I. The roots of Roundup–non-Hodgkin lymphoma litigation in the USA
The International Agency for Research on Cancer (IARC) issued its surprising classification of glyphosate-based herbicide (GBH) and glyphosate as a “probable human carcinogen” in March 2015,Footnote 1 via a press releaseFootnote 2 and short paper in The Lancet Oncology.Footnote 3 Substantial media coverage followed the IARC announcement in the USA. Most stories made reference to controversies arising from the ongoing US Environmental Protection Agency (EPA) glyphosate re-registration process and/or recent developments in the State of California in the wake of the State’s listing of GBH consumer products as possible carcinogens under Proposition 65.Footnote 4
The IARC classification decision led, within months, to the filing of lawsuits alleging that past use of Roundup had contributed to the plaintiffs’ cases of non-Hodgkin lymphoma (NHL). In the ongoing litigation over Roundup and NHL, the core issues in dispute arise at the nexus of cancer classification schemes, pesticide hazard and risk assessment policy, exposure assessment and risk mitigation requirements, legal standards, levels and burden of proof and evaluation of case-specific causality. Much of the expert witness testimony and arguments by attorneys presented to the juries during the three completed trials has focused on whether the EPA’s or IARC’s assessment of glyphosate oncogenicity is more relevant to deciding the merits of each plaintiff’s case.
After a multiyear review, the EPA concluded that glyphosate is not likely to pose an oncogenic risk.Footnote 5 The agency’s evaluation focused primarily on risks arising from exposure to pure glyphosate (not formulated Roundup) via the diet across the general population. Given the agency’s focus on dietary exposures and the risks of glyphosate, the lack of weight placed on the toxicology studies done on formulated products is defensible.Footnote 6 In addition, three factors led to, and sustained the EPA’s lack of concern over glyphosate dietary exposures:
-
The generally infrequent and low levels of glyphosate residues in food;
-
Glyphosate’s very high chronic reference dose;
-
The generally high levels of glyphosate fed to animals in two-year rodent cancer bioassays.
However, a different set of factors come into play when the EPA, or anyone, assesses the possible oncogenic risks from exposures to formulated GBHs during mixing and loading operations and application episodes. Mixer-loaders and applicators are exposed via dermal absorption to formulated products, which are a mixture of glyphosate and various surfactants and adjuvants. Such mixtures can markedly enhance the toxicity of GBH exposure.Footnote 7 This is because the surfactants in most GBHs facilitate the movement of glyphosate through the human epidermis and, once inside the body, through cell membranes. This accelerated movement of glyphosate increases the amount reaching the inside of cells, where it comes into contact with DNA and can trigger direct damage to DNA or oxidative stress, two genotoxicity mechanisms that are known to be associated with the formation of cancerous cell growths.Footnote 8
Despite these differences, the EPA did not conduct an applicator exposure risk assessment, nor render a judgement on the often much higher exposures and risks incurred by applicators, especially in the case of people spraying a GBH with handheld equipment. It chose not to do so because: “Dermal and inhalation endpoints were not selected since there was no toxicity observed in route-specific toxicity studies and there was no concern for increased quantitative susceptibility to offspring”.Footnote 9 No dermal exposure endpoints were selected because none was observed. This is not surprising, given that the EPA had not required registrants to conduct any “route-specific toxicity studies”, nor had it carried out such studies itself. The EPA has used a 3% dermal absorption “default value” in conducting glyphosate worker-exposure assessments since the early 1980s, a value weakly supported by studies involving topically applied, pure glyphosate in mouse and primate studies. Evidence presented at trial showed clearly that, by the early 2000s, Monsanto scientists were aware that the surfactants in Roundup-brand herbicides were likely increasing glyphosate’s dermal absorption rates above 3%, but they did not share these data and reasons supporting this conclusion with the EPA, nor other regulators.
The EPA relied mostly on registrant-commissioned, unpublished animal bioassays and genotoxicity studies done on pure glyphosate, and it did not conduct a thorough review of, nor place much weight on genotoxicity studies done with formulated GBHs, including Roundup.
The IARC, on the other hand, classified glyphosate and GBHs as “probable human carcinogens”. The IARC Working Group considered a greater diversity of routes of exposure, including several published studies reflecting applicator exposures to formulated GBHs. The IARC’s evaluation took account of, and placed considerable weight on in vivo studies from people exposed directly to GBHs.
As a result of these differences, the EPA’s assessment of glyphosate oncogenicity is primarily relevant to one route of exposure (dietary) and generally low rates of exposure, while the IARC’s evaluation spans all routes of exposure and the sometimes high levels of applicator exposure. In this paper, I explain the differences in the following: (1) the questions that the EPA and IARC set out to answer; and (2) the data that the EPA and IARC relied on in reaching their divergent classification decisions. I argue that a full accounting of these differences goes a long way towards explaining why and how the EPA and IARC reached diametrically opposed conclusions.
Throughout the first three trials, attempts to convince jurors that exposure to Roundup likely did or did not contribute to a plaintiff’s NHL were the primary focus of both expert testimony and the presentations of evidence by attorneys. In this body of litigation, two key pieces of the puzzle were clear: the plaintiffs had used Roundup frequently and had been exposed to it many times; and the plaintiffs had suffered and continued to suffer from NHL. The matter of dispute was whether and to what degree exposure to Roundup contributed to a given plaintiff’s NHL, taking into account other possible causes of a given plaintiff’s NHL.
Defence attorney presentations of evidence began, ended and never veered far from the EPA’s “not likely to pose oncogenic risk” classification. Plaintiff attorneys focused instead on the IARC’s synthesis of relevant data and its “probable carcinogenic” classification. They explained why the IARC’s assessment was more relevant to the exposures incurred by the plaintiffs. Plaintiff attorneys and experts did not directly dispute nor strive to question the EPA’s judgement that current, projected dietary exposures to glyphosate posed little if any cancer risk. However, they did explain in detail why the EPA’s analysis did not address nor encompass the much higher levels of exposure experienced by applicators, nor the fact that applicators are exposed to a mixture of chemicals, and not just pure technical glyphosate.
A factor unique to this sort of litigation warrants attention: the applicable level and burden of proof under US law and judicial precedent. To prevail on the question of liability and compensatory damages, plaintiff attorneys and experts must convince a jury that a preponderance of the evidence supports a conclusion that it is “more likely than not” that the plaintiff’s use of and exposure to Roundup contributed to his or her NHL.Footnote 10 Thus, it is not necessary to prove that a plaintiff’s NHL was caused solely, or even primarily, by exposures to Roundup. A jury can find in favour of a plaintiff if they feel that a preponderance of the evidence supports the conclusion that Roundup exposures accelerated the progression of a plaintiff’s NHL or made it more difficult to treat and control.
The legal standard to support an award of punitive damages is stricter and the nature of the evidence differs. To support a punitive damage award, there must be “clear and convincing” evidence that a defendant has acted with malice, oppression and/or a blatant disregard for public safety or the rights of others.Footnote 11 Both of these levels and burdens of proof are not nearly as onerous as the “beyond a reasonable doubt” threshold applicable in criminal cases.Footnote 12 These evidentiary burdens are well-known and accepted by lawyers and judges engaged in mass tort litigation, but they are not necessarily understood, or accepted, by non-lawyers. Some critics of the litigation argue that plaintiff attorneys and experts have not proven that a given plaintiff’s case of NHL was caused by his or her use of Roundup. In doing so, they impose on the proceedings an evidentiary and scientific threshold beyond that required by law. It is surely fair to question whether the level and burden of proof applicable in ongoing Roundup–NHL litigation is proper, but that debate should be pursued in a forum other than a courtroom in which a given Roundup–NHL case is tried.
In the spring of 2015, approximately 695,000 Americans were living with NHL,Footnote 13 and an estimated 74,200 will be newly diagnosed with the disease in 2019.Footnote 14 A substantial number of the individuals with NHL no doubt read or were told about the IARC’s classification of GBHs and glyphosate as “probable human carcinogens”. Some of these people likely recalled applying Roundup in the years prior to their diagnosis and consulted an attorney to explore whether the specifics of their case warranted joining the litigation. Two key factors taken into account in initial case reviews were the extent of a plaintiff’s use of Roundup and likely exposure levels, and the presence/absence of other known NHL risk factors.
Several law firms with experience in toxic chemical, mass tort litigation recognised the significance of the IARC’s classification decision and began assessing whether there was a plausible evidentiary and legal basis to pursue litigation against Monsanto, the sole manufacturer of GBHs under the Roundup brand from the mid-1970s through the early 2000s. Monsanto remained the major GBH manufacturer after the entrance of several basic and me-too manufacturersFootnote 15 into the GBH market in the 2000s. Bayer acquired Monsanto in June 2018, and today Bayer/MonsantoFootnote 16 is still by far the dominant player in the GBH market in the USA and globally.
The first Roundup–NHL case was filed by Enrique Rubio on 22 September 2015 in a US District Court in California. Three other early plaintiffs, Joselin Barrera, Elisa de la Garza and Judi Fitzgerald, filed their cases in Delaware in October 2015.Footnote 17 Since few firms had the resources to take on litigation of this scope and expense on their own, a group of law firms began the process in 2016 of coordinating the key tasks required by the litigation. This allowed the firms to share the technical burden and costs of pursuing litigation against then-Monsanto on behalf of individuals with NHL.
1. Outcomes of the first three trials
a. Johnson v. Monsanto
Three trials in this area have been completed to date; each is briefly discussed herein. The case that would be the first to come to trial was filed on 28 January 2016 by the Miller Firm in California State Court on behalf of Dewayne Johnson, a school district groundskeeper.Footnote 18 The Johnson case trial started on 9 July 2018 and was argued by Brent Wisner, an attorney working for the Baum Hedlund law firm based in Los Angeles, California. I was among the expert witnesses testifying on behalf of Mr Johnson. On 10 August 2018, the jury found in favour of Mr Johnson and awarded US$39.2 million in compensatory damages and US$250 million punitive damages (see the trial transcript and official jury form for the questions placed to the jury by the judge and the jury’s answersFootnote 19).
b. Hardeman v. Monsanto
Concomitant with the progression of the Johnson case through pre-trial motions, trial scheduling, jury selection and the trial itself, two other cases were moving through similar steps towards trial in other California courts. The Edwin Hardeman case was the first scheduled for trial as part of federal-district court multiple-district litigation (MDL) overseen by Judge Vincent Chhabria in San Francisco.Footnote 20 On 17 November 2016, Judge Chhabria named Weitz & Luxenberg, the Miller Firm and Andrus Wagstaff as the plaintiff’s co-lead counsel for the MDL. These co-lead counsel firms were joined by three additional firms on an MDL case management executive committee. The role of the lead counsel and executive committees was to oversee and coordinate the work of the plaintiff law firms and interactions with Judge Chhabria’s court as the cases within the MDL moved forwards.
Judge Chhabria decided to split the Hardeman trial into two phases: Phase 1 focused just on the plausibility of Roundup–NHL causality. Due to the limited focus of Phase 1, plaintiff attorneys were not allowed to introduce any documents or present any expert testimony on Monsanto’s behaviour, conduct and actions relative to studies presented to the EPA, or Monsanto-supported studies and reviews published in peer-reviewed journals. The judge’s stated goal was to ensure that the jury made its decision regarding Roundup–NHL causality based only on scientific evidence and without prejudice from information or testimony regarding Monsanto’s behaviour and efforts to shape regulatory decisions or policy.
The Hardeman Phase 1 trial began on 25 February 2019 and went to the jury on 14 March 2019. The Phase 1 verdict in favour of Mr Hardeman came on 19 March. On the next day (20 March), Phase 2 commenced, focusing on Monsanto’s behaviour and compensatory and punitive damages. After just a week, the second phase of the trial was completed and the case went to the jury. Their verdict and award were announced by Judge Chhabria on 27 March 2018.
The jury awarded Mr Hardeman US$5.1 million in compensatory damages and US$75 million in punitive damages. In response to a post-verdict motion by Bayer/Monsanto attorneys, Judge Chhabria modestly altered the compensatory damages award and reduced the punitive damages to US$20 million, for a total award to Mr Hardeman of US$25.3 million. In allowing US$20 million in punitive damages to stand, Judge Chhabria noted Monsanto’s efforts to shield the EPA from data raising new questions about Roundup’s safety and the company’s aggressive efforts to influence scientific discourse and the glyphosate-focused content of peer-reviewed journals.
Post-Hardeman, potential plaintiffs and law firms became more confident of the scientific merits of the litigation. As a result of the Johnson and Hardeman verdicts and awards, the number of cases filed rose steadily in 2018 and 2019 from a few thousand in 2017, to around 4500 at the time of the Johnson verdict in mid-2018, to over 42,000 in November 2019, as shown in Figure 1.
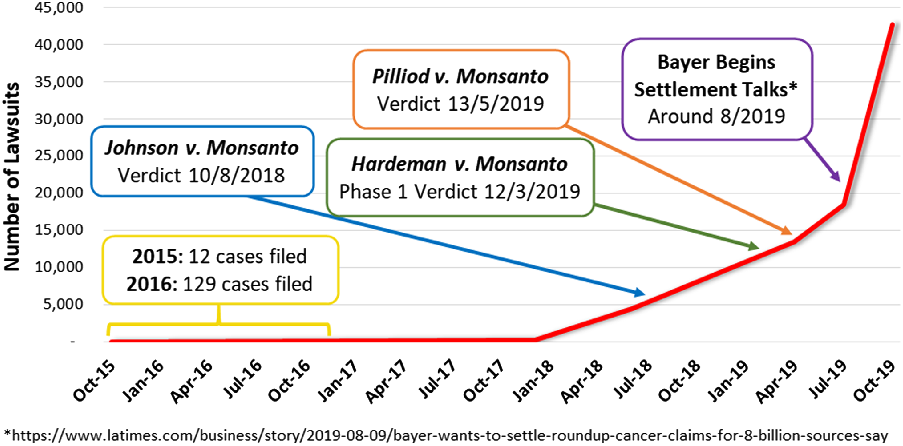
Figure 1: Non-Hodgkin lymphoma lawsuits, milestones and trends.
c. Pilliod v. Monsanto
Just weeks after the conclusion of the Hardeman federal MDL trial, a third case in California State Court began. Alva and Alberta Pilliod had both been diagnosed with the same form of NHL.Footnote 21 This married couple had sprayed Roundup several days per year on their rural California properties for over two decades. They often walked together, each with a handheld sprayer, spot-spraying berry bushes and other weeds and vegetation choking the trails they had created and maintained. On some occasions, they wore shorts and flip-flops as they sprayed Roundup on encroaching weeds along trails.
Judge Winifred Smith presided over the Pilliod trial. The plaintiff attorneys presented their case in about one month, beginning on 28 March 2019. The defence attorneys presented their case in about one week. Jury deliberations began on 5 May 2018, and the verdict and monetary awards were announced on 13 May. The Pilliods received a stunning punitive damage award of US$2 billion dollars. In response to post-trial motions, Judge Smith reduced the combined (Alva and Alberta) punitive damage award to US$69 million, citing Supreme Court guidance that classifies any punitive damage award greater than seven times the corresponding compensatory damages award as excessive and likely in need of judicial review and adjustment.
2. Why such large awards?
In her post-trial order, Judge Smith set forth what she regarded as part of the justification for a still significant US$69 million punitive damage award:
In this case there was clear and convincing evidence that Monsanto made efforts to impede, discourage, or distort scientific inquiry and the resulting science.Footnote 22
Comparable sentiments had been voiced earlier by Judge Curtis Karnow of the Superior Court of San Francisco County, California. He presided over several pre-trial motions setting forth the ground rules governing the Johnson trial. In his 17 May 2018 order in response to multiple pre-trial motions, Judge Karnow wrote:
The internal correspondence noted by Johnson could support a jury finding that Monsanto had long been aware of the risk that its glyphosate-based herbicides are carcinogenic, and more dangerous than glyphosate in isolation, but has continuously sought to influence the scientific literature to prevent its internal concerns from reaching the public sphere and to bolster its defenses in products liability actions.Footnote 23
Table 1 provides an overview of the compensatory and punitive damage awards in the above three trials as initially awarded by the jury, and later adjusted by the trial judges. The bottom two lines in Table 1 provide average compensatory, punitive and total damage awards per plaintiff, highlighting the sizable reduction in the punitive damage awards from an average of US$581 million per plaintiff by juries to US$32.2 million after adjustments by the three judges.
Table 1. Overview of compensatory and punitive damages in non-Hodgkin lymphoma–glyphosate cases
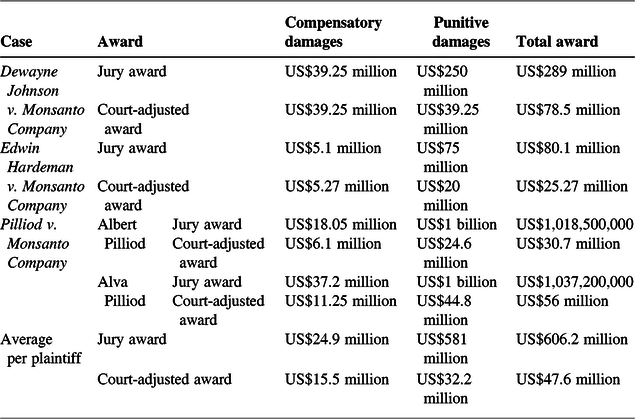
On average, across the first four plaintiffs whose cases have gone to trial, the total awards have been US$47.6 million per person. After reductions by the three judges, the average ratio of punitive-to-compensatory damage awards is about 2:1, well below the threshold of 7:1 set by the Supreme Court for review of punitive damage awards.
II. Impact of Roundup–NHL litigation on GBH risk assessment science
From the mid-1970s through early 2015, few scientists, doctors, pesticide users, regulators and environmentalists were concerned about Roundup use, exposures and risks to the general public. It was widely stated by industry experts that Roundup breaks down in the environment naturally to harmless, ubiquitous elements. Despite very limited testing of food for glyphosate residues by the US government,Footnote 24 there was no reason to expect the presence of glyphosate in food above very low, rarely detectable levels mostly from drift (except for raw soybeans). This is because the herbicide could only be applied before crop germination – and hence months before the edible portion of a crop is formed and ready for harvest. The frequency and levels of glyphosate residues in certain foods changed with the registration of pre-harvest crop desiccation uses of GBHs in the 1980s and 1990s and genetically engineered Roundup Ready (RR) crops in the second half of the 1990s. Since the mid-2000s, glyphosate dietary exposures have been common, and levels have been rising in the urine of Americans.Footnote 25
In addition, in the early 1980s, the EPA set a very high chronic reference dose (cRfD; also known as an “acceptable daily intake” (ADI) in much of the rest of the world) of 1.75 mg/kg/day.Footnote 26 At the time, only two widely used pesticide active ingredients had higher cRfDs (the imidazolinone herbicides imazapyr and imazethapyr had cRfDs equal to 2.5 mg/kg/day).Footnote 27 This means that the EPA then viewed glyphosate as less chronically toxic than all but two currently registered pesticides for which a cRfD had been established.
The IARC determination in March 2015 and the ensuing litigation triggered the first in-depth and independent assessment of Roundup/glyphosate uses and exposures in relation to several important risk factors:
-
Toxicity;
-
Mixer-loader and applicator dermal absorption and exposures;
-
Glyphosate absorption, distribution, metabolism and excretion;
-
Impacts of the surfactants in GBH formulations on toxicity and absorption;
-
The capacity of GBHs to damage DNA in ways that might lead to cancer, and especially NHL.
By “independent” assessment in the context of this litigation, I mean one that is carried out by an experienced and well-trained scientist who does not currently work for a GBH registrant and has not been significantly dependent on funds from a pesticide manufacturer during prior stages of their career. Both the IARC assessment and the reports and testimony by experts working on behalf of plaintiffs meet these criteria. So, too, do some of the experts who have worked for defendant attorneys in the Roundup–NHL litigation. Experts working for plaintiff attorneys are paid for the time invested in their work, but their careers have generally not been shaped by or been dependent on sustaining a mutually rewarding professional and financial relationship with a company with a major stake in the outcome of litigation.
In addition, the scientific work and insights gained by plaintiff experts were informed and shaped by a unique aspect of this litigation: access via the discovery record to Monsanto’s internal discussions of scientific concerns, risk assessment issues and findings from Monsanto-conducted proprietary research.
The record contains thousands of emails discussing what Monsanto could have done, should have done and eventually did in response to newly published data or their own internal studies that might alter regulatory risk assessments and lead to new restrictions on Roundup use. The issues and concerns regarding GBH human health risks that Monsanto has focused on for decades include elevated tumours in animal bioassays, dozens of positive genotoxicity assays, problems with published epidemiological studies reporting an association between Roundup exposures and cancer risk, the impact of GBH surfactants on applicator exposures and risk and the appropriate rate of dermal absorption to use in applicator exposure assessments. The way Monsanto responded to new science and risk concerns helped plaintiff experts identify possibly consequential scientific issues to focus on. Differences between what Monsanto scientists said in internal email chains about possible risk concerns compared to what Monsanto told regulators and/or published in peer-reviewed journals received substantial focus in plaintiff expert reports and trial testimony.
Over time, more discovery documents will be unsealed. When studied in conjunction with the already available and extensive body of both plaintiff and defendant expert reports, deposition transcripts, transcripts of trial testimony and trial exhibits, other scientists and the public will have the same opportunity as plaintiff experts to follow Monsanto’s evolving knowledge about Roundup and GBH risks. Those that do so can then reach their own conclusions regarding what the company did and did not do to steward the use of Roundup and sharpen the accuracy of Roundup risk assessments.
1. Insights abound in the massive discovery record
Several million documents have been turned over to the courts by Monsanto in response to queries from plaintiff attorneys, including millions that no regulator or member of the public has seen. Some documents shed light on what Monsanto knew, and was concerned about, regarding Roundup toxicity and exposures, while others outline and track the impact of the often elaborate efforts undertaken by the company and its surrogates to undermine published science, and scientists, questioning Monsanto’s view of Roundup safety.
Because the majority of these documents remain under seal, it is not possible to cite and discuss their content. However, many of the significant documents have been unsealed and are available from several sources.Footnote 28 These documents include:
-
Essentially all official communications between Monsanto and the EPA over the life history of Roundup, including Monsanto’s internal speculation regarding the basis for new risk concerns raised by regulators and how to assuage such concerns.
-
The results of Monsanto-conducted or commissioned studies on glyphosate or Roundup, including internal studies and preliminary reports never disclosed to regulators (including some drafts of study reports containing more worrisome results than final reports).
-
Thousands of documents addressing Monsanto’s marketing strategies, efforts and materials.
-
Monsanto efforts to influence the flow of information to the scientific community and attitudes and opinions regarding Roundup safety among scientists, occupational health specialists, the media, politicians and farmers.
-
Stewardship policies, programmes and commitments in terms of product safety and worker health, including Monsanto’s input during the evolution of the FAO Code of Conduct for pesticide manufacturers and the company’s obligations as a signatory.
-
Internal discussions and debates among Monsanto scientists, the company’s regulatory officials and corporate leaders over what the company should do in response to new, negative information in published studies and/or new concerns raised by regulators (usually European regulators, especially since the early 1990s) or scientific bodies (eg the IARC).
Until the four lead law firms began hiring experts in the science areas central to the Roundup–NHL litigation, few if any independent scientists and physicians in the USA had ever had the funding, time or access to data needed to carry out an in-depth assessment of what was known about Roundup-induced human health risks. During 2016–2018, plaintiff attorneys and experts benefited greatly from access to the discovery record.Footnote 29 Individuals working for the lead law firms conducted systematic topical searches, often at the request of plaintiff experts, and then shared relevant documents with the experts working in a given area.Footnote 30
A set of revealing Monsanto documents and internal email exchanges have been identified that discuss the concerns of Monsanto scientists and regulatory officials arising from both the company’s internal studies and published science in peer-reviewed journals. Many have been unsealed by judges, and they are often referred to as the “Monsanto Papers”.Footnote 31 These documents include internal Monsanto exchanges that address possible risk-driven problems emerging in ongoing US EPA and European glyphosate re-registration reviews. Some of these discussions date back to the early 1980s and primarily focus on adverse glyphosate and GBH health and safety data and cancer risk.
Some key milestones in the history of GBH risk assessment addressed in the “Monsanto Papers” have been:
-
The EPA’s conclusion in 1984 that a Monsanto-commissioned mouse study showed a statistically significant increase in a rare tumour (renal tubular adenomas) in male mice, leading to an Office of Pesticide Programs – Toxicology Branch-recommended “possible human carcinogen” classification of glyphosate that remained in place from 1984 through 1991.Footnote 32
-
Publication of multiple peer-reviewed papers beginning in the 1990s reporting glyphosate and/or GBH genotoxicity.Footnote 33
-
Epidemiological studies reporting statistically significant linkages between GBH use, exposures and the risk of cancer, especially NHL.Footnote 34
Each of the above milestones triggered concerted efforts by Monsanto to raise questions about the design, conduct and interpretation of studies reporting a possible new risk concern following exposure to Roundup. Such efforts often lasted for years, even decades. Several have played out multiple times, such as in the back-and-forth following the March 2015 IARC classification, and most recently in the course of each of the three trials. Common examples of Monsanto actions taken to raise doubts about adverse data include citing inappropriate tumour historical control data,Footnote 35 arguing that a tumour observed in a cancer bioassay was not treatment related for specious reasons in violation of EPA cancer risk assessment guidelinesFootnote 36 and dismissing a positive result in an in vivo genotoxicity assay because of an inappropriate method of administration of the test substance or excessively high dose rates.
But unlike all past venues and cycles in which glyphosate and GBH health impacts have been discussed, the US Roundup–NHL litigation has been the first time that the rules of scientific engagement were set by courts to ensure an equal opportunity by plaintiff and defence lawyers and experts to present their best cases possible. The judges overseeing each of the trials rigorously enforced evidentiary and equal time rules.
These trials marked the first time scientists independent of GBH registrants and regulators had the opportunity to assess such an extensive portion of the full record of glyphosate risk assessment science, including Monsanto concerns in the wake of negative study results or new questions from regulators. Other documents reviewed by plaintiff experts and presented to the juries describe Monsanto plans and actions to undermine confidence in any published finding that raised a new or more serious risk concern over Roundup use and exposures. Significantly, the record convincingly shows that Monsanto’s near-universal response to new adverse data was to attack the messenger and the science, rather than to conduct new and more sophisticated studies capable of dismissing or confirming – and possibly quantifying – potential new areas of risk.
In short, the Roundup–NHL litigation has provided the venue and funding for the first open, rigorous and data-driven assessment of Roundup–NHL risks conducted by scientists with roughly equal access to and familiarity with the pertinent evidence. Each of the plaintiff experts responsible for addressing a critical scientific or medical area – NHL diagnosis and treatment, toxicology, epidemiology, genotoxicity, animal bioassays and risk assessment – spent hundreds if not thousands of hours in the course of preparing for and participating in the first three trials.
For each expert on both sides of the litigation, the process started with preparation of often near-book-length expert reports answering questions put to them by counsel.Footnote 37 Each expert was then deposed under oath by Bayer/Monsanto or plaintiff attorneys, who had the right to review and question the basis of any factual statements or opinions offered in an expert’s report. Such videotaped depositions typically lasted a day, but some spanned up to three days. Over the last three years, most plaintiff experts have been deposed eight or more times, beginning with their pre-Johnson trial deposition. Experts must defend their methods and analytical procedures, and they typically are asked to explain any deviations from the methods preferred and used by Monsanto scientists and/or regulators. Often, plaintiff experts are asked to explain why an alternative analysis or judgement is wrong (eg IARC versus EPA on genotoxicity). Whenever possible, attorneys attempt to back experts into a corner in order to generate testimony that can be used at trial to characterise the expert’s methods and analysis as outside of the scientific mainstream and purposefully biased. But importantly, everyone involved in the process knows that if such an attempt is made at trial, the expert whose work is called into question will have an opportunity to respond and defend his or her work.
In my work on this litigation, I concluded early on that the differences in the IARC and EPA analyses of the GBH and glyphosate genotoxicity databases were critical to understanding why the IARC and EPA reached such different cancer classification decisions. My first expert report stated this conclusion and set out why I had reached this opinion. During my first depositions, Monsanto attorneys allotted a significant share of their time to questioning me about this opinion. For example, after putting a proprietary genotoxicity study in front of me, I would be asked something like, “Dr Benbrook, are you familiar with this study?” Often, the answer was “no”. The next series of questions might repeat this question-and-answer process on several more internal genotoxicity studies that I had not seen or reviewed. Then I would be asked something like, “Now that you are aware of these x number of studies, do they change your opinion?”
Questions of this sort are clearly legitimate and I should have been able to answer them. For this reason, I studied in greater detail the full body of genotoxicity assays available on glyphosate and GBHs and conducted an in-depth comparison of the EPA’s and IARC’s assessments of the relevant genotoxicity and mechanistic data. The results of my ongoing analytical work in this area were explained in my second and third expert reports, filed respectively as part of the Hardeman and Pilliod cases. My analysis of this component of the glyphosate and GBH cancer classification decision process served as the basis for a paper I wrote on this topic and published in Environmental Sciences Europe.Footnote 38
Some of the experts in the Roundup–NHL litigation had, furthermore, been involved with glyphosate and GBH use, regulation and risk issues for many years, if not decades. For example, in 1982–1983, I served as the Staff Director for a Congressional subcommittee with jurisdiction over federal pesticide law and regulation. Roundup was among the pesticides that received considerable attention by the subcommittee. Glyphosate and Roundup oncogenicity, risk assessment, use and regulation have continued to arise throughout my career. Dr Christopher Portier, the plaintiff expert addressing in detail the results of mouse and rat cancer bioassays, has also been involved with Roundup/GBH oncogenicity over several decades.Footnote 39 Other examples could be cited among both defence and plaintiff experts.
Throughout the trials, the first challenge facing both attorneys and experts was explaining to juries the basic concepts, tools and approaches used to assess pesticide risk. Their second task was to explain the relevance and reliability of a given study’s reported findings. Last, they had to knit together multiple study results and lines of evidence into a hopefully coherent and persuasive “weight of evidence” judgement regarding the potential link – or lack thereof – between exposure to Roundup and NHL.
III. The case presented to juries
One of the core questions addressed throughout all three trials was whether the EPA was right in its judgement that glyphosate is unlikely to pose oncogenic risk,Footnote 40 or whether the IARC was correct in reaching its “probable human carcinogen” classification.Footnote 41 Over several weeks, juries heard plaintiff and defendant experts explain and critique the results and implications of the extensive animal cancer bioassay database (14 roughly 2-year studies, 6 in mice and 8 in rats), the hundreds of genotoxicity assays conducted on glyphosate and GBHs and the epidemiological database composed of over a dozen cohort studies, case–control studies and meta-analyses.
Monsanto experts presented testimony generally praising the design and conduct of studies that supported their assertion of no credible or persuasive data supporting a link between glyphosate or GBH exposures and NHL.Footnote 42 In the case of dozens of studies that produced data supportive of a possible link between GBH use and NHL, they focused their testimony on perceived flaws in study design, inappropriate dosage levels or routes of administration, inappropriate statistical inferences, sources of bias or other criticisms. Consistently, Bayer/Monsanto attorneys and experts cited the views of regulators on specific studies and findings, but only when such views were aligned with Monsanto’s views.
Plaintiff experts addressed the findings of most of the same studies analysed by the Monsanto team. Often plaintiff experts acknowledged the existence of study flaws pointed out by Monsanto experts, but argued that reliable findings and/or inferences could still be derived from a given study.Footnote 43 They also often highlighted the number and diversity of studies in each key area (animal bioassays, genotoxicity and epidemiology) reporting statistically significant results supportive of a Roundup–NHL link. They also pointed out that both Monsanto and the EPA deviated from applicable EPA cancer risk assessment policies and guidelines in advancing various reasons to dismiss dozens of positive results (eg historical controls, too-high doses, inappropriate statistical tests, errant histopathology).
During trial testimony, juries heard about an EPA Scientific Advisory Panel (SAP) convened in December 2016 to review the EPA’s and the IARC’s reviews of the glyphosate and GBH cancer data. The purpose of the SAP meeting was to shed light on why the EPA and IARC reached such different conclusions. After three days of detailed presentations and debate, the SAP members came to consensus on few important conclusions. One unanimous conclusion was that the EPA had not followed its own cancer risk assessment guidelines in its review of glyphosate oncogenicity.Footnote 44 Key deviations addressed by the SAP occurred in the EPA’s assessment and use of historical control data, requiring a positive tumour response in both male and female treatment groups to regard a statistically elevated tumour in one sex as biologically relevant, a variety of issues arising around the presence/absence of a maximum tolerated dose and whether dozens of tumour types that were elevated in treatment groups based on linear statistical tests but not under pairwise comparisons were “treatment related”.
Nearly all of the 14 cancer bioassays produced statistically elevated levels of one or more tumours in one or both sexes.Footnote 45 Each of the six mouse studies reported evidence of treatment-related lymphatic tumours. Some of the rat studies revealed elevated levels of carcinomas. Most published epidemiology studies reported an elevated risk of NHL associated with more frequent GBH use, as did all of the meta-analyses discussed during the trials. Dozens of published genotoxicity assays were reviewed that reported one or more positive assay, including a small number of important positive studies in exposed human populations.
Throughout the trials, Bayer/Monsanto lawyers asserted that there was no evidence supporting a link between exposure to Roundup and NHL, despite the fact that experts for both plaintiffs and the defence had spent weeks discussing hundreds of discrete study findings supporting a possible role of Roundup in the initiation and/or progression of NHL. Explaining away such a diversity of experimental results supporting a role of GBH exposures in the plaintiffs’ NHLs was among the hurdles faced by the Bayer/Monsanto trial teams.
1. Differences in the EPA and IARC assessments of GBH exposures and oncogenicity
Beyond the EPA versus IARC interpretations of specific GBH–NHL relevant study findings, there were several other factors and considerations described to the three juries that likely played a role in tipping the weight of evidence:
-
1. The failure of Monsanto and the EPA to acknowledge or address the known higher risks following exposures to formulated GBHs compared to exposures to pure technical glyphosate.
-
2. The mostly registrant-generated genotoxicity studies and data relied on by the EPA, in contrast to the IARC’s reliance on genotoxicity studies published in peer-reviewed journals, including a significant number of studies focused on GBHs instead of just technical glyphosate.
-
3. The significant differences that exist in the levels of applicator GBH exposures among people applying Roundup via a handheld wand (as is the case with the first three sets of plaintiffs), in contrast to the operators of a modern pesticide sprayer with a glass–steel cab and air filtration system.
-
4. The virtual absence of requirements for personal protective equipment (PPE) on Roundup labels (including, for example, “wear gloves when mixing and loading or applying this product”).
-
5. The importance of multiple exposure episodes per year, over multiple years, including a certain percentage of high-exposure episodes caused by application equipment problems, leaky hoses and valves, spills, wind, spray patterns and equipment clean-up and repair.
Regarding Factor 1, substantial published data presented at trial support the conclusion that formulated GBHs are more toxic than pure technical glyphosate, especially GBHs containing polyethoxylated tallowamine (POEA)-based surfactants.Footnote 46 Multiple internal Monsanto emails express the same conclusion, and they are cited and accessible via expert reports and trial testimony and exhibits. Nearly all GBHs sold in the USA by Monsanto since the mid-1970s have contained POEA-based surfactants, which have been phased out in GBHs in Europe due to safety concerns.Footnote 47
The most important reason why the POEA-based surfactants in US Roundup products are more toxic than technical glyphosate is the proclivity of the POEA in Roundup to increase dermal (and weed leaf) absorption rates. Once through the human epidermis, POEA-based GBHs also promote the movement of glyphosate through cell membranes, where it can then damage DNA via oxidative stress or some form of chromosomal or genetic aberration, including possibly epigenetic changes.Footnote 48
Regarding Factor 2, the EPA’s assessment of the genotoxicity of Roundup and other GBHs was limited predominately to unpublished, registrant-submitted studies. Some 94 of the 95 registrant-submitted glyphosate and GBH genotoxicity assays were negative.Footnote 49 Nearly half were reverse bacterial mutation studies (ie Ames tests) unlikely to produce positive results because bacteria lack mitochondria, and hence are not subject to oxidative stress caused by alterations in mitochondrial function, as is the case within mammalian cells.Footnote 50
The IARC, however, focused on a much larger and more diverse body of published genotoxicity assays. Nearly 76% of the 191 assays reviewed by the IARC reported one or more positive genotoxicity response. The dramatic differences in the genotoxicity databases relied on by the EPA and IARC are summarised in Figures 2 and 3, and they explain in large part why the EPA and IARC reached such different conclusions in the case of glyphosate and GBH genotoxicity. Incidentally, over two dozen genotoxicity studies conducted with glyphosate, GBHs or both have been published since the EPA and IARC conducted their reviews; all but one reported one or more positive assay.Footnote 51
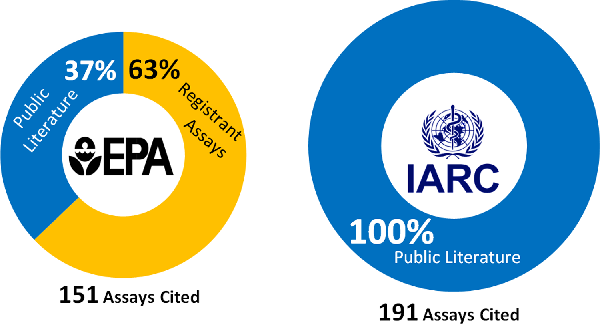
Figure 2: Number and type of assays cited by the US Environmental Protection Agency (EPA) and the International Agency for Research on Cancer (IARC).

Figure 3: Number of regulatory versus public literature assays cited by the US Environmental Protection Agency or the International Agency for Research on Cancer with one or more positive result for genotoxicity.
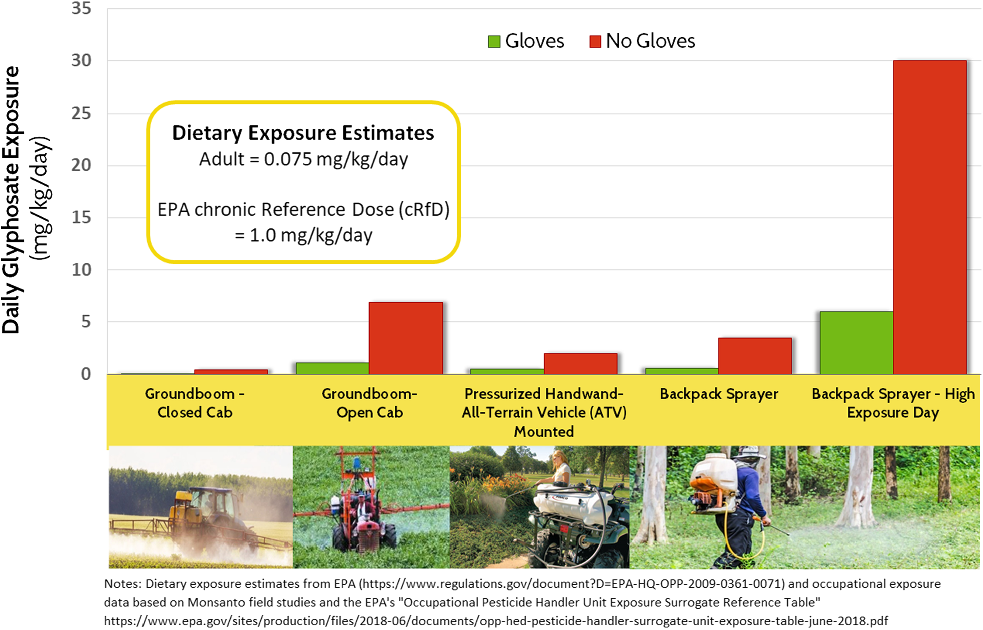
Regarding Factor 3, the absence of high-quality mixer-loader and applicator exposure studies that compare various types of application equipment and varying combinations of PPE is one of the most glaring deficiencies in the overall glyphosate and GBH database. Exposure estimates cited or made by experts during the first three trials relied predominantly on the predictive operator exposure model (POEM)Footnote 52 and a Monsanto-commissioned UK field study that partially relied on POEM simulations. The UK study estimated exposures to different parts of the body per hour of spraying by application method and type of equipment, by rate of application and conditions during the spray application, and in the presence/absence of PPE. It compared estimated exposures following six hours of spraying relative to levels of exposure regarded by UK regulators as acceptable. Multiple scenarios resulted in applicator exposures well over acceptable limits, especially when applications were made without gloves.
The exposure assessment branch of the EPA’s Office of Pesticide Programs has developed detailed surrogate reference values for worker exposures based on type of application, methods of application and PPE.Footnote 53 While it is an imperfect basis for quantifying mixer-loader and applicator exposures, crude approximations of exposures to glyphosate following a workday across several common application scenarios can be calculated. Figure 4 compares simulated occupational exposures based on different application equipment and scenarios, noting as well the EPA’s most recent estimate of adult dietary exposures to glyphosate and acceptable daily exposures (glyphosate’s cRfD).Footnote 54
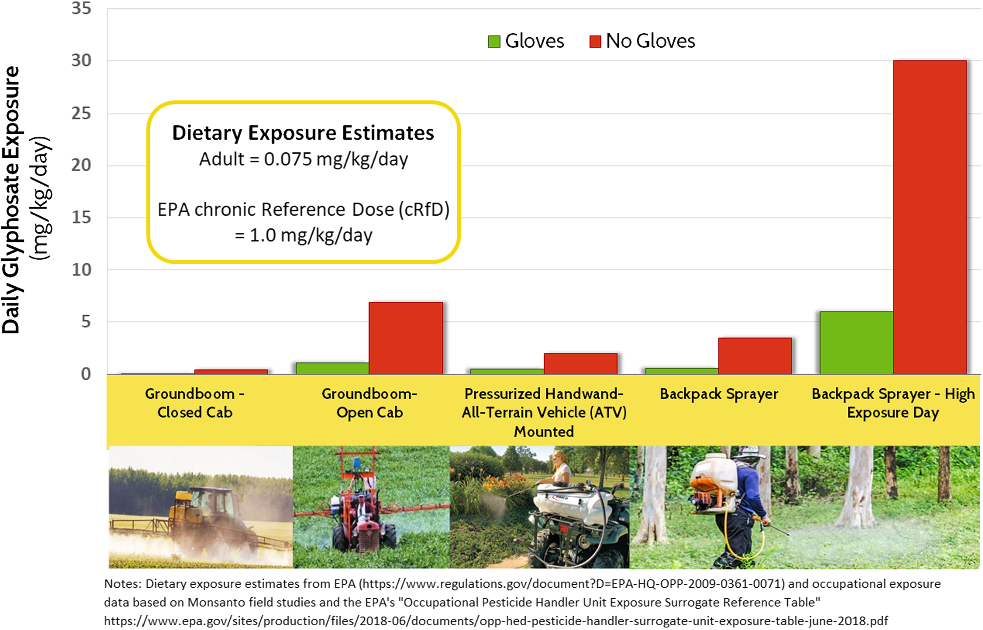
Figure 4: Rough estimates of glyphosate dietary and occupational exposures (see notes). EPA = Environmental Protection Agency.
Some insights are clear: handheld and backpack sprayers result in far higher exposures than in the case of tractors or spray rigs with glass–steel cabs and air filtration systems. On days when something goes wrong while applying a GBH through a handheld wand (a leaky valve, gusts of wind, pinhole leaks in a hose, excessive application rate) exposures can be vastly higher.
Regarding Factor 4, the EPA’s glyphosate oncogenicity risk assessment does not encompass, nor address high-exposure occupational scenarios. It does not take into account the added risks stemming from repeated use of a GBH during a spray season, nor users who spray for several hours per day. It does not consider the added risks facing people who use – and are exposed to – a GBH for many continuous years.
In addition, current glyphosate and GBH risk assessments do not take into account nor address several factors that can markedly heighten an individual’s GBH-related risks. Individuals with a compromised immune system or battling another form of cancer should exercise added caution when applying a GBH, or they should avoid occupational exposures altogether. Pregnant women should also take added precautions to avoid any exposure to a GBH, and indeed exposures to most pesticides. Such contraindications are common on drug labels, and for good reasons. Should these same reasons apply occupational exposures to pesticides and associated label precautions?
IV. Implications for pesticide use, risk assessment and regulation
Intense global focus on glyphosate and GBH uses, exposures and risks has brought into sharp relief systemic shortcomings in pesticide hazard assessment, how exposures and risks are quantified and how excessive or rising risks are mitigated. The science base supporting the quantification of applicator and occupational exposures, and especially those using handheld wands to direct GBH sprays, is profoundly deficient. The uncertainty embedded in current GBH applicator dermal exposure estimates is a striking example of how both the public and private sectors have failed to produce credible, real-world exposure and risk estimates.
There is growing agreement among independent pesticide risk assessment scientists, physicians and occupational health experts that pesticide product labels should disclose fully all of the ingredients in pesticide formulations. For example, Mesnage etal (2019)Footnote 55 advanced multiple reasons why it is time to drop “Confidential Business Information” (CBI) protection of co-formulants, arguing that the need for policies and science advancing public and environmental health is more compelling than the need for pesticide formula CBI protection. Other policy tools and interventions can be deployed to reward innovation in pesticide formulation chemistry.Footnote 56
Pesticide manufacturers and their supporters argue that it is not feasible to put all pesticide formulations through a full battery of toxicological studies. I agree. But it would be feasible and beneficial to subject two or three distinctly different but common formulations of high-volume pesticides to genotoxicity, sub-chronic and, in some cases, chronic toxicity testing. When an initial set of such studies produces no basis for added concern, no further testing of similar formulations would be required. But when different formulations produce significantly different results, steps should be taken by industry and regulators to phase out higher-risk formulations, while continuing to invest in the search for lower-risk formulations.
Today’s human health and environmental impacts stemming from agricultural GBH uses and the emergence and spread of GBH-resistant weeds are inextricably bound to the scope and frequency of GBH uses. Impacts on monarch butterfly habitats, aquatic ecosystems, beneficial insect habitats, pollinators and human health are not linear relative to pounds of active ingredient applied in a given watershed or region. The scale and frequency of use of pesticides must become a variable that is considered by regulators when re-registering pesticides that have gained substantial market share. It is ridiculous to continue basing pesticide regulation on the myth that if one acre of wheat in Kansas can be safely treated with Pesticide X, then all acres of wheat in the state – or the world – can also be treated safely, year in and year out.
Political leaders and regulators should consider the merits of a simple, clear change in policy: pesticide companies and farmers must find ways to use herbicides such that residues in foods as eaten are rare, and, when detected, very low. In the case of many insecticides and fungicides, such a universal “no residues in food” goal is not achievable without significant costs and at least short-term disruption of pest management systems. But it surely is readily attainable in the case of herbicides, most of which are applied very early in the crop production cycle.
Glyphosate residues could be essentially eliminated from human foodstuffs by ending all pre-harvest crop desiccation uses, in conjunction with some additional restrictions on labelled GBH uses on RR crops. Collectively, such dietary exposure-driven limits and risk mitigation measures would likely have a modest impact on the role of GBHs in agriculture, including in RR weed management systems, and no impact on applicator exposures and risk among those applying a GBH with handheld or small-scale equipment. Regulation-driven efforts to slow the emergence and spread of glyphosate-resistant weeds may eventually be put in place and, if meaningful, would impact GBH use more significantly than restrictions stemming from the need to get glyphosate out of the human food supply.
Last, the regrettable history of GBH testing and regulation points to the need for a much-expanded role for independent science – and scientists – in conducting studies that are essential to the pesticide risk assessment process. Monsanto seems to have an uncanny ability to design and conduct studies that are almost always negative using the same experimental methods that frequently result in positive studies when done by independent scientists. This highlights the need for greater diversity in who conducts the basic studies supporting the pesticide regulatory decision-making process.