Ca supplements, which have been widely recommended for the treatment and prevention of osteoporosis, have recently been associated with increased cardiovascular risk. In meta-analyses of randomised control trials by our group, Ca supplements, with or without vitamin D, were associated with an increased risk of myocardial infarction and stroke( Reference Bolland, Avenell and Baron 1 , Reference Bolland, Grey and Avenell 2 ). These findings have been confirmed by others( Reference Mao, Zhang and Tang 3 ). The mechanism by which Ca supplements increase cardiovascular risk is currently uncertain. As most of the studies suggest that dietary Ca is not associated with cardiovascular risk, we have hypothesised that the mechanism involves the increase in serum Ca that follows the ingestion of a supplement, as this is smaller or negligible after dietary Ca( Reference Karkkainen, Wiersma and Lamberg-Allardt 4 ). The elevation in serum Ca following a 500–1000 mg dose of supplemental Ca is approximately 1 sd of baseline values, is present for >8 h and is not diminished with long-term use of Ca( Reference Bristow, Gamble and Stewart 5 ). An elevation of this magnitude is likely to be clinically relevant, as a 1 sd increase in serum Ca is associated with an increased risk of cardiovascular events in cohort studies( Reference Slinin, Blackwell and Ishani 6 , Reference Jorde, Sundsfjord and Fitzgerald 7 ).
As Ca is involved in a diverse range of biological processes, there are a number of possible mechanisms by which Ca supplementation and the associated elevations in serum Ca could influence cardiovascular risk. Although an increase in vascular calcification has been suggested as the mechanism, the rapid increase in myocardial infarction risk after supplementation is initiated (within 1 year)( Reference Bolland, Avenell and Baron 1 , Reference Bolland, Grey and Avenell 2 ) suggests that a faster process is involved. Ca appears to acutely influence blood pressure, based on data following Ca infusions( Reference Nilsson, Rastad and Johansson 8 , Reference Kamycheva, Jorde and Haug 9 ), and thus this is a potential mechanism. Further, Ca is involved in blood coagulation, and platelets express the Ca-sensing receptor( Reference House, Kohlmeier and Chattopadhyay 10 ), raising the possibility that changes in serum Ca could influence blood coagulability. However, very few studies have examined whether these indices of cardiovascular risk are affected acutely following the ingestion of a Ca supplement, at the time when serum Ca concentrations are elevated.
We recently carried out a randomised controlled trial in post-menopausal women, comparing the acute and 3-month effects of different Ca supplements on serum Ca and bone turnover. We found that serum Ca was elevated for at least 8 h following 1000 mg of Ca supplementation( Reference Bristow, Gamble and Stewart 5 ). As secondary, exploratory end points in this trial, we examined the acute and 3-month effects of Ca supplements on blood pressure and their acute effects on blood coagulation. As the commonly used laboratory measures of blood coagulation (prothrombin time and activated partial thromboplastin time) are measured in plasma collected into Ca chelator-containing tubes, they are unsuitable for examining the influence of physiological changes in serum Ca on whole-blood coagulation( Reference James and Roche 11 ). We, therefore, measured blood coagulation by thromboelastography (TEG), a point-of-care method that provides a global assessment of the coagulation system in native whole blood.
Methods
Participants
Participants were 100 women, who were at least 5 years post-menopausal. We excluded women if they had a history of CVD, if their 5-year cardiovascular risk was >15 % (assessed using tables published by the New Zealand Guidelines Group, www.nzgg.org.nz), if they had a major ongoing systemic illness, if they had taken any medication known to affect Ca or bone metabolism in the past year or if they were currently taking >2000 IU/d (50 µg/d) of vitamin D. In the Ca group, nine women (10 %) received low-dose vitamin D supplements, as well as two (12 %) in the control group (P=0.84). One participant reported regular usage of >500 mg/d of supplemental Ca and was required to undergo a 6-month period of withdrawal before being enroled to the study. Thus, none of the participants were using Ca supplements at the time of the study. In the Ca group, nineteen women (25 %) received anti-hypertensive medication, as well as six (30 %) in the control group (P=0.82). We recruited women from those who had volunteered to participate in a previous osteoporosis study by our group, but had been ineligible to do so because of normal bone mineral density (women had been recruited for that study via advertisement and mail-outs using electoral rolls). The flow of participants is presented in Fig. 1.

Fig. 1 Flow of participants through the study. Only participants allocated to the calcium citrate or placebo groups had blood coagulation measured. One participant in the control group and two participants in the calcium citrate group did not have blood coagulation measured due to technical problems with the device. These participants did not contribute to the coagulation data of the study and were not included in the analysis of blood coagulation data. One participant in the Ca group declined to have blood pressure measured. This participant did not contribute to the blood pressure data of the study and was not included in the analysis of blood pressure data. MCH, microcrystalline hydroxyapatite.
Study design
The study design has been described previously( Reference Bristow, Gamble and Stewart 5 ). In brief, women were randomly assigned to the treatment group with 1 g/d of Ca (twenty randomised to each of four groups receiving citrate, carbonate or two preparations of microcrystalline hydroxyapatite (MCH), respectively) or to a placebo group with no Ca supplementation for 3 months. Randomisation was carried out by a computer-generated variable-block schedule prepared by staff who were not in contact with the participants. Calcium carbonate and calcium citrate (Jost Chemical Co.) were purchased from Hawkins Watts, and the MCH preparations were supplied by Waitaki Biosciences. All the interventions, including the placebo, were administered as identical encapsulated powders, from bottles labelled with the participant number by staff who were not in contact with participants. Participants and study staff were blinded to treatment allocation, although study staff were informed whether a participant was allocated to either the calcium citrate or the placebo group (but not which of these), as coagulation tests were only performed for these two groups. All four Ca treatment arms were pooled for the comparison of blood pressure effects with placebo. The comparison of coagulation factors was performed for the calcium citrate and placebo arms only (Fig. 1).
On day 1 of the study, participants attended our research clinic after fasting overnight. Blood pressure was measured, and subsequently a venous cannula was inserted and a blood samples were collected between 07.00 and 09.00 hours. The first dose of treatment (as 1 g of Ca or placebo) was administered with a glass of water. A light breakfast (peaches in juice and toast with margarine, jam, marmalade or honey) was provided following intervention. Blood pressure was measured, and subsequent blood samples collected at 2, 4, 6 and 8 h after the supplement was administered. A light lunch (fruit salad in juice and bread with margarine, jam, honey, marmalade or peanut butter) was provided after the 4 h sampling procedures were completed, and an optional snack (plain biscuits and decaffeinated tea) was provided after the 6 h sampling procedures were completed. Water and non-caffeinated tea without milk were allowed ad libitum.
After day 1, participants took their allocated supplements at home in two divided doses, with their morning and evening meals. We telephoned participants midway through the study to encourage compliance. After 3 months, participants returned to our clinic having taken their final dose of treatment the evening before, and a final fasting blood pressure measurement was taken. This study was conducted in accordance with the Declaration of Helsinki and was approved by the New Zealand Northern Regional X Ethics Committee. Written informed consent was obtained from all the participants. This study is registered with the Australia New Zealand Clinical Trials registry (ACTRN12611000232932).
Measurements
Blood pressure was measured using a Dinamap automatic monitor (Johnson & Johnson). After a 5 min period of rest in a sitting position, three recordings were taken at 3 min intervals, as programmed automatically by the device. Across the cohort, the first recording at each time point was found to be significantly higher than the following two recordings; therefore, the mean of the second and third recordings was used in the analyses.
Blood coagulation was measured by TEG on participants allocated to the calcium citrate or placebo group. The calcium citrate group was selected for these measurements because they are time-consuming to perform and because this group was expected to have substantial increases in serum Ca levels( Reference Thomas, Need and Tucker 12 ). TEG is based on the motion of a pin suspended in a rotating cup containing a blood sample. As blood begins to clot, the increased viscosity of the blood causes the pin to move along with the cup. This motion allows calculation of various indices of blood coagulation, including the time to clot initiation (denoted the R-time), rate of clot formation (α-angle and K-time) and the strength of the final clot (maximum amplitude)( Reference Karon 13 ). From these four values, the coagulation index can be calculated, and describes a patient’s overall coagulation status – more positive values indicating increased coagulation and more negative values indicating reduced coagulation. Blood coagulation was measured using a TEG 5000 Thromboelastograph Hemostasis Analyzer as per the manufacturer’s guidelines. At each time point, 2–3 ml of blood was drawn from the venous cannula into a syringe and discarded. A further 1–2 ml of blood was then drawn into a syringe and gently transferred to a test tube. From this, 360 µl of blood was immediately transferred into a pre-warmed cup loaded into the TEG device. The TEG tracing was initiated 4 min after blood was drawn from the cannula.
Statistical analyses
A mixed effects model repeated measures ANCOVA with change from baseline to each time point for each end point as the dependent variable and with the baseline value of the end point variable as the covariate (unstructured covariance) was used to construct an appropriate error term for each of the comparisons between treatment and control at each time point. The assumption of normality was verified for each dependent variable. The main effects of treatment allocation and time and a time by treatment allocation interaction effect were fitted. Contrasts were pre-specified to limit the number of pair-wise comparisons that were performed to those between treatment arms at each time point (i.e. four comparisons at each time point for each variable), and a false discovery rate-adjusted P value was calculated to preserve an overall 5 % significance level for each end point. Day 1 and day 90 data were analysed independently. Mean values with their standard errors changes from baseline are plotted (without covariate/mixed model adjustment). Analyses were performed using SAS (version 9.4; SAS Institute Inc.). Comparison between variables at baseline was made using Student’s t tests, as all the variables listed satisfied the requirements for normality and homogeneity of variances. All the tests were two-tailed, and P<0·05 was considered to be significant.
Results
The baseline clinical and biochemical characteristics of the participants are presented in Table 1. Changes in serum Ca, phosphate and parathyroid hormone levels in this study have been reported elsewhere( Reference Bristow, Gamble and Stewart 5 ).
Table 1 Baseline characteristics of participants (Mean values, standard deviations and ranges)

TEG, thromboelastography.
* Overall assessment of coagulability calculated from R-time, K-time, α-angle and maximum amplitude.
† Time to clot initiation.
‡ Rate of clot formation.
§ Strength of the final clot.
Blood pressure
Changes in blood pressure are presented in Fig. 2. Systolic blood pressure and diastolic blood pressure were significantly lower than baseline at all time points between 2 and 8 h in both the control and the Ca groups (all time points P<0·001). There was a consistent pattern in the change in blood pressure over 8 h in both the groups. Both systolic and diastolic pressures showed dips at 2 and 6 h, the two postprandial time points. When we examined changes from baseline at individual time points, the reductions in systolic blood pressure from baseline were smaller in the Ca group compared with the placebo at 2 (P=0·003), 4 (P=0·02) and 6 h (P=0·01). Similarly, the reductions in diastolic blood pressure were smaller in the Ca v. placebo group at 2 h (P=0·004) and close to significant at 4 (P=0·07) and 6 h (P=0·06). Restricting the analyses to those not using anti-hypertensive medication produced the same pattern of results. After 3 months of supplementation, systolic and diastolic blood pressures were not different from baseline in the Ca or placebo groups (all P>0·31) and were not different between the groups at this time point (all P>0·80). Comparable effects on blood pressure were seen across the four different types of Ca supplement groups.
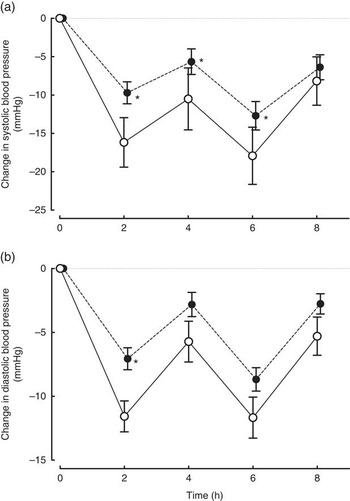
Fig. 2 Changes (a) systolic and (b) diastolic blood pressures in post-menopausal women over 8 h after the ingestion of 1000 mg of Ca (![]() , n 76) or a placebo containing no Ca (
, n 76) or a placebo containing no Ca (![]() , n 20). Values are means with their standard errors. Changes from baseline in systolic blood pressure were significantly different between the Ca and placebo groups between 2 and 6 h (all P<0·02) and diastolic blood pressure at 2 h (P=0·004). * Significantly different from the control group (P<0·02).
, n 20). Values are means with their standard errors. Changes from baseline in systolic blood pressure were significantly different between the Ca and placebo groups between 2 and 6 h (all P<0·02) and diastolic blood pressure at 2 h (P=0·004). * Significantly different from the control group (P<0·02).
Blood coagulation
Blood coagulability tended to increase over 8 h in both the groups, reflected by an increase in the coagulation index at all time points between 2 and 8 h in the Ca (all time points P<0·007) and control (all P<0·042) groups (Fig. 3). The increase from baseline in the coagulation index was significantly greater in the Ca group compared with the control group at 4 h (P=0·03). This appeared to be primarily due to a reduction in the time to clot initiation, as this was significantly shorter in the Ca group compared with the control group at 4 h (P=0·03). The other TEG variables from which the coagulation index is calculated (reflecting the rate of clot formation and strength of the final clot) were not different between the Ca and control groups at any time point (data not shown).

Fig. 3 Changes in the thromboelastographic measures of blood coagulation (a) coagulation index and (b) time to clot initiation (R-time) in post-menopausal women over 8 h after the ingestion of 1000 mg of Ca as citrate (![]() , n 16) or a placebo containing no Ca (
, n 16) or a placebo containing no Ca (![]() , n 19). Changes from baseline in the coagulation index and time to clot initiation were significantly different between the calcium citrate and placebo groups at 4 h (both P=0·03). * Significantly different from the control group, P=0·03. Values are means with their standard errors.
, n 19). Changes from baseline in the coagulation index and time to clot initiation were significantly different between the calcium citrate and placebo groups at 4 h (both P=0·03). * Significantly different from the control group, P=0·03. Values are means with their standard errors.
Discussion
Blood pressure
Blood pressure declined from baseline over 8 h in both the Ca and control groups. This reduction is likely due to the normal diurnal variation in blood pressure, which is characterised by a surge in the morning( Reference Pickering, Shimbo and Haas 14 ). The consistent pattern we observed in blood pressure dip at 2 and 6 h, the time points following the two meals, suggests that feeding also influenced blood pressure in our study. In healthy older adults, the ingestion of a meal has been shown to result in a 11 mmHg reduction in systolic blood pressure, similar to the magnitude of the reduction in the control group of the present study( Reference Lipsitz and Fullerton 15 ).
The reduction in blood pressure over 8 h tended to be smaller in the Ca group compared with the control group, with the difference between groups being significant at some time points (between 2 and 6 h for systolic blood pressure and at 2 h for diastolic blood pressure). These differences were 5 mmHg or more, large enough to be biologically significant. To our knowledge, our study is the first randomised placebo-controlled trial to examine the acute effects of Ca supplements on blood pressure. Two recent, small, uncontrolled studies examined the changes in blood pressure at one time point following Ca supplementation( Reference Burt, Mangelsdorf and Srivastava 16 , Reference Yaron, Roach and Izkhakov 17 ). In both the studies, serum Ca increased 2 or 3 h after the Ca supplementation, but blood pressure did not change from baseline. However, in the absence of a control group, those studies were unable to address the effect of Ca supplementation on blood pressure, independent of the endogenous diurnal rhythm. Both previous studies were performed after fasting( Reference Burt, Mangelsdorf and Srivastava 16 , Reference Yaron, Roach and Izkhakov 17 ), which may also have contributed to the differences between their findings and ours.
The long-term (weeks to months) effects of Ca supplementation on blood pressure have been examined in a number of clinical trials, and the relationship between dietary Ca intake and blood pressure has been assessed in epidemiological studies. In meta-analyses of these studies, Ca supplementation and higher dietary Ca intakes have been associated with modest but significant reductions in blood pressure( Reference Van Mierlo, Arends and Streppel 18 – Reference Dickinson, Nicolson and Cook 20 ). In contrast, a separate body of evidence supports a direct relationship between serum Ca and blood pressure. In epidemiological studies, increased serum Ca concentrations have been associated with higher blood pressure( Reference Jorde, Sundsfjord and Fitzgerald 7 , Reference Sabanayagam and Shankar 21 ) and pulse pressure( Reference Mateus-Hamdan, Beauchet and Rolland 22 , Reference Hagström, Ahlström and Ärnlöv 23 ). In a small trial, ionised Ca increased by 0·32 mmol/l after Ca infusion and systolic blood pressure increased by 7 mmHg( Reference Nilsson, Rastad and Johansson 8 ). In a similar study, ionised Ca increased step-wise by 0·10 and 0·20 mmol/l after Ca infusion and systolic blood pressure increased by 5 and 10 mmHg( Reference Kamycheva, Jorde and Haug 9 ). Furthermore, in patients on dialysis, increases in serum Ca and smaller reductions in blood pressure during dialysis have been observed with the use of high-Ca dialysates( Reference Locatelli, Cavalli and Tucci 24 ).
The differing relationships of serum Ca and Ca intake with blood pressure can be explained by the fact that high dietary Ca intakes and Ca supplementation do not result in sustained higher serum Ca concentrations, due to the tight regulation of extracellular Ca levels to a physiological set-point( Reference Houillier, Froissart and Maruani 25 ). However, it is well-known that extracellular Ca concentrations can be increased to the upper levels of their normal range or above in the period immediately following the ingestion of a Ca supplement( Reference Bristow, Gamble and Stewart 5 , Reference Heaney, Dowell and Bierman 26 ). Therefore, it is possible that Ca supplements might have different effects on blood pressure acutely, when serum Ca concentrations are elevated, compared with >8–12 h later when serum Ca concentrations have returned to their baseline levels. The finding of the present study of a difference in blood pressure between the Ca and placebo groups only within 8 h following supplementation, but not after 3 months of supplementation (when blood pressure was measured in the morning after an overnight fast), is consistent with this hypothesis.
If Ca supplements do result in an acute elevation in blood pressure, or less of a reduction in blood pressure from fasting or morning values, then there are several possible mechanisms through which this might be mediated. In rats, the administration of a calcimimetic produces an acute rise in blood pressure( Reference Fryer, Segreti and Widomski 27 , Reference Odenwald, Nakagawa and Hadtstein 28 ), suggesting the Ca-sensing receptor, which is expressed throughout the vasculature( Reference Smajilovic and Tfelt-Hansen 29 ), may be involved. Changes in extracellular Ca and/or Ca-regulatory hormones could influence blood pressure through alterations in the renin–angiotensin–aldosterone system( Reference Vaidya, Brown and Williams 30 ). A change in extracellular Ca levels could influence intracellular Ca levels, and thus increase the tone of vascular smooth muscles. The postprandial reduction in blood pressure that occurs in older adults might be attenuated by an increased release of vasoactive gut hormones such as glucagon-like peptide 1( Reference Gentilcore, Bryant and Wishart 31 ), which may in turn be influenced by the Ca content of the meal( Reference Gonzalez and Stevenson 32 ). Correspondingly, the ingestion of a Ca supplement with breakfast attenuated the usual postprandial fall in vascular tone in one small trial( Reference Soares, Kuriyan and Kurpad 33 ).
Blood coagulation
Blood coagulability increased in both the Ca and placebo groups over 8 h, which may have been due to diurnal variations in activators and inhibitors of coagulation. Alternatively, phlebotomy and sample collection may have influenced our TEG results( Reference Chitlur and Lusher 34 ) – for example, any increase in difficulty in drawing blood from the cannula over 8 h may have resulted in activation of clotting factors and platelets.
There was a greater reduction in the time to clot initiation and correspondingly a greater increase in the coagulation index at 4 h in the Ca group compared with the control. To our knowledge, this is the first study to examine the acute effects of Ca supplements on blood coagulation. In a study in rats, hypercalcaemia significantly shortened the time to clot initiation( Reference Hilgard 35 ). In a study of citrated and re-calcified human blood samples, ionised Ca was inversely associated with the time to clot initiation and was positively associated with the strength of the clot( Reference James and Roche 11 ). Across the physiological range of Ca values, the time to clot initiation was linearly and inversely related to ionised Ca concentration. If Ca supplements and/or changes in extracellular Ca did influence clotting, then this might be mediated through increased activation of the clotting cascade, in which Ca is an essential cofactor. Platelets, which have an essential role in the initiation of coagulation, also express the Ca-sensing receptor( Reference House, Kohlmeier and Chattopadhyay 10 ), suggesting extracellular Ca concentrations could influence their activity. It has recently been demonstrated that platelet activation is greater in patients with primary hyperparathyroidism than it is following surgical cure of that condition( Reference Yilmaz 36 ), suggesting that elevated serum Ca might directly activate these cells.
Strengths of the present study include its randomised controlled design and measurement of blood pressure and blood coagulation at multiple time points. Limitations of this study include the relatively small size of the control group (n 20), which was due to the primary outcome (change in markers of bone turnover) being a comparison between five groups( Reference Bristow, Gamble and Stewart 5 ). The differences we identified in blood pressure and blood coagulation only reached significance at some time points. Nonetheless, the effects demonstrated are novel, and are large enough to be pathophysiologically significant, and also provide a basis for further research examining the acute effects of Ca supplements on indices of cardiovascular risk. As this study was conducted in a group of older women, the changes in blood pressure and coagulation may not apply to other age groups or to men.
In conclusion, this study has identified two putative mechanisms by which Ca supplements might impact on cardiovascular risk – through acute increases in both blood pressure and in blood coagulation immediately following dosing. The potential importance of these findings and the fact that the changes are only significant at some time points both suggest that confirmation by independent studies is required. However, these preliminary observations could be important in helping us understand the adverse effects of Ca supplements on vascular health.
Acknowledgements
This work was supported by the Health Research Council of New Zealand. Some of the analyses in this study were funded by a Sir Charles Hercus Health Research Fellowship awarded to Mark J. Bolland. S. M. B. is the recipient of a University of Auckland Doctoral Scholarship. The funders had no role in the design, analysis or writing of this article.
The author contributions to the manuscript are as follows: S. M. B. and I. R. R. designed the study; S. M. B., A. S. and A. H. conducted the study; G. D. G. analysed the data; S. M. B., I. R. R. and G. D. G. wrote the manuscript; and S. M. B. and I. R. R. had primary responsibility for the final content. All the authors critically reviewed and approved the final version of the manuscript.
There are no conflicts of interest to declare.







