INTRODUCTION
Hepatitis A virus (HAV) infection is a common disease, particularly in children and is endemic in areas with substandard hygiene and sanitation. The virus accounts for 25% of all clinically evident acute hepatitis and affects 10 million persons annually worldwide [Reference Wasley, Fiore and Bell1, Reference Kumar2]. Persons affected with the disease typically complain of low appetite and malaise and present with jaundice. Hepatitis A is a self-limiting disease with signs and symptoms lasting for a few weeks and is not known to cause chronic hepatitis. A previous infection is detected by the presence of serum IgG hepatitis A antibody (anti-HAV) which persists for years and possibly confers lifelong immunity against all strains of HAV. Since HAV rarely causes fulminant hepatitis, the fatality rate associated with the infection is extremely low [Reference Kumar2].
Once common in the USA, HAV infection has declined significantly in the era of hepatitis A vaccination [Reference Wasley, Fiore and Bell1, 3–Reference Samandari, Bell and Armstrong7]. With the introduction of the vaccine in 1995 and the recommendations by the Advisory Committee on Immunization and Practices (ACIP) to target the HAV infection-vulnerable population, the annual incidence rates of clinical disease started to decline from 12/100 000 in 1995 to 2·6/100 000 in 2003 and 1·0/100 000 in 2007 [3, Reference Daniels, Grytdal and Wasley4]. In 1996 ACIP recommended vaccinating children aged ⩾24 months in high-risk communities; in 1999 ACIP recommended more extended routine coverage for the same age group; and in 2006, ACIP recommended vaccination for all children in the USA, starting at age 12–23 months. Routine childhood vaccination resulted in the precipitous decline of incidence in children, who historically were disproportionately burdened by the disease [Reference Wasley, Fiore and Bell1, 3, Reference Wasley, Samandari and Bell5–Reference Jenson8].
While developed countries have low hepatitis A endemicity, in the USA, young children and Mexican American or Hispanic children are at high risk of HAV infection [Reference Hayes-Bautista9–Reference Weinberg12]. More than 15 years have passed since the hepatitis A vaccine was introduced in the USA. The fervour to decrease HAV infection by targeting regions endemic for hepatitis A may have afforded herd immunity thus altering disease epidemiology. In 2007–2008, the NHANES included serological testing for anti-HAV that provided population-based seroprevalence data. We performed an analysis of the hepatitis A data made available in the NHANES 2007-2008 for subjects aged 2–19 years in order to assess the current determinants of anti-HAV seroprevalence in this age group as well as to assess epidemiological profile changes 15 years after hepatitis A vaccination was started.
METHODS
NHANES 2007–2008
The National Health and Nutrition Examination Survey (NHANES) provides cross-sectional information on health and nutrition status that can be generalized to the US civilian non-institutionalized population. The survey uses a complex, stratified, multistage probability sampling of households and obtains data from consenting participants through questionnaires and standardized health examinations. Health examinations include physical and laboratory tests conducted in mobile examination centres (MECs). In NHANES 2007–2008, data were collected from 10 149 participants, weighted to represent close to 300 million persons in the USA. NHANES oversampled Hispanics, African Americans, participants aged ⩾60 years, and low-income persons to obtain a sample size sufficient for analysis of these demographic categories [13].
Subjects in this study
Of the 10 149 participants of all ages, those between 2 and 19 years were eligible for a qualitative determination of total serum hepatitis A antibody (n=3306). Of those eligible for anti-HAV testing, 2621 (79·3%) had confirmed results, one indeterminate, and 684 (20·7%) did not have a specimen for analysis [14].
Laboratory methods and questionnaire
Solid-phase competitive enzyme immunoassay (EIA) was used to qualitatively determine anti-HAV in serum or plasma. The intensity of the yellow-orange colour change generated at the final step of the assay was measured by a spectrophotometer at 492 nm cut-off. A commercial assay kit was used to test for anti-HAV (HAVAB-EIA solid-phase EIA kit, cat. no. 789524, Abbott Laboratories, USA). Samples with absorbance ⩽492 nm were considered reactive to anti-HAV and labelled positive for the antibody while samples with >492 nm absorbance were labelled negative for anti-HAV. Solid-phase competitive EIA does not distinguish seropositivity acquired from hepatitis A infection or hepatitis A immunization [14].
The NHANES sample person and family questionnaire provided demographic information of the participants. For the analysis, age was categorized as follows: 2–4, 5–9, 10–14, 15–19 years. NHANES categorized race/ethnicity as non-Hispanic White, non-Hispanic Black, Hispanic-Mexican American, Hispanic-Other Hispanic, and Other. In this study, 2497 (95·3%) participants had information on both race and anti-HAV status. The category ‘Other’ was excluded from the analysis. Participants indicating place of birth other than USA were considered foreign-born. For determination of socio-economic status, the ratio of family income to the family's appropriate poverty threshold as measured by the U.S. Census Bureau was used. This variable was categorized as follows in the analysis: <1·00, ⩾1·00 to <2·00, ⩾2·00 to <3·00, ⩾3·00 to <4·00, and ⩾4·00. If a family's total income is less than the family's threshold income value, the index is <1 and that family, and every individual in it, are considered poor.
The participants' hepatitis A vaccination status was determined using vaccination records and self-report. Subjects aged ⩾16 years were interviewed directly. A proxy was interviewed for those aged <16 years and those unable to answer questions. Vaccination status was grouped as no vaccination, a single dose, or two doses of vaccine. Other variables included in the analysis were water source and the year the participant's home was built. Water source was categorized into company-provided source and well, or other sources.
Statistical analysis
Prevalence estimates were weighted to account for the method of sampling used by NHANES and to ensure that the resulting estimates represent the US population. We used logistic regression to calculate odds ratios (OR) with 95% confidence intervals (CI) for hepatitis A vaccination status, adjusted for age, gender, race, poverty level, country of birth, and year home was built. All of these variables were included in the multivariate models. Education and family size were also examined in relation to anti-HAV status but they were not associated with the outcome and therefore were not included in the models. Since anti-HAV seropositivity may be acquired through either vaccination or previous natural infection, we conducted a subgroup analysis for only those who were not vaccinated to assess seropositivity due to natural infection. Results with P<0·05 were considered significant. Stata v. 10 (svy module) was used to calculate prevalence and odds ratios accounting for weighting and complex sampling of the survey.
RESULTS
Based on the results from the 2621 participants tested for anti-HAV, the overall prevalence of anti-HAV antibodies in the US population aged 2–19 years was 39% (95% CI 32·6–45·3) (Table 1). Out of 2314 subjects with a known vaccination status, 1132 (48·9%) had not been vaccinated. Of the latter, 23·7% (95% CI 17·5–29·9) were positive for anti-HAV and therefore considered to be positive via previous HAV infection.
Table 1. Prevalence, crude and adjusted odds ratios for selected determinants of seroprevalence of antibody to hepatitis A virus in participants aged 2–19 years, NHANES 2007–2008
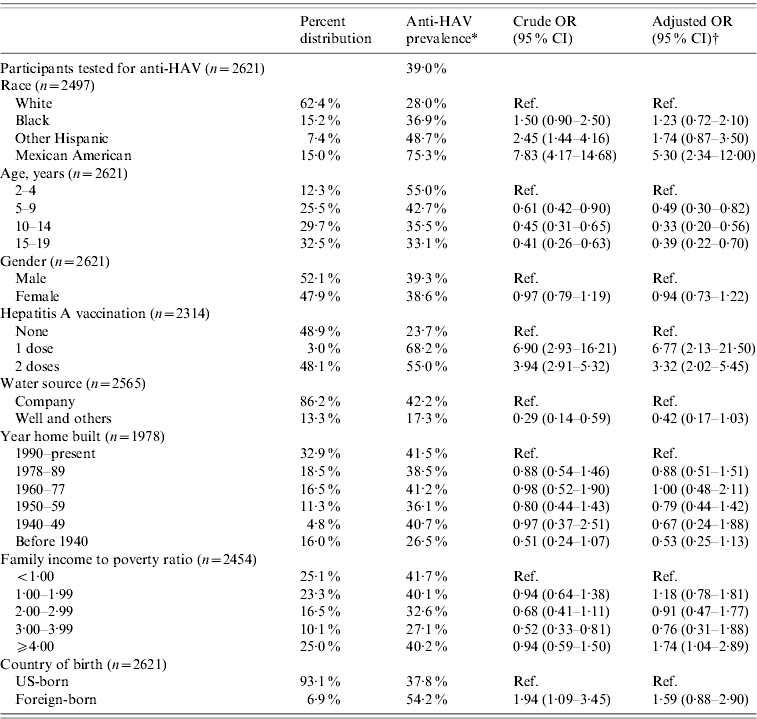
OR, Odds ratio; CI, confidence interval.
* Refers to participants testing positive for anti-HAV which may have been acquired through hepatitis A vaccination or previous infection.
† Odds ratios were adjusted for age, gender, race, poverty level, hepatitis A vaccination, water source, year home built, and country of birth.
Demographics
Race/ethnicity was strongly associated with anti-HAV seroprevalence. Seventy-five percent of Mexican Americans, 49% of Other Hispanics, 37% of African Americans, and 28% of Whites were positive for anti-HAV. Being Mexican American increased the odds for anti-HAV seroprevalence fivefold that of Whites (OR 5·30, 95% CI 2·34–12·00) after adjusting for age, gender, poverty level, year home was built, water source and country of birth (Table 1). In the subgroup analysis representing the non-vaccinated population, the odds for anti-HAV seroprevalence among Mexican Americans compared to Whites remained almost the same (OR 5·49, 95% CI 1·93–15·64) (Table 2).
Table 2. Prevalence, crude and adjusted odds ratios for selected determinants of hepatitis A virus infection in non-vaccinated participants aged 2–19 years, NHANES 2007–2008
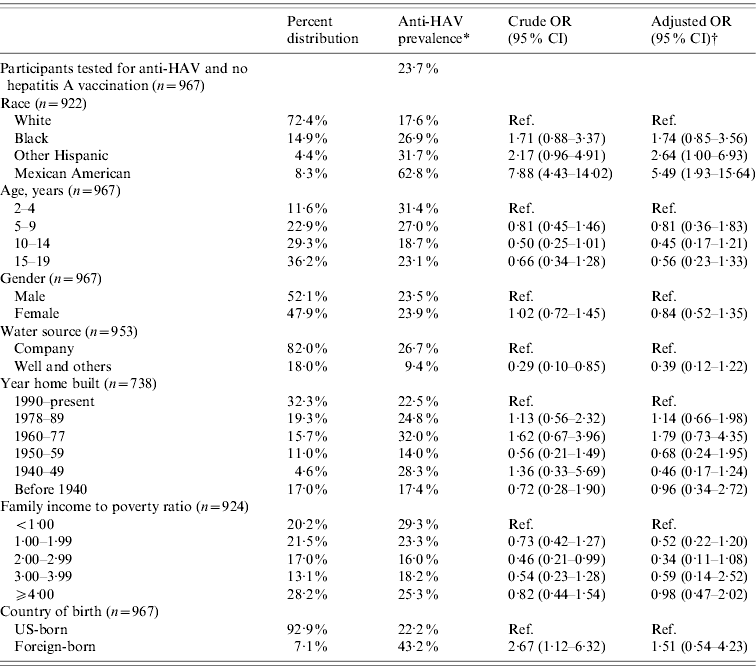
OR, Odds ratio; CI, confidence interval.
* Refers to participants testing positive for anti-HAV which is more likely acquired through previous hepatitis A infection.
† Odds ratios were adjusted for age, gender, race, poverty level, water source, year home built, and country of birth.
The prevalence of anti-HAV decreased with age. Fifty-five percent aged 2–4 years, 43% aged 5–9 years, 36% aged 10–14 years, and 33% aged 15–19 years had anti-HAV detected in their blood samples. Compared to the 2–4 years group, persons aged 5–19 years had 2–3 times lower odds of anti-HAV seroprevalence (5–9 years: OR 0·49, 95% CI 0·30–0·82; 10–14 years: OR 0·33, 95% CI 0·20–0·56; 15–19 years: OR 0·39, 95% CI 0·22–0·70) (Table 1). Persons aged 5–19 years had lower odds even after removing exposure to hepatitis A vaccination; however, the 95% confidence intervals included the null value (Table 2). Figure 1 shows anti-HAV positivity and HAV vaccine status proportions by age groups for the past three rounds of NHANES.
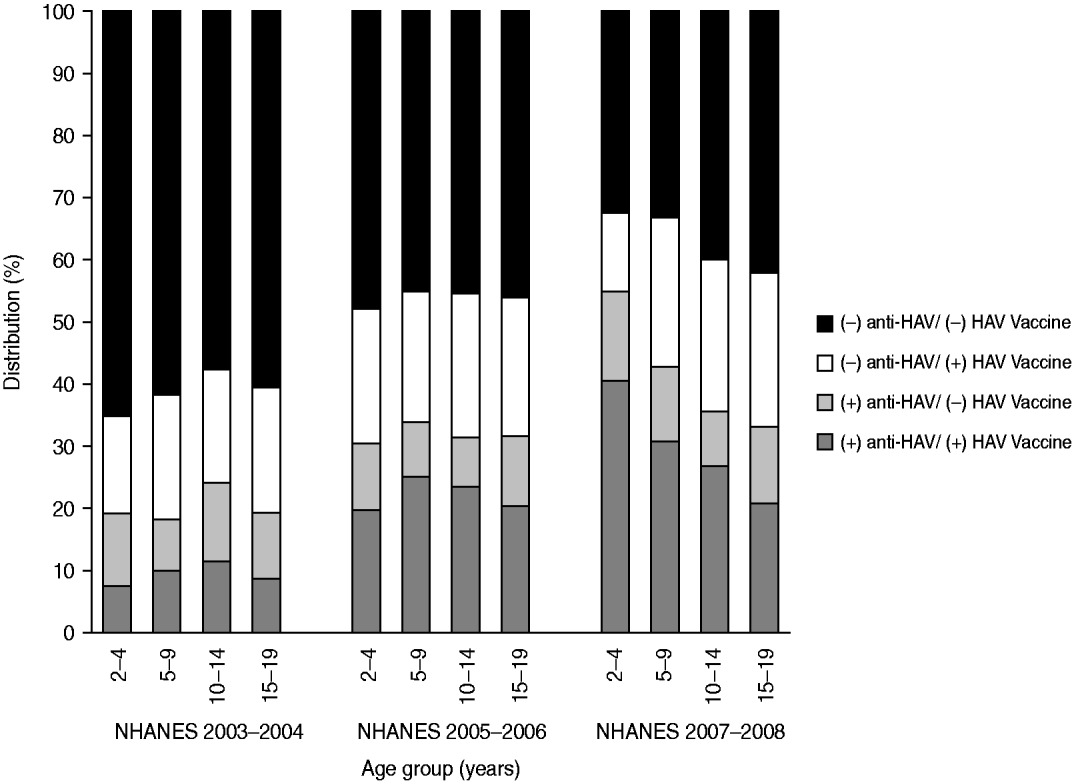
Fig. 1. Percent distribution of anti-HAV status: NHANES 2003–2004, 2005–2006, and 2007–2008.
Gender did not appear to influence anti-HAV seroprevalence. Although females, both for the entire population and the non-vaccinated subpopulation, had lower odds compared to males, this was not statistically significant (all: OR 0·94, 95% CI 0·73–1·22; non-vaccinated: OR 0·84, 95% CI 0·52–1·35).
Hepatitis A vaccination
Compared to non-vaccinated persons, those who reported having received one and two doses of hepatitis A vaccine had over six and three times greater odds of testing positive, respectively (one dose: OR 6·77, 95% CI 2·13–21·50; two doses: OR: 3·32, 95% CI 2·02–5·45). Mexican Americans and Other Hispanics reported higher proportions of hepatitis A vaccination than Whites and African Americans. Seventy-two percent of Mexican Americans aged 2–19 years reported receiving at least one dose of hepatitis A vaccine compared to 71% of Other Hispanics, 53% of African Americans, and 42% Whites.
Other contextual factors (water and housing characteristics, poverty, country of birth)
Persons using water from sources other than company providers had lower odds for anti-HAV seroprevalence although this difference was not significant. In general, persons living in homes built before the 1960s also had lower odds of anti-HAV seroprevalence compared to those living in homes built in 1990 onwards; however, these differences were also not significant (Tables 1 and 2).
The association between poverty and overall anti-HAV seroprevalence was not significant (Table 1). However, in the non-vaccinated population, the odds for anti-HAV seropositivity were generally lower for those above the poverty threshold compared to persons living in poverty (Table 2). Persons born outside the USA had 50–60% greater odds of testing positive for anti-HAV although this was not statistically different from US-born persons (all: OR 1·59, 95% CI 0·88–2·90; non-vaccinated: OR 1·51, 95% CI 0·54–4·23).
DISCUSSION
The results of this study indicate that in the USA, being Mexican American and having received hepatitis vaccination were the strongest predictors of anti-HAV seropositivity in the 2–19 years age group. Moreover, a younger age appeared to be a strong predictor of anti-HAV seropositivity.
Race and demographics
Mexican Americans had the highest rates of vaccination in this study. Seventy-two percent of Mexican Americans aged 2–19 years in the USA reported having received at least one dose of hepatitis A vaccination. This result is consistent with previous reports showing higher hepatitis A vaccination coverage in Hispanic children [Reference Fiore15, Reference Bell16]. The latest (2006) ACIP recommendation to routinely vaccinate children aged 2–18 years in regions with ⩾20 clinically evident HAV infection cases/100 000 led to the identification of 11 states (Alaska, Arizona, California, Idaho, New Mexico, Nevada, Oklahoma, Oregon, South Dakota, Utah, Washington) – all in the western region, as priority areas for vaccination [17]. The western region has the highest percentage of Hispanic-origin dwellers – 19·1% and 24·3% in the 1990 and 2000 censuses, respectively. Of these 11 western states, four had at least 10% Hispanic population in the 1990 census and by 2000 the proportion of Hispanics in these states had at least doubled. Starting at the 2000 census, the country of origin was specified for Hispanics where Mexican Americans represented 58·5% of the US Hispanic population [18]. The correspondence between geographic distribution of the Hispanic population and the hepatitis A vaccination priority areas explains the higher percentage of vaccinated Mexican Americans and Other Hispanics which contributed to the high anti-HAV seroprevalence in these groups.
In an attempt to examine the seroprevalence attributable to natural infection, we conducted a subgroup analysis limited to those who reported not having received vaccination. In this subgroup analysis results remained largely the same. Mexican Americans had significantly higher odds of anti-HAV seropositivity acquired through previous HAV infection. This finding was consistent with earlier anti-HAV prevalence studies conducted prior to the introduction of hepatitis A vaccination that reported higher seroprevalence in Mexican Americans compared to non-Hispanic participants [Reference Hayes-Bautista9, Reference Bell16]. Similar results were seen in prevalence surveys involving children living in Texas along the USA/Mexico border and families of migrant farm workers in Florida. The higher prevalence of infection in Mexican American and Hispanic communities may be attributed to greater exposure to HAV through higher exposure to HAV infection in these communities or frequent travel to HAV-endemic countries in Central and South America and Mexico [Reference Hayes-Bautista9, Reference Tapia-Conyer11, Reference Weinberg12, Reference Jong19]. A study of cases of hepatitis A reported to the San Diego County Health Department in Hispanic children aged ⩽18 years linked infection to cross-border travel to Mexico and its associated foodborne exposure [Reference Weinberg12, Reference Owen20]. In addition to cross-border travel, other risk factors were identified for schoolchildren living in a USA–Mexico border community which include being in first grade of elementary education, low maternal educational attainment, >6 months residence in Mexico, household crowding, and substandard sanitation system [Reference Redlinger, O'Rourke and VanDerslice21]. The same reasons may explain the high prevalence of HAV infection in non-vaccinated 2- to 19-year-old Mexican Americans.
The results of this study suggest that in the range of this study (2–19 years) younger individuals were more likely to be seropositive. This is contrary to earlier studies describing hepatitis A epidemiology where age was one of the strongest predictors of anti-HAVseropositivity [Reference Bell16, Reference Sac22–Reference Omar and Hashish25]. Indeed, in countries where HAV vaccination is not provided, and in the USA prior to vaccination, seropositivity rates would increase with age because antibodies acquired through natural infection provide lifelong immunity. With the introduction of vaccines and decreasing natural infection, the main source of antibodies will be vaccines rather than natural infection and age-patterns shift. Our analysis of the most recent NHANES (2007–2008) showed an unexpected distribution of seropositivity across age groups. This led us to compare results with previous surveys (2003–2004 and 2005–2006, Fig. 1). We found slightly higher seroprevalence rates across the younger three age groups in the first survey, decreasing seropositivity across the older three age groups in the second survey and decreasing seropositivity across all groups in the most recent survey (NHANES 2008). This trend could be attributed to either higher rates of infection in younger children that add to the vaccine seropositivity; to weaning of vaccine effect after a number of years, hence leading to lower positivity rates in older children or teenagers who received their vaccine many years before; to use of more effective and immunogenic vaccines for the younger children; or to higher vaccination rates in younger children. Nevertheless, these explanations are unlikely, since most reports indicate that antibodies generated via vaccination have a long life [26]; however, some studies have quantitatively documented decreasing antibody levels over time after hepatitis A vaccination arguing for the need of booster doses [Reference Van Damme27, Reference Hammitt28]. We are not aware of any reports showing that the newer vaccines are more immunogenic or effective. To study whether younger children are being vaccinated at higher rates, we conducted a subgroup analysis in those who did not report vaccination. In this subgroup analysis anti-HAV seropositivity was also higher in younger children which may seem to refute the hypothesis. However, it should be borne in mind that reporting of vaccination is subject to non-differential misclassification. Therefore, it is still possible that younger children are being vaccinated at higher rates and due to misclassification of reporting the results show higher rates of seropositivity in those who do not report having received a vaccine. However, a similar misclassification effect on prevalence would be expected to be seen in earlier surveys, which was not the case.
Hepatitis A vaccination
Hepatitis vaccination was expected to be a strong predictor of anti-HAV positivity. Available since 1995, the hepatitis A vaccine was routinely administered to children living in the West and Southwest regions where hepatitis was endemic causing the reversal of disease age distribution; infection rates are now higher in adults particularly in men aged 25–39 years [Reference Wasley, Fiore and Bell1, 3, Reference Wasley, Samandari and Bell5, Reference Armstrong and Bell6, Reference Jenson8, Reference Bell16, Reference Armstrong29].
The general recommendation in the USA for children is two doses of hepatitis A vaccine administered at least 6 months apart [3, 17]; however, the results of this study did not indicate lower seropositivity rates in those who received a single dose of hepatitis vaccine. The World Health Organization (WHO) recognizes that all hepatitis A vaccination schedules are efficacious in eliciting sufficient antibody levels for protection against HAV infection and that high antibody levels are readily achieved by a single dose [26]. Further studies are needed to evaluate the protection conferred by a single dose of hepatitis A vaccination.
We found that in each of the three NHANES studies 13–25% of participants who reported having been vaccinated for hepatitis A were seronegative, with a larger percentage for older participants (Fig. 1). This result may be attributed largely to misclassification where participants erroneously report receiving hepatitis A vaccination. However, if participant reports were accurate for the most part, seronegativity despite hepatitis A vaccination may reflect vaccine failure or a shorter than expected vaccine-conferred immunity. No changes in case definition or in laboratory cut-off values for seropositivy occurred in the three surveys.
Contextual factors
Poverty encompasses a number of socioeconomic status indicators such as education, sanitation, household characteristics, and income. In this study, poverty was not significantly associated with anti-HAV seropositivity. On the contrary, members of the wealthiest group (family income to poverty ratio of ⩾4·00) have almost the same anti-HAV seroprevalence as the poorer participants (Table 1). This increased risk appears to be inversely related to immunization since the anti-HAV prevalence in the wealthiest subgroup drops from 40% (Table 1) in the total group to 25% (Table 2) in those denying being immunized. However, the protective effect of family income against seropositivity acquired through natural infection, although not significant, can be seen in non-vaccinated persons. In developing countries where hepatitis A is endemic, the role of poverty in hepatitis A disease transmission is firmly established [Reference Sac22–Reference Omar and Hashish25]. In the USA, immigration and international travel, as previously discussed, were factors more strongly associated with the spread of communicable diseases like hepatitis A [Reference Hayes-Bautista9–Reference Weinberg12, Reference Jong19, Reference Redlinger, O'Rourke and VanDerslice21, Reference Hayes-Bautista, Baezconde-Garbanati and Hayes-Bautista30–Reference Mutsch32]. The country's accessibility to migrants from developing nations and proximity to places where hepatitis A is endemic may better explain the geographic and ethnic distribution of the disease than poverty. Although a study on HAV infection in the USA using NHANES III identified poverty as a significant predictor of HAV infection prevalence [Reference Bell16], the confounding introduced by the revisions on the policies and recommendations on hepatitis A vaccination may have reduced the ability of poverty to predict seropositivity. The higher odds among foreign-born persons for seropositivity due to previous infection, although not statistically significant, alludes to a relatively better public health strategy like access to potable water, efficient sewage disposal system, and better housing among US-born persons. While company-provided water is the major water source in the USA, the stronger association it had with anti-HAV seropositivity than other water sources like wells for non-vaccinated persons, may have been due to chance secondary to a lack of a sufficient sample for analysis. The inclusion of the year participants' homes were built was based on the assumption that communities with older homes are more vulnerable to spread of hepatitis A due to older sewage systems that may be potential sources of contamination. This is particularly relevant for participants denying vaccination; however the results showed that participants living in older homes did not have elevated risks for anti-HAV seroprevalence.
Strengths and limitations
This study has several strengths. NHANES is representative of the entire non-institutionalized US population, and serum samples from over 2600 participants were analysed for anti-HAV seropositivity. Data were available for potential confounders and subgroup analyses; however, very low numbers of other Hispanic and Mexican Americans limited our ability to draw conclusions from the results of the non-vaccinated group. However, this study has limitations too. Most notably vaccination was based on self-report which may be subject to misclassification.
CONCLUSIONS
In summary, the results of this study showed that Mexican Americans, while receiving higher rates of vaccination, were still at higher risk of HAV infection than other groups. Therefore racial/ethnic disparities with regard to HAV exist and efforts to expand vaccination, especially to Mexican Americans may be needed. Older children and teenagers were less likely to be seropositive against HAV than younger children, which may indicate a higher rate of vaccination in younger children, increased infection rates or gradual lower levels of antibodies years after vaccination. All these explanations seem unlikely. In addition, herd immunity is unlikely to explain the increased relative HAV prevalence in younger children in NHANES compared to earlier surveys. The latter may need to be investigated and, if needed, booster vaccines may need to be considered. Finally, the reasons why HAV-vaccinated subjects tested negative to anti-HAV need to be further explored.
DECLARATION OF INTEREST
None.





