Rotaviruses (RV) are double-stranded RNA viruses of the family Reoviridae, which are the most common viral agents causing viral gastroenteritis and diarrhoea in infants and young children worldwide. Each year, approximately 1·4 billion episodes of RV gastroenteritis (RVGE) occur in children under 5 years of age in developing countries, and half a million children die( Reference Parashar, Burton and Lanata 1 , Reference Tate, Burton and Boschi-Pinto 2 ). Vaccination is the main public health intervention to prevent RV infection. Systematic reviews of vaccine effectiveness and vaccination-impact studies in industrialised countries (USA, Europe and Australia) have demonstrated an effectiveness of 85–100 % associated with decreased hospitalisations for RVGE( Reference Giaquinto, Dominiak-Felden and Van Damme 3 ). Vaccination-impact studies have demonstrated that the burden of RVGE has been reduced significantly since the introduction of RV vaccination( Reference Giaquinto, Dominiak-Felden and Van Damme 3 ). However, efficacy trials in developing countries in Africa and Asia showed that vaccine efficacy was lower than that observed in other countries, typically 40–70 %( Reference Cherian, Wang and Mantel 4 ). Although the efficacy of RV vaccines correlates closely with the national per capita income( Reference Holmgren and Svennerholm 5 ), it is unclear why vaccination is less efficacious in developing countries( Reference Perez-Schael, Salinas and Tomat 6 ). This reduced vaccine efficacy coupled with the high cost and barriers to a widespread distribution of RV vaccines( Reference Clemens 7 ) suggest that other means for preventing RV should continue to be investigated.
Human milk (HM) probably contains anti-RV components, as there is evidence that breast-fed infants have a lower incidence of diarrhoeal diseases compared with formula-fed infants( Reference Plenge-Bönig, Soto-Ramírez and Karmaus 8 , Reference Newburg, Ruiz-Palacios and Morrow 9 ). Furthermore, HM contains a large number of functional components that actively protect the infant from pathogenic infection and facilitate the development of a beneficial microbiota( Reference Donovan 10 ). A unique aspect of HM is the particularly high concentration (5–12 g/l) of oligosaccharides (HMO)( Reference Morrow and Rangel 11 ). HMO have complex carbohydrate structures derived from twelve core structures made up of glucose, galactose and N-acetylglucosamine, with a lactose unit at the reducing end and fucose or sialic acid (SA) at the non-reducing end( Reference Morrow and Rangel 11 , Reference Kunz, Rudloff and Baier 12 ). Epithelial cells express specific glycans that act as receptors for specific pathogens( Reference Martin-Sosa, Martin and Hueso 13 , Reference Morrow, Ruiz-Palacios and Jiang 14 ). It has been shown that sialylated oligosaccharides inhibit the attachment of uropathogenic Escherichia coli strains( Reference Martin-Sosa, Martin and Hueso 13 ) to the host cells, and fucosylated HMO are associated with the protection of breast-fed infants against diarrhoea caused by Campylobacter ( Reference Ruiz-Palacios, Calva and Pickering 15 ). Due to the structural similarities of HMO to cell-surface glycans, HMO could serve as enterocyte receptor analogues for pathogens, and competitively inhibit the binding of pathogens to the intestinal epithelium, thereby protecting infants from diarrhoea( Reference Newburg, Ruiz-Palacios and Morrow 9 – Reference Morrow and Rangel 11 ).
Therefore, the objective of the present study was to evaluate the anti-RV activity of HMO. We tested HMO isolated from HM, pure neutral HMO and acidic HMO (aHMO) in established in vitro systems for assessing RV binding to the epithelial cells and RV infectivity/replication. Additionally, we developed an acute RV infection piglet model to determine which HMO fraction would be most efficacious in vivo. We hypothesised that sialylated HMO would reduce the binding and infectivity of sialic-acid-dependent RV both in vitro and in vivo.
Experimental methods
Oligosaccharides
The oligosaccharides used in the present study were as follows: lacto-N-neotetraose (LNnT; Abbott Nutrition); 2′-fucosyllactose (V-Labs); SA (V-Labs); 3′-sialyllactose (3′-SL; V-Labs); 6′-sialyllactose (6′-SL; V-Labs). In addition, a complex mixture of HMO (isolated HMO; iHMO) was isolated from preterm HM, provided by Dr Paula Meier (Rush University, Chicago, IL), as described by Li et al. ( Reference Li, Bauer and Chen 16 ). A range of oligosaccharide concentrations was tested in the focus-forming unit (FFU) assay and the binding assay as described below. The concentrations used in situ were based on the in vitro results.
Rotavirus preparation
A sialidase-sensitive group A porcine RV OSU strain (P9[7], G5) was obtained from the American Type Culture Collection. The virus was propagated in MA-104 cells as described previously( Reference Rolsma, Gelberg and Kuhlenschmidt 17 ). For the in situ assays, clarified RV OSU-infected cell-culture lysates were used. For the in vitro assays and for radioiodination, RV OSU was further purified by caesium chloride gradient centrifugation as described previously( Reference Rolsma, Gelberg and Kuhlenschmidt 17 ). A sialidase-insensitive group A human RV strain (Wa) (P1A[8], G1) (American Type Culture Collection) was propagated in MA-104 cells, isolated and purified as described for the OSU strain with the following two modifications. The Wa strain was cultured in the presence of 0·5 μg trypsin/ml; however, instead of using caesium chloride, the final gradient purification was accomplished using 15–60 % OptiPrep (Greiner Bio-One, Inc.) discontinuous step gradients. After centrifugation for 1 h in a NVT65 rotor (Beckman Coulter) at approximately 159 000 g (41 000 rpm), the single band at approximately 30 % down the gradient was collected and OptiPrep was removed by filtration through Vivaspin 500 100K MWCO (Sartorius Group). Viruses were treated with crystallised trypsin (final concentration 10 μg/ml; Sigma Chemical Company) for 30 min at 37°C before use in the in vitro or in situ assays.
In vitro assays
Focus-forming unit assay
The FFU assay was performed in MA-104 cells (BioWhittaker), a well-established model for studying RV infectivity in vitro ( Reference Andres, Donovan and Kuhlenschmidt 18 , Reference Rolsma, Kuhlenschmidt and Gelberg 19 ). MA-104 cells were grown to confluence in twenty-four-well plates. In a preliminary experiment, the trypsin-treated OSU strain or the RV Wa strain (103 infectious units) was mixed with oligosaccharides at either 1 or 10 mg/ml for 30 min before the application to the confluent MA-104 cells. Before the infection, MA-104 cells were rinsed twice with PBS (pH 7·3), and then the oligosaccharide/RV mixture was applied to the cells in duplicate at 100 μl/well. In the oligosaccharide dose–response assay, cells were treated with the oligosaccharide/RV mixture at 37°C. 3′-SL and 6′-SL were tested at 2, 4, 6, 8, 10 and 16 mg/ml and iHMO at 2, 4, 6, 8, 10, 12, 16, 20 and 25 mg/ml. RV was held constant at 103 FFU/well. After 30 min, the oligosaccharide/RV mixture was removed from the cells, replaced with serum-free minimum essential medium (MEM) and incubated for 24 h at 37°C in a 5 % CO2 incubator. In the timing of the exposure assay, the confluent MA-104 cells were treated with HMO before or after exposure to RV or continuously (before, during and after the RV infection). For the HMO treatment before the infection, cells were exposed to 3′-SL and 6′-SL at a concentration of 4 mg/ml for 24 h or iHMO at 12 mg/ml for 4 h prior, HMO was then removed and the cells washed before the addition of RV in MEM. For the HMO treatment after the RV infection, cells were treated with RV for 30 min, and then RV was removed from the cells, the cells were washed and replaced with MEM containing 3′-SL, 6′-SL for 24 h or iHMO for 4 h at 37°C in a 5 % CO2 incubator.
Quantification of the RV-infected cells was performed by peroxidase immunocytochemical detection of virus progeny as described previously( Reference Andres, Donovan and Kuhlenschmidt 18 , Reference Rolsma, Kuhlenschmidt and Gelberg 19 ). Images of the stained cells (twenty-five images/well) were captured with a SPOT RT camera (Diagnostic Instruments, Inc.) attached to a Nikon Eclipse TS100 inverted microscope at a 10 × magnification (Nikon, Inc.), equipped with a motorised microscope stage (Optiscan™; Prior Scientific). Viral infectivity, characterised as the number of brown-stained cells per well, was quantified by counting from digitised images using MetaMorph software (Molecular Devices Corporation). Data were expressed as a percentage of control.
Virus binding assay
125I-radiolabelled RV and the binding assay were prepared and performed as described previously( Reference Rolsma, Gelberg and Kuhlenschmidt 17 ). Briefly, confluent monolayers of MA-104 cells were dissociated with 0·05 % trypsin in 0·53 mm-EDTA (Sigma Chemical Company), and then the single cell suspension was washed twice with serum-free MEM and suspended to a density of 4 × 106 cells/ml. 125I-radiolabelled RV OSU (approximately 150 000 dpm, 150 ng viral protein) in 15 μl was mixed in a 75 × 100 mm glass tube containing 250 μl 3′-SL (2, 4 and 8 mg/ml), 6′-SL (1, 2 and 4 mg/ml) or iHMO (8, 12, 16, 20 and 25 mg/ml) or medium alone for 30 min at 4°C. Following the addition of 500 μl of MA-104 cell suspension (2 × 106 cells), the tube was rotated end over end at 5 rpm. At time 0 and 30 min, aliquots of the mixture were layered over 200 μl of silicone/mineral in 400 μl polypropylene microcentrifuge tubes and centrifuged through the oil for 20 s at 15 000 g to separate the cells containing bound 125I-radiolabelled RV OSU from the free 125I-radiolabelled RV OSU remaining above the oil. The bottom of the plastic microcentrifuge tube containing the cell pellet was cut off, placed in a glass tube and radioactivity in the cell pellets was enumerated in a Packard Cobra gamma counter (Packard Instruments). After correction for binding at the 0 time point, data were expressed as a percentage of control.
In situ rotavirus infection model
All animal procedures were approved by the Institutional Animal Care and Use Committee of the University of Illinois. Piglets (n 9) were obtained from the University of Illinois Swine Research Center after receiving 48 h of colostrum. Piglets were individually housed in cages, maintaining six piglets separated by Plexiglas partitions (Evonik Industries) in environmentally controlled rooms (25°C) with radiant heaters attached to the tops of the cage to maintain an ambient temperature of 30°C. Piglets were fed a non-medicated, sow-milk replacer (Milk Specialties Global Animal Nutrition) twenty times daily at a rate of 360 ml/kg body weight per d until 21 d of age.
At 21 d of age, piglets were fasted 8 h before being sedated by an intramuscular injection of Telazol (tiletamine HCl and zolazepam HCl; Pfizer Animal Health) reconstituted with ketamine (2·2 mg/kg; Bioniche Pharma USA LLC) and xylazine (2·2 mg/kg; Akorn, Inc.). Piglets were maintained under isoflurane (98 % O2/2 % isoflurane) for 6 h. Upon initiation of sedation, a midline laparotomy was performed, and six 10 cm loops of ileum were isolated in situ as described by Di Lorenzo et al. ( Reference Di Lorenzo, Bass and Krantis 20 ). Beginning at the ileal–caecal junction, 10 cm sections were measured and loops were formed by tying the intestine with suture (Deknatel Silk Surgical Suture; Teleflex Medical OEM). Approximately 1 cm intervals were maintained between each loop. The following treatments were injected into the loops in a 2 ml volume: media, 2 mg/ml of neutral HMO (LNnT), 2 mg/ml of aHMO mixture (40 % 6′-SL/10 % 3′-SL/50 % SA) or each treatment+sialidase-sensitive porcine RV OSU strain (1 × 107 FFU). The RV OSU strain was activated by crystallised trypsin before the injection. This trypsinisation is required to permit RV penetration of cell membranes following cell attachment. Since the in vitro work showed that HMO did not decrease the infectivity of the human RV Wa strain, we chose to focus on the RV OSU strain for the in situ study. Given the limited number of loops that could be reasonably created within each piglet, we chose to pool the SA-containing HMO rather than test them individually in situ. Additionally, this is how aHMO would be present in the intestinal milieu of a HM-fed infant. For aHMO, we chose to mimic the total SA content of HM( Reference Kunz, Rudloff and Baier 12 , Reference Wang, Brand-Miller and McVeagh 21 – Reference Martín-Sosa, Martín and García-Pardo 26 ). 6′-SL and 3′-SL were added at the reported ratio to each other and the remaining total SA was provided as free SA, assuming that some lipid or protein-bound SA could be released by sialidases in the intestine( Reference Dickson and Messer 27 ) or by microbial fermentation( Reference Brand-Miller, McVeagh and McNeil 28 ). The order of the six treatment loops within a piglet was randomised between the piglets. After 6 h of treatment, piglets were euthanised by an intracardiac injection of sodium pentobarbital (60 mg/kg body weight, euthanasia-5 solution; Vortex Pharmaceuticals). The intestine was removed and flushed with ice-cold saline. Samples (1–2 cm) of ileal loops were snap-frozen in liquid N2, as well as opened longitudinally, scraped free of mucosa and mucosal samples were snap-frozen in liquid N2.
Cytokine and non-structural protein-4 mRNA expression
Total RNA was isolated from ileal mucosal samples (0·1 g) using the Qiagen, RNeasy Plus Mini Kit (Qiagen) following the manufacturer's protocol. RNA was quantified and quality was assessed using a Nanodrop 1000 (Thermo Scientific) and a 2100 Bioanalyzer (Agilent Technologies, Inc.), respectively. All samples had an RNA integrity number greater than 6. RT was performed with 3 μg total RNA in a reaction volume of 20 μl (High Capacity cDNA Reverse Transcription Kit; Applied Biosystems). RT products were analysed by quantitative real-time PCR using TaqMan® custom porcine-specific primers and probes (non-structural protein-4; NSP4) or gene expression assay kits for NF-κB, IL-8, TNF-α, chemokine (C–C motif) ligand 2 and IL-1β (Applied Biosystems) (Table 1). Reference complementary DNA ribosomal protein L19 was used as an endogenous control (Applied Biosystems). All reactions were analysed using the 7900HT Fast Real-Time PCR Detection System instrument and SDS 2.3 software (Applied Biosystems). Universal reaction conditions were used as follows: 2 min at 50°C and 10 min at 95°C and then forty cycles of 15 s at 95°C and 1 min at 60°C. Each reaction was run in triplicate. Cytokine expression was standardised to ribosomal protein L19 mRNA and expressed as fold difference relative to the medium control loop. NSP4 expression was standardised to ribosomal protein L19 mRNA and expressed as normalised quantity, since the control loop contained no NSP4 mRNA expression.
Table 1 TaqMan gene expression assay kits and custom primers for quantitative real-time PCR
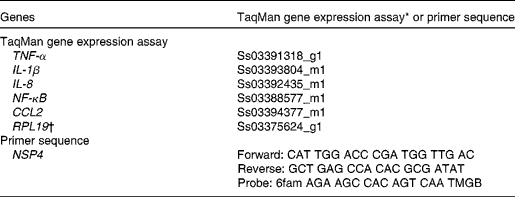
CCL2, chemokine (C–C motif) ligand 2; RPL19, ribosomal protein L19; NSP4, non-structural protein-4.
* Applied Biosystems.
† Reference complementary DNA.
Statistical analyses
All statistical analyses were performed using SAS (version 6.09; SAS Institute). Data were analysed by one-way ANOVA using the general linear model procedure in SAS. For in vitro data, differences between treatment means within each oligosaccharide were analysed by Duncan's test. For in situ PCR data, outliers were identified using Cook's D test and normality was checked using the Shapiro–Wilk test. PCR data were analysed using a PROC MIXED, randomised block analysis, to determine the effects of the HMO and RV loop treatments, and their interactions. The interactions were removed from the model if not significant. In the event of a significant main effect, a post hoc least significant difference test was used to compare differences among the treatments. All data are reported as means and standard deviations. Statistical significance was set at P< 0·05.
Results
In vitro studies
The composition of the isolated crude HMO as determined by HPLC-chip/time-of-flight MS has been reported previously( Reference Li, Bauer and Chen 16 ). Approximately 25·1 % of HMO were neither fucosylated or sialylated; LNnT/lacto-N-tetraose (LNT) accounted for 16·1 % of total iHMO. Fucosylated oligosaccharides accounted for 30·9 % of total iHMO, of which 2′-fucosyllactose consisted of 5·8 % of total iHMO. Sialylated oligosaccharides comprised 31·6 % of total iHMO, of which 3′-SL and 6′-SL comprised 1·1 and 2·1 % of total iHMO, respectively. HMO containing both fucose and SA accounted for 12·4 % of total iHMO( Reference Li, Bauer and Chen 16 ).
Focus-forming unit assay
To initially screen the effective concentration range of oligosaccharides against RV infectivity in MA-104 cells, 1 and 10 mg/ml concentrations were tested in the FFU assay. The infectivity of the RV OSU strain was not inhibited by any oligosaccharides at 1 mg/ml. At 10 mg/ml, a significant inhibition of OSU infectivity was observed for 3′-SL (85 % inhibition), 6′-SL (90 % inhibition) and iHMO (33 % inhibition) (P< 0·05). The LNnT and 2′-fucosyllactose treatments at either 1 or 10 mg/ml did not reduce RV OSU infectivity (data not shown). Human RV Wa strain infectivity was not inhibited by any oligosaccharides at either dose; therefore, no further analyses were conducted using the RV Wa strain.
To define the optimal HMO concentration to inhibit the RV OSU strain in vitro, 3′-SL and 6′-SL were screened from 2 to 16 mg/ml and iHMO from 2 to 25 mg/ml (Fig. 1). Compared with the control, a dose-dependent reduction of RV infectivity was observed between 2 and 6 mg/ml for 3′-SL (20–74 % inhibition, P< 0·05) and 6′-SL (61–83 % inhibition, P< 0·05). In addition, 6′-SL was significantly more effective than 3′-SL at 2 and 4 mg/ml. At 6 mg/ml, the percentage of inhibition by 6′-SL and 3′-SL was similar, and no further reduction in RV infectivity was observed at concentrations greater than 4 mg/ml for 6′-SL and 6 mg/ml for 3′-SL. iHMO did not significantly reduce RV infectivity at concentrations between 2 and 6 mg/ml, whereas a significant reduction (26 % inhibition) was reached at the concentration of 8 mg/ml. No further inhibition was detected at concentrations greater than 20 mg/ml. Approximately 50 % inhibition of RV infectivity was achieved at 4 mg/ml for 3′-SL, 2 mg/ml for 6′-SL and 12 mg/ml for iHMO, respectively (P< 0·05).
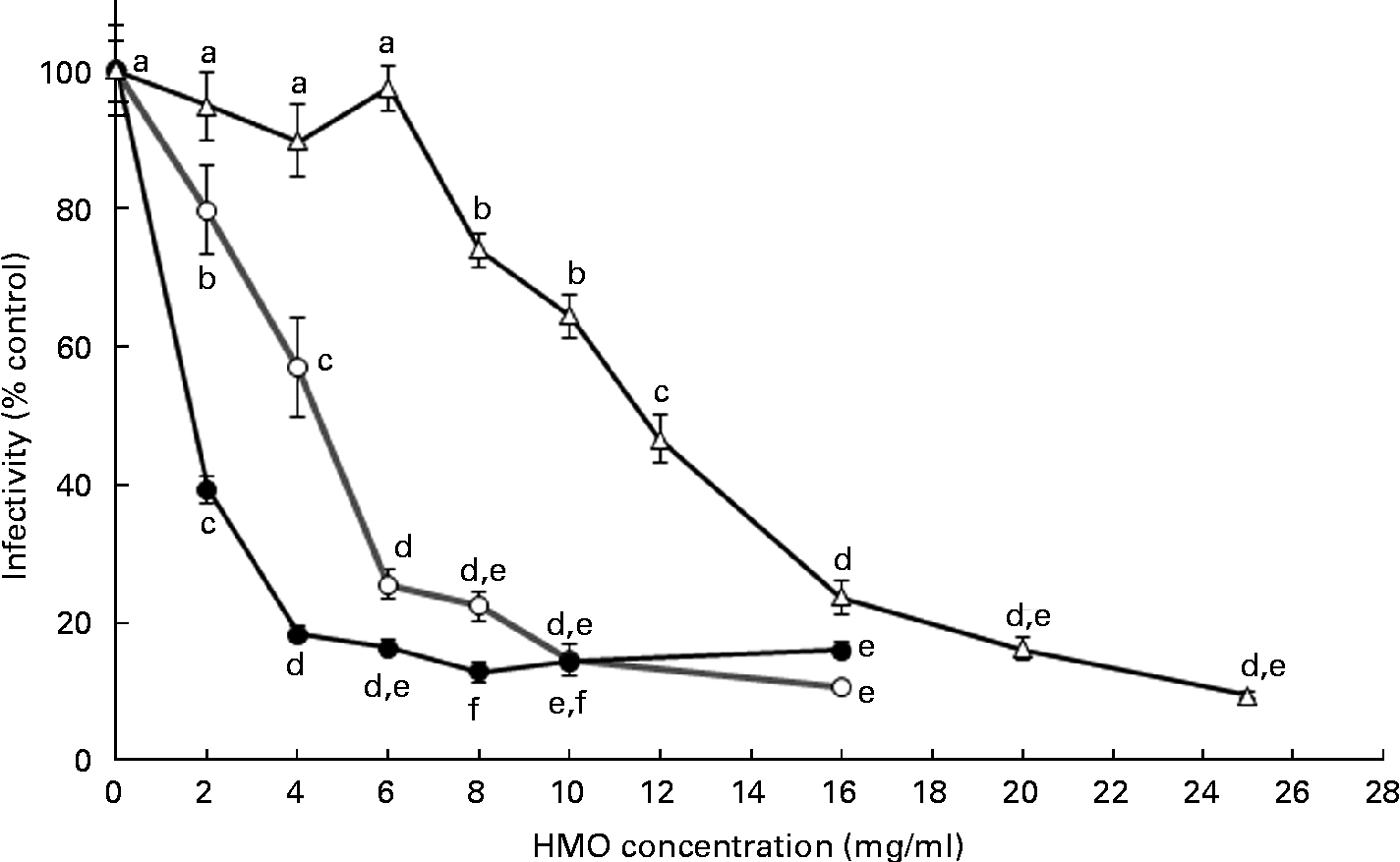
Fig. 1 Dose-dependent inhibition of porcine rotavirus OSU strain infectivity in MA-104 cells by sialylated human milk oligosaccharides (HMO) and isolated HMO (![]() ). Values are means as a percentage of control, with standard deviations represented by vertical bars (n 6). a,b,c,d,eMean values with unlike letters within each oligosaccharide were significantly different (P< 0·05).
). Values are means as a percentage of control, with standard deviations represented by vertical bars (n 6). a,b,c,d,eMean values with unlike letters within each oligosaccharide were significantly different (P< 0·05). ![]() , 3′-Sialyllactose;
, 3′-Sialyllactose; ![]() , 6′-sialyllactose.
, 6′-sialyllactose.
Data in Fig. 1 reflect exposure to HMO only during the RV infection phase. Therefore, to investigate the effect of the timing of exposure to HMO on RV infectivity, MA-104 cells were incubated with 3′-SL (4 mg/ml), 6′-SL (4 mg/ml) or iHMO (12 mg/ml) before and/or after the RV infection, or were present in the media before, during and after the RV infection (Fig. 2). In all the treatment scenarios, 6′-SL significantly reduced RV infectivity by 16–96 % when present at any phase (P< 0·05). Neither 3′-SL nor iHMO reduced RV infectivity when the cells were treated before the infection; however, exposure to these treatments after the RV infection reduced RV infectivity compared with the control (P< 0·05). Continuous exposure to any HMO treatment was most effective in reducing RV infectivity.
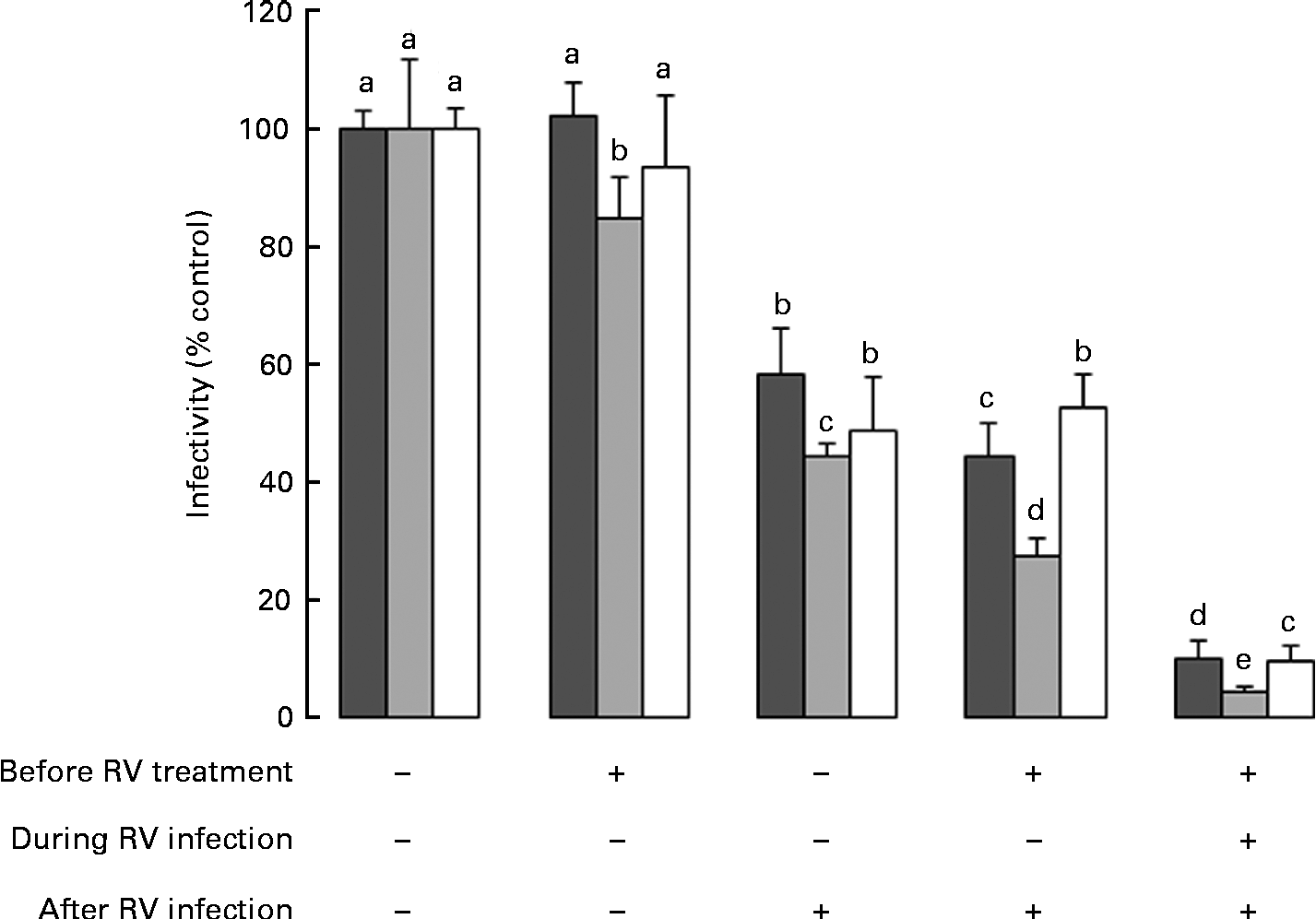
Fig. 2 Impact of the timing of exposure to human milk oligosaccharides (HMO) on the infectivity of the porcine rotavirus (RV) OSU strain in MA-104 cells. Values are means as a percentage of control, with standard deviations represented by vertical bars (n 6). a,b,c,d,eMean values with unlike letters within each oligosaccharide were significantly different (P< 0·05). ■, 3′-Sialyllactose; ![]() , 6′-sialyllactose; □, isolated HMO.
, 6′-sialyllactose; □, isolated HMO.
Virus binding assay
The ability of the oligosaccharides to block the binding of RV to MA-104 cells was tested using the 125I-labelled RV OSU strain (Fig. 3). Both 6′-SL and 3′-SL significantly reduced RV cellular binding at 1 and 2 mg/ml, respectively (P< 0·05), while iHMO inhibited virus binding at concentrations between 8 and 25 mg/ml (P< 0·05). A 50 % inhibition of RV binding was observed at the concentration of 4 mg/ml for 3′-SL, 2 mg/ml for 6′-SL and 16 mg/ml for iHMO, respectively.
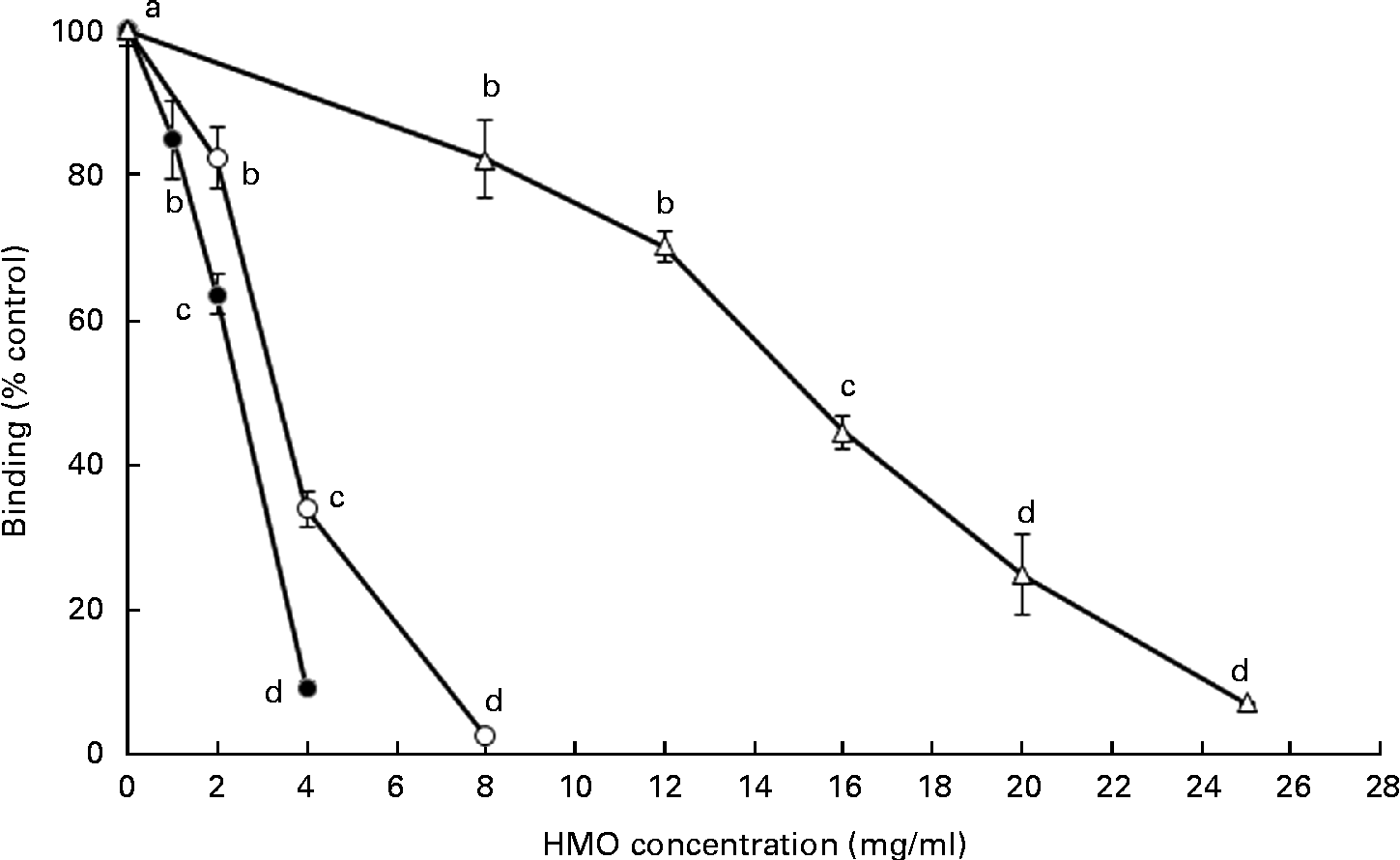
Fig. 3 Dose-dependent inhibition of porcine rotavirus OSU strain binding to MA-104 cells by sialylated human milk oligosaccharides (HMO) and isolated HMO (![]() ). Values are means as a percentage of control, with standard deviations represented by vertical bars (n 5). a,b,c,dMean values with unlike letters within each oligosaccharide were significantly different (P< 0·05).
). Values are means as a percentage of control, with standard deviations represented by vertical bars (n 5). a,b,c,dMean values with unlike letters within each oligosaccharide were significantly different (P< 0·05). ![]() , 3′-Sialyllactose;
, 3′-Sialyllactose; ![]() , 6′-sialyllactose.
, 6′-sialyllactose.
In situ rotavirus infection model
Body weight and formula intake
Piglets were healthy and grew normally throughout the study. Average body weights of piglets at 2 and 21 d of age were 1·9 (sd 0·4) and 5·5 (sd 0·5) kg, respectively. Formula intake at 21 d of age was 1·89 (sd 0·32) litres/d.
Ileal mucosa mRNA expression
Ileal mucosa from the loops treated with either aHMO (40 % 6′-SL/10 % 3′-SL/50 % SA) or LNnT+RV had lower RV replication, as assessed by NSP4 mRNA expression, than that from the RV-treated loop (P= 0·007) (Fig. 4). Ileal loops not exposed to RV had no NSP4 expression. There were no differences in the ileal muscosa mRNA expression of NF-κB, TNF-α, chemokine (C–C motif) ligand 2, IL-8 or IL-1β among the treatments (Table 2).
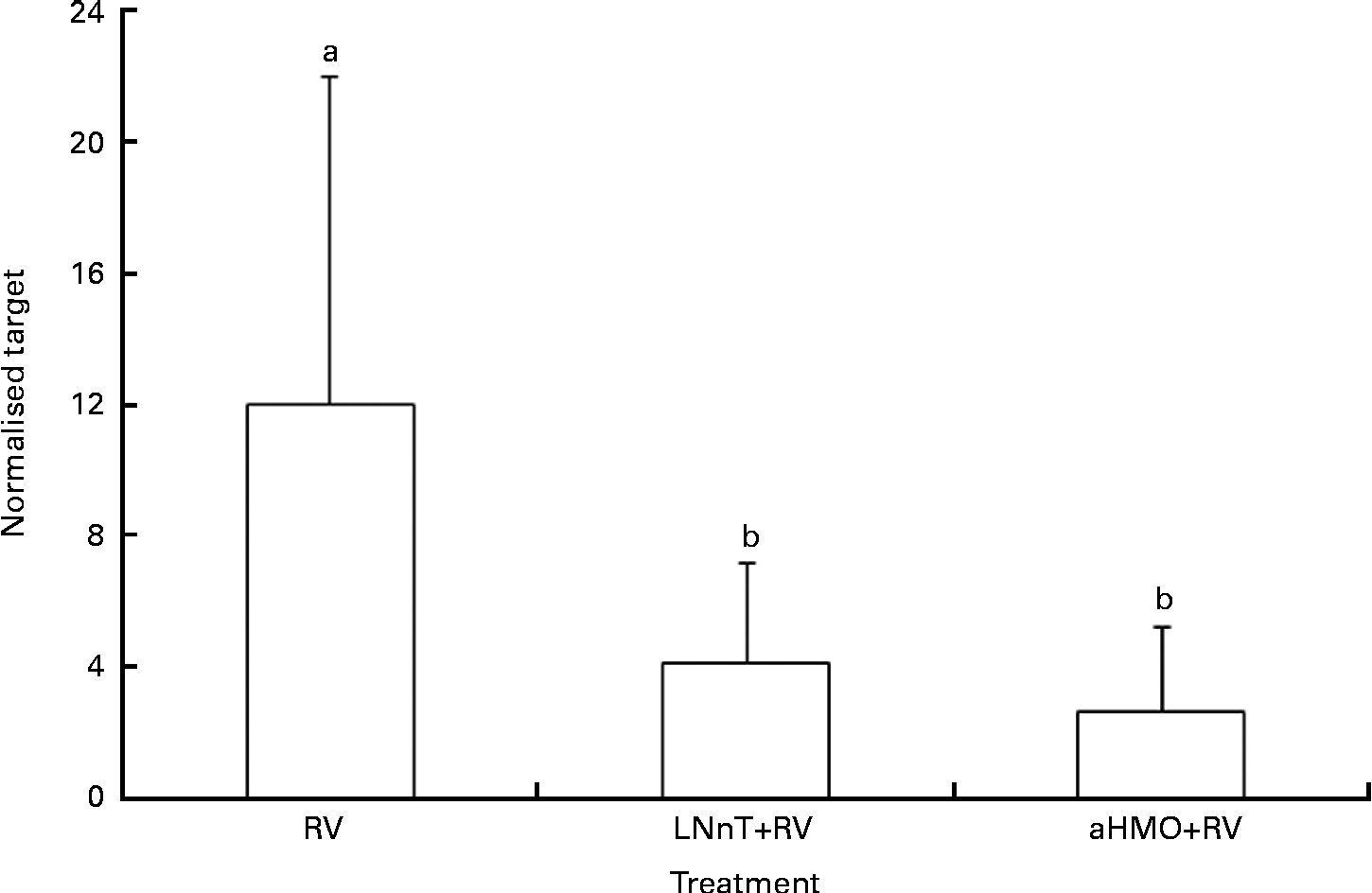
Fig. 4 Acidic human milk oligosaccharide (aHMO) mixture (40 % 6′-sialyllactose/10 % 3′-sialyllactose/50 % sialic acid) and lacto-N-neotetraose (LNnT) reduce mucosal non-structural protein-4 (NSP4) mRNA expression in the ileal mucosa of acutely rotavirus (RV)-infected piglets. Values are means, with standard deviations represented by vertical bars. The expression levels of NSP4 were standardised to ribosomal protein L19 mRNA and expressed as normalised target. a,bMean values with unlike letters were significantly different (P< 0·05).
Table 2 Cytokine and transcription factor mRNA abundance in piglet ileal mucosa is unaffected by the rotavirus (RV) or human milk oligosaccharides (HMO) treatments* (Mean values and standard deviations)
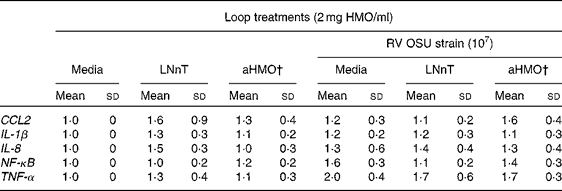
LNnT, lacto-N-neotetraose; aHMO, acidic HMO; CCL2, chemokine (C–C motif) ligand 2.
* Cytokine expression was standardised to ribosomal protein L19 mRNA and expressed as fold difference relative to the medium control loop.
† aHMO mixture comprised 40 % 6′-sialyllactose/10 % 3′-sialyllactose/50 % sialic acid.
Discussion
Breast-feeding reduces the incidence of RVGE( Reference Plenge-Bönig, Soto-Ramírez and Karmaus 8 ), a major cause of morbidity and mortality worldwide. HM contains high concentrations of HMO, which are known to block the attachment of pathogens to the intestinal cells( Reference Martin-Sosa, Martin and Hueso 13 – Reference Ruiz-Palacios, Calva and Pickering 15 ). However, the anti-RV activity of specific HMO has been insufficiently studied. RV strains are generally classified as sialidase-sensitive or -insensitive according to their dependence on the presence of SA on the cell surface for efficient binding and infectivity( Reference Brand-Miller, McVeagh and McNeil 28 , Reference Isa, Arias and Lopez 29 , Reference Haselhorst, Fleming and Dyason 30 ). The present study tested the hypothesis that HMO, particularly sialylated HMO, would reduce RV infectivity by inhibiting the binding of a SA-dependent RV to the epithelial cells. Consistent with this hypothesis, the present results demonstrated that infectivity of porcine RV OSU strain was inhibited by sialylated HMO (3′-SL and 6′-SL) and by the iHMO mixture in vitro, but not by neutral oligosaccharides (LNnT and 2′-fucosyllactose). We did not include free SA in the FFU or binding assays as previous work in our laboratory demonstrated no inhibition of porcine RV OSU strain( 31 ), although Koketsu et al. ( Reference Koketsu, Nitoda and Sugino 32 ) have shown that 1 mg/ml of free SA inhibited the binding of SA-dependent simian SA-11 RV strain to MA-104 cells by 40 %.
These results are in agreement with previous studies showing that the infectivity of SA-dependent strains of RV was reduced in the presence of SA-containing compounds( 31 , Reference Koketsu, Nitoda and Sugino 32 ). Bergner et al. ( Reference Bergner, Kuhlenschmidt and Hanafin 33 ) reported that a sialyllactose-containing neoglycolipid reduced RV OSU strain binding to MA-104 cells and in newborn piglets. Similarly, sialyloligosaccharides isolated from egg yolk hindered the infectivity of a SA-dependent strain of RV isolated from simians (SA-11), both in vitro and in vivo ( Reference Koketsu, Nitoda and Juneja 34 ). Consistent with this structural specificity for the inhibition of binding, the infectivity of the human RV Wa strain was not prevented by 3′-SL or 6′-SL, which is known to be a SA-insensitive RV strain( Reference Ciarlet and Estes 35 ). RV Wa strain infectivity was also not inhibited by neutral HMO or the complex mixture of HMO isolated from HM. Since many RV strains that infect humans are non-SA-dependent( Reference Ciarlet and Estes 35 ), it is possible that other HM components are responsible for the lower incidence of RVGE in breast-fed infants( Reference Plenge-Bönig, Soto-Ramírez and Karmaus 8 , Reference Newburg, Ruiz-Palacios and Morrow 9 ). For example, HM contains antibodies against RV, and proteins in the milk-fat-globule membrane, including lactadherin, have been shown to inhibit RV replication( Reference Bojsen, Buesa and Montava 36 – Reference Kvistgaard, Pallesen and Arias 38 ). Thus, HM contains components that may provide broad-spectrum protection against both SA-dependent and -independent strains.
The dose–response experiment showed that both 6′-SL and 3′-SL reduced RV OSU infectivity by 50 % at concentrations of 2 and 4 mg/ml, respectively. The results are consistent with Rolsma et al. ( Reference Rolsma, Kuhlenschmidt and Gelberg 19 ), who found that the amount of 3′-SL to inhibit RV OSU binding by 50 % in MA-104 cells was greater than that of 6′-SL. Thus, 6′-SL is more effective in obstructing RV OSU infectivity than its isomer 3′-SL, possibly due to a greater affinity of sialylated HMO for SA-containing cellular receptors.
The concentration of the iHMO mixture needed to inhibit RV infectivity was in the range of 8–20 mg/ml, which is within the range of the concentrations of HMO (5–12 g/l)( Reference Kunz, Rudloff and Baier 12 ), implying that physiological concentrations of HMO could have the potential to inhibit RV infection. Analysis of iHMO by MS demonstrated that sialylated HMO comprised 31·6 % of total HMO( Reference Li, Bauer and Chen 16 ), which supports the lower efficacy of iHMO, if SA-containing HMO were the most effective in impeding RV OSU infectivity.
The mechanisms by which 3′-SL, 6′-SL and HMO inhibited RV OSU strain infectivity were investigated in two ways. First, the effect of the timing of exposure to HMO on RV infectivity was determined. All three oligosaccharides inhibited RV infectivity when MA-104 cells were treated during and after the infection, indicating that HMO could modulate RV infectivity by blocking entry into the cell and/or by affecting viral replication. A slight inhibition of RV infectivity was also observed when MA-104 cells were pretreated with 6′-SL before the infection, which was not the case for 3′-SL- and HMO-treated cells. These data show that continual exposure to HMO was most effective in inhibiting the infection, which is what a breast-fed baby would experience. However, iHMO were also effective when the cells were exposed post-RV exposure, suggesting an opportunity to use HMO therapeutically in oral rehydration solutions or specialised formulae for children already infected with RV to reduce the severity and duration of the disease.
Second, whether 3′-SL, 6′-SL and HMO directly blocked RV binding to the host cells was investigated using the 125I-labelled RV OSU strain. Comparing the results of the FFU infectivity assay v. the binding assay, there was a concordance between the inhibition of RV binding and infectivity/replication for 3′-SL and 6′-SL, supporting the blocking of RV binding as the primary mechanism of action by SA-containing HMO. These findings are also consistent with Yolken et al. ( Reference Yolken, Willoughby and Wee 39 ), who showed that SA-containing glycoproteins inhibited RV binding and replication in vitro and in vivo. In contrast, at a physiological concentration (12 mg/ml), the iHMO mixture was less effective in inhibiting RV binding (30 % inhibition) than replication (53 % inhibition), implying that HMO also acts at a post-binding step. Recent studies have shown that HMO can reduce intestinal cell proliferation and induce apoptosis( Reference Kuntz, Rudloff and Kunz 40 , Reference Kuntz, Kunz and Rudloff 41 ); thus, HMO may reduce the ability of RV to replicate within the cells by altering intestinal cell turnover.
To determine whether HMO inhibit RV infectivity in vivo, we developed a novel piglet model of acute RV infection in which multiple 10 cm loops were created in the ileum in situ. HMO with or without the RV OSU strain was introduced into the loops and samples were collected at 6 h post-infection. Expression of RV NSP4 gene in ileal mucosa was used as an indicator of RV viral replication in vivo. The triple-layered RV contains a genome of eleven segments of double-stranded RNA that codes for six structural and five non-structural proteins. NSP4 is one of the first viral genes transcribed in the host cell. In addition, it is important in the pathophysiology of RV in that it can lead to a mobilisation of intracellular Ca( Reference Hyser, Collinson-Pautz and Utama 42 ), and purified NSP4 can induce diarrhoea in young mice when injected intraperitoneally or intraileally( Reference Ball, Tian and Zeng 43 ).
Mucosa from RV-infected ileal loops displayed a high level of RV NSP4 mRNA expression. However, no NSP4 replication was detected in the loops not treated with RV. Thus, viral infection was confined to the challenged loops and absent from the adjacent loops. The advantage of this approach is that it is possible to compare host responses to RV-infected and non-infected ileum within the same animal. Additionally, due to the low volume required within the loops, this model provides an ideal screening tool for the efficacy of multiple components that may only be available in limited quantities, such as HMO.
We observed that RV-infected ileal loops co-incubated with either aHMO (40 % 6′-SL/10 % 3′-SL/50 % SA) or LNnT had significantly lower NSP4 mRNA expression than the loop exposed to RV alone. The inhibition of RV replication by aHMO in situ confirmed the present in vitro observation that SA-containing HMO are effective inhibitors of RV infectivity. Our aHMO mix included free SA, 3′-SL and 6′-SL. We demonstrated herein that 3′-SL and 6′-SL reduce RV OSU strain binding and replication in vitro. The mixture used in the in situ model contained 50 % SA and may have different effects on RV infectivity as HMO bound SA in the same concentration. Although our previous work did not show the inhibition of RV OSU strain by free SA( 31 ), Koketsu et al. ( Reference Koketsu, Nitoda and Sugino 32 ) showed that 1 mg/ml of free SA, which is comparable with that included in the aHMO mix, inhibited the binding of another SA-dependent RV strain (SA-11) by 40 %. Therefore, we cannot rule out that free SA also contributed to reduced RV replication in situ.
On the basis of the present in vitro observations, we anticipated that LNnT would not inhibit RV infectivity. However, NSP4 expression was also reduced when RV was co-incubated with LNnT. It is possible that LNnT within the milieu of the ileum is able to inhibit RV binding or entry into the cell, an effect which could not be observed in the in vitro FFU assay. Although this result was unexpected, there are several potential explanations for this observation, through altering the host immune response, inhibiting the binding of RV or altering the intraluminal milieu. First, HMO are immunomodulatory due to their ability to interact directly with intestinal epithelial cells( Reference Kunz and Rudloff 44 ). Terrazas et al. ( Reference Terrazas, Walsh and Piskorska 45 ) demonstrated that injection of LNnT into mice increased a population of cells (Gr1+CD11b+F4/80+) that secreted anti-inflammatory cytokines (IL-10, transforming growth factor-β) as early as 2 h post-injection. Furthermore, co-culturing these cells with naive CD4+ T cells suppressed T-cell proliferation and programmed naive CD4+ T cells to secrete lower levels of interferon-γ coincident with increased IL-13 production. Thus, injection of LNnT generated anti-inflammatory mediators that suppressed T helper 1 (Th1)-type and inflammatory responses( Reference Terrazas, Walsh and Piskorska 45 ). To date, there is no direct evidence for the inhibition of RV binding or infectivity by LNnT. However, both sialylated and non-sialylated LNnT structures were found to be present in lipo-oligosaccharides of Neisseria meningitidis and may play a role in cellular recognition by this pathogen( Reference Tsai 46 ). Lastly, LNnT may be fermented by the microbiota in the ileum, leading to the production of SCFA in the intraluminal environment. We recently demonstrated that LNnT was fermented by piglet gut microbiota( Reference Li, Bauer and Chen 16 ). LNnT produced larger amounts of gas, total SCFA, acetate and butyrate than did other substrates (polydextrose/galacto-oligosaccharides, short-chain fructo-oligosaccharides or iHMO)( Reference Li, Bauer and Chen 16 ). Previous studies have shown that SCFA inhibit the replication of bovine enterovirus, but were almost ineffective against other viruses such as poliovirus type 1, coxsackievirus B5, encephalomyocarditis virus and human rhinovirus 1B( Reference Ismail-Cassim, Chezzi and Newman 47 ). Ismail-Cassim et al. ( Reference Ismail-Cassim, Chezzi and Newman 47 ) showed that lauric acid could bind to bovine enterovirus. Virions with lauric acid bound to it were able to attach to cells, but failed to undergo cell-mediated uncoating. The inhibitory effect is reversible with chloroform and may result from a hydrophobic interaction between the fatty acid and a specific site on the virus particle( Reference Ismail-Cassim, Chezzi and Newman 47 ). One or more of these actions may be at work in the LNnT-treated loops, thereby preventing RV infection/replication and the subsequent increase in NSP4 mRNA expression. Although these mechanisms may be at play, further research is needed to determine the direct mechanism of inhibition by LNnT.
RV infection leads to an up-regulation of cytokine gene expression. Following a RV infection in MA-104 cells, an increase in the protein abundance of the NF-κB transcription factor was detected at 2 h( Reference Rollo, Kumar and Reich 48 ) and the maximum rate of viral RNA synthesis was achieved at 3 h( Reference Stacy-Phipps and Patton 49 ). Work in our laboratory in the RV-infected piglet showed an increase of NF-κB nuclear protein abundance in the jejunal mucosa at 4 h post-infection( Reference Andres, Taylor and Helregel 50 ). Accordingly, we anticipated that NF-κB and cytokine mRNA expression would be up-regulated at 6 h following an RV infection in the ileum. However, no statistically significant differences in gene expression were observed between the RV-infected and non-infected loops for any gene tested. This may be due to the fact that we measured NF-κB mRNA expression, rather than nuclear protein abundance. Since NF-κB present in the inactive form in the cytosol is bound to inhibitor of κB (IκB), it would not require time for transcription or translation and could be more rapidly detected than NF-κB mRNA. Therefore, 6 h may have been insufficient time for the acute RV infection in situ to cause transcription factor and cytokine expression differences in the ileum.
In summary, SA-containing HMO and SA-containing HMO with SA effectively inhibit the infection caused by SA-dependent RV OSU strain both in vitro and in vivo. In vitro findings support several possible mechanisms: by inhibiting RV binding to the host cells and/or modulating the process of RV entry into the cell or viral replication. The present paper reports for the first time that both neutral (LNnT) and aHMO with free SA significantly decreased RV replication in the in situ model. Although the mechanisms of RV infectivity inhibition remain to be further investigated, the present study demonstrates the potential of HMO to reduce RV infection. Thus, these results suggest that HMO could be useful for preventing RV infection in young animals and human infants. The novel in situ loop model provides a sensitive system in which to rapidly screen HMO and other ingredients for anti-RV activity. This model can thus be used to guide the design of in vivo feeding studies in piglets or human infants.
Acknowledgements
The present in vitro study was supported by the NIH grant R01 HD061929 and the piglet study was supported by Abbott Nutrition (Columbus, OH). S. N. H. was supported by a NIH Ruth L. Kirschstein National Research Service Award (T32 DK59802) to the Division of Nutritional Sciences. X. C. was supported by the CSC postgraduate scholarship program. We thank Sharon Kim for propagating the RV Wa strain and the Donovan Laboratory for assistance with piglet care. S. M. D. and M. S. K. received grant funding from Abbott Nutrition. The authors' contributions are as follows: S. M. D., T. B. K. and M. S. K. designed the in vitro study; S. M. D., S. S. C. and S. N. H. designed the in situ study; X. C., T. B. K. and M. L. conducted the in vitro experiments; S. N. H. and M. H. M. conducted the in situ experiments; X. C., M. L. and S. N. H. analysed and interpreted the data; X. C., M. L. and S. N. H. drafted the manuscript; S. M. D., T. B. K. and M. S. K. reviewed the manuscript. All authors read and approved the final manuscript. S. M. D. served as a paid consultant for Abbott Nutrition. No other authors have conflicts of interest to disclose.








