco; Becton Dickinson & Company, Franklin Lakes, NJ, USA) for Lactobacillus, reinforced clostridial medium for Bifidobacterium and C. perfringens, and Luria Bertani medium for E. coli. The calculated log colony-forming units per ml of each serial dilution was plotted against the cycle threshold to create a linear equation to calculate colony-forming units per g of dry faeces. Although the standard curves are meant to represent a group of bacteria, our quantitative PCR assays were based on a single bacterial strain within each group. Because operon copy number is different among strains, a potential bias in our assay exists. Because our design used each dog as its own control, however, dietary effects that are truly occurring should be identified using these assays.
Calculations and statistical analysis
Apparent total tract macronutrient digestibility values were calculated using the following equation: nutrient intake (g DM/d) − nutrient output (g DM/d)/nutrient intake (g DM/d) × 100. Data were analysed using the MIXED procedure of SAS (version 9.2; SAS Institute, Inc., Cary, NC, USA). Faecal score data were compared using the GLIMMIX procedure of SAS. The statistical model included period and dog as random effects, whereas treatment was a fixed effect. Data were analysed using the type 3 test of the MIXED procedure. All treatment least squares means were compared using pre-planned contrasts that tested for linear and quadratic effects of polydextrose supplementation. Means were separated using a protected least squares difference with a Tukey adjustment. Outlier data were removed from analysis after analysing data using the UNIVARIATE procedure to produce a normal probability plot based on residual data and visual inspection of the raw data. Outlier data were defined as data points 3 or more standard deviations from the mean. A probability of P < 0·05 was accepted as being statistically significant and P ≤ 0·10 accepted as a trend.
Results
Dietary ingredient and chemical composition data are presented in Table 1. DM, organic matter, CP, acid-hydrolysed fat and gross energy concentrations were consistent among the diets. Total dietary fibre content decreased with increasing polydextrose concentrations because polydextrose is soluble and not detected by the total dietary fibre assay.
Nutrient intakes, apparent total tract macronutrient digestibilities and faecal characteristics are presented in Table 2. Food refusals were minimal. Dogs consumed 0·0, 1·3, 2·7 and 3·9 g polydextrose/d for the 0·0, 0·5, 1·0 and 1·5 % polydextrose treatments, respectively. All data were reflective of the 4 d collection period. Polydextrose did not alter food intake, faecal output, or apparent total tract DM and organic matter digestibility. Apparent total tract CP digestibility, however, tended to decrease (P < 0·10) linearly with increasing dietary polydextrose concentrations. There was a trend for a linear decrease (P < 0·10) in fresh faecal DM percentage and increased (P < 0·05) faecal scores (looser stools) with increasing dietary concentrations of polydextrose. However, no diarrhoea was observed.
Table 2 Food intake, faecal characteristics and apparent total tract macronutrient digestibility of adult dogs fed diets containing polydextrose
(Mean values with their standard errors)

CP, crude protein; OM, organic matter; AHF, acid-hydrolysed fat.
* Faecal score scale: 1 = hard, dry pellets; 2 = dry, well-formed stool; 3 = soft, moist, formed stool; 4 = soft, unformed stool; 5 = watery, liquid that can be poured.
Faecal pH, NH3, SCFA, branched-chain fatty acid, phenol and indole concentrations are presented in Table 3. Faecal pH decreased (P < 0·05) linearly with increasing polydextrose. Faecal acetate, propionate and total SCFA concentrations increased (P < 0·05) linearly with increasing dietary polydextrose. Polydextrose had a curvilinear effect on faecal indole concentrations, in which concentrations tended to decrease (P < 0·10) linearly with increasing polydextrose. Polydextrose had a quadratic effect (P = 0·05) on faecal isobutyrate concentrations, in which concentrations increased with 0·5 and 1·0 %, but decreased with 1·5 % in comparison with the control. However, other faecal protein catabolites were not changed.
Table 3 Faecal pH, ammonia, SCFA, branched-chain fatty acid (BCFA), phenol and indole concentrations of adult dogs fed diets containing polydextrose
(Mean values with their standard errors)

Faecal microbial concentrations are presented in Table 4. Faecal C. perfringens decreased (P < 0·05) linearly with increasing dietary polydextrose concentrations, but E. coli, Lactobacillus spp. and Bifidobacterium spp. were not affected by the inclusion of polydextrose in the diet.
Table 4 Faecal microbial populations of adult dogs fed diets containing polydextrose
(Mean values with their standard errors)
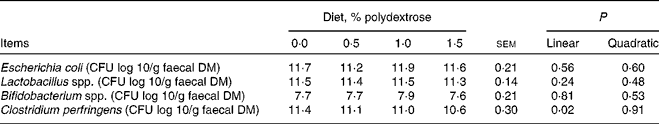
CFU, colony-forming units.
Discussion
The consumption of prebiotics has been shown to improve gastrointestinal health by selectively stimulating the growth and activity of ‘beneficial’ intestinal bacteria, decreasing faecal pH, increasing faecal bulk and relieving constipation(Reference Mussato and Mancilha33, Reference Roberfroid34). Previously, polydextrose has been studied in vitro, in human subjects, and in dogs used as a model for humans and shown to possess prebiotic properties. Probert et al. (Reference Probert, Apajalahti and Rautonen15) evaluated the prebiotic potential of adding polydextrose at 1 % (w/v) and 2 % (w/v) of an anaerobic medium using human faecal inoculum. In that study, six fermentations were carried out including polydextrose (Litesse Ultra, a high-grade form of polydextrose; Danisco, Terre Haute, IN, USA), lactitol monohydrate, lactitol monohydrate:polydextrose (50:50), short-chain fructo-oligosaccharides, polydextrose (using a pooled inoculum) and oligofructose (using a pooled inoculum) for a period of 48 h. Those researchers concluded that SCFA concentrations, namely acetate and butyrate, were increased with the addition of polydextrose. Those researchers also concluded that the addition of polydextrose led to increased bifidobacteria during all four fermentations. In another in vitro study, Mäkivuokko et al. (Reference Mäkivuokko, Kettunen and Saarinen17) examined the effects of adding polydextrose to dark chocolate using two in vitro simulation techniques, including gastric and small-intestinal digestion simulation, adapted from Fuller(Reference Fuller35), and an automated four-stage colon simulator(Reference Mäkivuokko, Nurmi and Nurminen36). Those researchers concluded that SCFA production was highest for acetate, butyrate, propionate and total SCFA in each vessel of the cocoa mass +2 % polydextrose v. baseline and no added polydextrose. Those researchers also concluded that the addition of polydextrose to cocoa mass digestion increased in vitro SCFA concentrations in the colon simulations v. fermented cocoa mass digestion without polydextrose. The in vitro studies displayed the prebiotic potential of polydextrose, with increased SCFA concentrations and increased numbers of beneficial bacteria(Reference Probert, Apajalahti and Rautonen15, Reference Mäkivuokko, Kettunen and Saarinen17).
Jie et al. (Reference Jie, Bang-yao and Ming-jie14) evaluated the effects of feeding 0, 4, 8 or 12 g polydextrose/d to healthy adult human subjects. That study consisted of a 28 d feeding phase and fresh faecal collection (within 1 h of defecation) on days 1 and 28. Those researchers concluded that dietary intake of polydextrose increased the ease of defecation (scale of − 3 to 3; 0 g polydextrose/d: − 0·21 to 0·41; 4 g polydextrose/d: − 0·18 to 1·36; 8 g polydextrose/d: 0·20 to 1·88; 12 g polydextrose/d: − 0·14 to 2·35) and faecal output (as-is g/d; 0 g polydextrose/d: 103 to 106; 4 g polydextrose/d: 106 to 115; 8 g polydextrose/d: 101 to 128; 12 g polydextrose/d: 98 to 142), probably due to its water-holding capacity. Subjects also reported softer stools and improved ease of defecation after a couple days of ingestion. Those researchers reported that faecal pH was decreased in subjects consuming 8 or 12 g polydextrose/d (6·71 and 6·37, respectively) v. control subjects (7·04). Also, in subjects consuming 8 or 12 g polydextrose/d, faecal butyrate (8 g/d: 1·31 mg/g; 12 g/d: 1·41 mg/g) and acetate (8 g/d: 4·70 mg/g; 12 g/d: 5·12 mg/g) were increased compared with the control subjects (0·94 and 4·12 mg/g, respectively). That human study displayed the prebiotic potential of polydextrose, with decreased faecal pH, increased SCFA concentrations, decreased concentrations of carcinogenic metabolites and increased numbers of beneficial bacteria in the faeces following consumption(Reference Jie, Bang-yao and Ming-jie14).
The prebiotic potential of polydextrose has not been well studied in pets, but Knapp et al. (Reference Knapp, Parsons and Swanson37) used the dog as a model for humans to test tolerance and glycaemic/insulinaemic responses of polydextrose. In that study, dogs were fed extruded diets containing 0, 14 or 28 g polydextrose/4184 kJ. Those diets were formulated so that dogs consumed test carbohydrates at 0, 100 or 200 %, the adequate intake of dietary fibre for humans(38). In that study, dogs consumed between 19 and 39 g polydextrose/d. Those researchers reported that a portion of the non-digested polydextrose was highly fermentable and that faecal scores increased as intake of polydextrose increased (scale of 1–5, where 1 = dry, hard pellets and 5 = watery, liquid that can be poured; control: 2·9/5, 100 % adequate intake: 4·2/5, 200 % adequate intake: 4·6/5). Because the dogs in that study were fed to imitate what adult humans should consume, it is not surprising that the high dosage of polydextrose led to increased faecal score. The results of the Knapp et al. (Reference Knapp, Parsons and Swanson37) study were used to establish the polydextrose inclusion levels in the present study that were expected to avoid tolerance problems, yet were practical from a commercial standpoint. In the present study, dogs were consuming about 3·5 g polydextrose/d for the diet with the highest concentration of polydextrose (1·5 %). To maintain a desirable faecal score quality, the present results suggest that polydextrose should not exceed 1·5 % in canine diets.
The results of the present study demonstrate that polydextrose beneficially alters faecal pH and fermentative end products, with little effect on food intake, nutrient digestibility and faecal microbiota, at dietary concentrations up to 1·5 %. Apparent total tract macronutrient digestibility values for all diets were consistent with what is expected with extruded diets in which high-quality ingredients are used. Apparent total tract CP digestibility tended to decrease as the concentration of polydextrose increased in the diet. This response is common in diets containing fermentable fibres and was probably due to the increased fermentable substrate and formation of bacterial biomass when compared with the control diet (0 % polydextrose), which has been observed previously(Reference Middelbos, Fastinger and Fahey39). In addition to the potential for decreased CP digestibility, increasing the concentration of dietary fibre may lead to a decrease in faecal quality (i.e. looser stools); high fibre inclusion can have a laxative effect and cause cramping, bloating and flatulence. The fermentable nature of polydextrose is evident in the present dog study due to the decreased faecal pH, increased faecal SCFA concentrations and decreased faecal protein catabolites that were observed. The dogs in the present study, however, did not have changes in Bifidobacterium spp. or Lactobacillus spp. Further research is needed to determine which bacterial groups in the intestinal tract of dogs are capable of fermenting polydextrose, causing the increased faecal SCFA concentrations and decreased faecal pH observed in the present study.
In conclusion, the results of the present study demonstrate the beneficial fermentable properties of polydextrose. In the present study, polydextrose appeared to be fermentable, which was evident by the increased concentrations of faecal SCFA, primarily acetate and propionate, and by the decrease in faecal pH, without affecting food intake or faecal output. The inclusion of polydextrose also decreased some protein catabolites, in particular faecal indole concentrations. Faecal C. perfringens concentrations were decreased by including polydextrose in the diet, but other bacteria measured were unaffected. While many beneficial effects were observed by the inclusion of polydextrose, based on the present study and previous dog studies, we would recommend feeding 1·5 % polydextrose or less to adult dogs to avoid any adverse effects. For example, faecal scores were increased (softer stools) when dietary polydextrose was included at 1·5 %. Polydextrose appears to act as a highly fermentable fibre, providing benefits through fermentation and laxation, but requires further research to test its potential as a prebiotic in dogs.
Acknowledgements
The present study was funded by the USDA-CSREES (project ILLU-538-396). The authors also acknowledge Tate and Lyle for donating the polydextrose that was used in this study. The contributions of the authors are as follows: A. N. B. and K. S. S. contributed to the experimental design; A. N. B. and A. K. W. performed the animal experiment; A. N. B. and A. K. W. performed the laboratory analyses; A. N. B. performed the statistical analyses; A. N. B. and K. S. S. wrote the manuscript. The authors declare that they have no conflict of interest.






