Introduction
The South Asian tropical island of Sri Lanka is rich in amphibian diversity (Meegaskumbura et al., Reference Meegaskumbura, Bossuyt, Pethiyagoda, Manamendra-Arachchi, Bahir, Milinkovitch and Schneider2002). Of the country's 119 described amphibian species 104 (c. 87%) are endemic and > 90% are restricted to rainforests (Surasinghe, Reference Surasinghe2009; Wickramasinghe et al., Reference Wickramasinghe, Bandara, Vidanapathirana, Tennakoon, Samarakoon and Wickramasinghe2015). Sri Lanka's amphibians are threatened by deforestation, environmental pollution and road traffic (Pethiyagoda et al., Reference Pethiyagoda, Manamendra-Arachchi, Bahir, Meegaskumbura and Bambaradeniya2006; Karunarathna et al., Reference Karunarathna, Henkanaththegedara, Amarasinghe and De Silva2013). These anthropogenic stressors have contributed to the extinction of 18 amphibian species, and declining populations of nearly half of the extant species (MOE, 2012).
The stream-dwelling toad genus Adenomus contains two species, Kelaart's torrent toad A. kelaartii and the Kandian torrent toad A. kandianus (Meegaskumbura et al., Reference Meegaskumbura, Senevirathne, Wijayathilaka, Jayawardena, Bandara, Manamendra-Arachchi and Pethiyagoda2015a). The latter was considered to be extinct for 136 years (Stuart et al., Reference Stuart, Hoffmann, Chanson, Cox, Berridge, Ramani and Young2008) until its rediscovery at Peak Wilderness Sanctuary in 2012 (Wickramasinghe et al., Reference Wickramasinghe, Vidanapathirana and Wickramasinghe2012). Currently, this species is known from only two localities in the Central Highlands (> 1,400 m; Gabadage et al., Reference Gabadage, De Silva, Botejue, Bahir, Surasinghe and Madawala2014) and it is categorized as Critically Endangered on the IUCN Red List (IUCN SSC Amphibian Specialist Group, 2012). The species’ molecular phylogeny, bioacoustics, osteology, and larval and adult morphology have been studied (Meegaskumbura et al., Reference Meegaskumbura, Senevirathne, Wijayathilaka, Jayawardena, Bandara, Manamendra-Arachchi and Pethiyagoda2015a,Reference Meegaskumbura, Wijayathilaka, Abayalath and Senevirathneb).
In the literature on A. kandianus, details about the species’ natural history, habitat associations, behaviour and population status are limited. This paucity of ecological information impedes conservation action. We therefore studied the population abundance, behaviour, body size variation with sex and sexual maturity, and microhabitat associations and environmental correlations of A. kandianus. Our findings will help inform future conservation action for this Critically Endangered species and provide a foundation for future research.
Study area
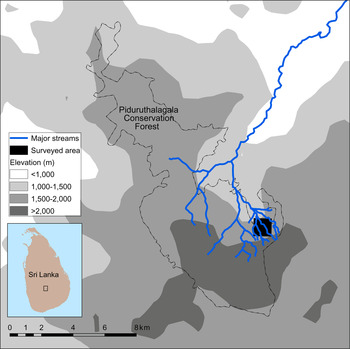
The study area is located partly in the buffer zone of Pidurutalagala (Mount Pedro) Conservation Forest in Sri Lanka's Central Highlands (Fig. 1), one of the two localities from where A. kandianus is known (Gabadage et al., Reference Gabadage, De Silva, Botejue, Bahir, Surasinghe and Madawala2014). Although the forest is under governmental jurisdiction, its buffer zone is imperilled by a multitude of anthropogenic disturbances, the most detrimental being clear-cutting (Plate 1). It is one of the largest montane protected areas in Sri Lanka (7,625.29 ha) and is also a UNESCO World Heritage Site (UNESCO, 2016). The mean annual precipitation is > 3,000 mm, and mean annual temperature is 18 °C (Gabadage et al., Reference Gabadage, De Silva, Botejue, Bahir, Surasinghe and Madawala2014).

Fig. 1 The location of the study site in Pidurutalagala Conservation Forest in the Central Highlands of Sri Lanka.
Methods
Survey design
We conducted 1-week-long field excursions annually during 2011–2013 and twice in 2014. Surveys were conducted in the morning (07.00–14.00) and evening (18.00–20.00) to account for both diurnal and nocturnal activities. Across the entire survey period we surveyed both during (April, May, August) and outside (October, December) the monsoon season. In addition to transect surveys we made opportunistic observations. Statistical analyses were based only on the transect surveys. We placed 15 100 × 5 m belt transects along stream habitats, which covered stream channels and adjacent woodlands, with a minimum distance of 40 m between neighbouring transects. The four-person survey team walked along the transects, actively searching and visually scanning for amphibians. Active searching included examining leaf litter, superficial roots and tree trunks, and understorey vegetation, lifting movable rocks and woody debris, and searching the stream channel (riffles and pools) and cascades using hand (50 × 30 cm) and seine nets (1 × 3 m). For each individual found we recorded sex (based on the presence or absence of nuptial pads and gular sacs), snout–vent length (using a digital vernier caliper), stage of maturity (based on snout–vent length: juvenile ≤ 21 mm, adult ≥ 29 mm), and blotted wet weight (using a Taylor spring scale). Measuring blotted wet weight eliminates excess water on skin surface. All captured individuals were released to the wild without harm at the point of capture.
Environmental variables
Where toads were found we recorded the following environmental variables: stream width, canopy cover (using a canopy densiometer), water and air temperature (using a standard mercury thermometer), relative humidity and light intensity (using a Digitech QM1594 6-in-1 multifunction environment meter), and pH (using a Digitech QM–1670 pH meter). We measured the length of boulders at the intermediate axis, and the above-water height of emergent boulders, pool depth, and the Euclidean distance from each toad to the nearest cultivated land. We obtained rainfall data from the nearest weather station (National Meteorological Department, Sri Lanka). Additionally, we observed the surrounding habitat and recorded any evidence of anthropogenic disturbance.
Behavioural observations
We noted the behaviour of each toad at the time of observation, as well their microhabitat and substrate use.
Statistical analysis
To test for differences in relative abundance among age–sex categories (male, female, juveniles) we ran a one-way ANCOVA (analysis of covariance) in which abundance was considered to be the response variable, the age–sex category was the treatment, and rainfall and sampling dates were covariates. Pairwise differences in abundance of age–sex groups were tested using a Tukey HSD (honest significant difference) test. To test for differences in body size among age–sex categories we used one-way ANOVA (analysis of variance) in which the age–sex category was considered to be the treatment and snout–vent length and blotted wet weight were response variables. A post-hoc Tukey test was performed to discern pairwise differences among age–sex groups for blotted wet weight and snout–vent length. We constructed a linear regression model to test for a significant relationship between the snout–vent length and blotted wet weight. We used a χ2 test of independence to detect any influence of sex on behavioural acts or habitat use. Based on a χ2 test for goodness of fit, we tested if the behavioural acts and habitat use were distributed evenly among individuals. To test for the importance of various environmental variables on the abundance of toads, we constructed a stepwise multiple regression model (a mixed-model approach) in which all the environmental variables were considered to be predictor variables and the abundance of toads was the response variable. Statistical analyses were conducted using various packages (ANCOVA: aov; linear regression: lm; and χ2 test: chisq.test) in R v. 2.15.0 (R Development Core Team, 2011).
Results
The transects covered a total area of 0.1 km2, in which we recorded A. kandianus only in a 10 × 500 m (0.005 km2) stretch of a medium-sized fast-flowing perennial montane stream. The toads were found only along the stream channel, including undercut banks, and never in the woodlands. Our opportunistic surveys of other neighbouring streams, wetlands and forests did not reveal any persistent populations of the species.
Total abundance
We recorded a total of 169 individuals, with a mean of 24.5 males, 13 females and 6.75 juveniles per year (Table 1). The overall population density was 0.85 ± SD 0.57 individuals per 100 m2. Abundance differed significantly among the age–sex groups (F = 3.69, P < 0.05); effects of rainfall (F = 8.07, P < 0.05) and date surveyed (F = 10.09, P < 0.05) were significant covariates. The abundance of males was significantly higher than that of females (mean difference = 1.33, P < 0.05). The differential mean abundance between males and juveniles (mean difference = 0.83, P > 0.05) and between females and juveniles was not significant (mean difference = 0.50, P > 0.05). The population was male-skewed (male : female = 2 : 1) throughout the study, except in 2013 (1 : 3).
Table 1 Mean number of females, males and juveniles of Adenomus kandianus recorded per transect in Pidurutalagala Conservation Forest, Sri Lanka (Fig. 1), in each of 4 sampling years (2011–2014).

Behaviour and microhabitat use
Behavioural acts we observed included perching on boulders, mating in a variety of microhabitats (boulders, submerged in water), swimming, and seeking refuge (Table 2). Age–sex category had a significant influence on behavioural acts (χ 2 = 93.62, P < 0.05). Behavioural acts were not observed uniformly among toads (χ 2 = 46.71, P < 0.05). Perching was the most commonly observed behavioural act among males, followed by mating. Among females, mating was the most dominant behavioural act (Table 2). In a number of instances juveniles emerged from undercut banks at dusk (18.00–19.00) and perched at the stream edge in groups of 2–4. Neither males nor females were observed in refuge. The juveniles had only partially developed webbing in their feet and thus were weak swimmers. Adults had complete webbing and were strong swimmers, and swam perpendicular to the flow.
Table 2 Percentage of female, juvenile and male individuals of Adenomus kandianus recorded mating, perching, seeking refuge, and diving and swimming during surveys in Pidurutalagala Conservation Forest (Fig. 1).

The toads were found in five microhabitats: (1) on moss-covered boulders (most of the time, underneath leaf litter on the boulders), (2) in pools with decaying organic matter, (3) in pools with a sandy substrate, (4) in riffles with a rocky–cobble–pebble substrate, and (5) in undercut banks, among leaf litter and roots of riparian vegetation (Table 3). Toads did not occur uniformly across all microhabitats (χ 2 = 25.89, P < 0.05); microhabitat use was dependent on the age–sex category (χ 2 = 95.22, P < 0.05). Both males and females were found mostly on moss-covered boulders (Table 3). A substantial proportion of adults were also found in in-stream pools. A few adults were observed actively swimming across fast-flowing riffles. Neither adults nor juveniles were found in the riparian zone or in woodlands. Juveniles were found almost exclusively at the edge of the stream channel, within the leaf litter or finely branched roots of the riparian vegetation in undercut banks. We did not observe any juveniles cohabiting with adults in the same microhabitat.
Table 3 Percentage of female, juvenile and male individuals of Adenomus kandianus occupying various microhabitats in Pidurutalagala Conservation Forest (Fig. 1).

Advertisement calls and mating (amplexus) were recorded only during April–May in each year. On a given day, calling started at 18.00 and continued until 20.00. Toads were calling from mid-stream, while perching on boulders. Precipitation was associated with increased incidence of observed mating; for example, on a rainy day in 2012 we observed 17 amplexed pairs in a single in-stream pool along with 15 advertising males, the only breeding congregation we recorded throughout our survey. Amplexus was observed in a variety of microhabitats: underwater, on emergent parts of moss-covered boulders, and in the leaf litter on moss-covered boulders. We found no egg masses during our survey. Amplexus lasted for 4 hours on average but some amplexed pairs remained in position at the same location for 8 hours. On each of five different occasions we observed five amplexed pairs swimming continuously for 3.5 minutes (Plate 1). On 10 occasions we observed non-mating toads swimming as a group. Individuals remained underwater for a mean of 20 minutes.

Plate 1 (a) Adenomus kandianus underwater, camouflaged against the rocky streambed, (b) male (on top) and female Adenomus kandianus in amplexus, (c) and (d) plantation agriculture and deforestation around Pidurutalagala Conservation Forest, and (e) the stream segment at Pidurutalagala Conservation Forest, one of two known localities of Adenomus kandianus.
Body size variation
Both snout–vent length and blotted wet weight differed significantly among age–sex categories (Table 4). Females were significantly larger than males and juveniles in terms of both snout–vent length and blotted wet weight. Males were significantly larger than juveniles in terms of snout–vent length. There was a strong linear length–weight relationship among all age–sex categories (linear regression model, R 2 = 0.9012; F = 356.9; P < 0.0001; Fig. 2).

Fig. 2 Length–weight relationship for adult females, adult males and juveniles of Adenomus kandianus in Pidurutalagala Conservation Forest, Sri Lanka, based on a linear regression of snout–vent length (SVL) against blotted wet weight (R 2 = 0.9012, F = 356.9, P = 0.0001).
Table 4 Differences in snout–vent length and blotted wet weight among females, males and juveniles of Adenomus kandianus, with ANOVA and Tukey statistics.

* P < 0.05
Environmental variables
The environmental variables indicated an association between A. kandianus and microhabitats with higher canopy cover (> 68%), cooler air temperatures (< 26°C), lower light intensity (< 33 lux) and high humidity (> 71%) (Table 5). The low-order montane stream in which we found the species was cool (17°C), fast-flowing and medium-sized (6 m in width), with multiple pools and riffles and near-neutral pH (6.14). The stream channel contained boulders of various sizes (1–8 m in length), which were continually splashed by the turbulent flow. The stream substrate comprised cobbles and pebbles, leaf litter and sand. Given the reduced flow, pool habitats had accumulated silt, sediment and organic matter (coarse woody debris).
Table 5 Mean values of environmental variables measured when individuals of Adenomus kandianus were found during surveys in Pidurutalagala Conservation Forest (Fig. 1), with partial correlation coefficients between the abundance of toads and environmental variables extracted from a stepwise multiple regression model.

* P < 0.05
The best predictors of the abundance of A. kandianus were the Euclidean distance to the nearest cropland, and the ambient temperature (stepwise multiple regression, R 2 = 0.997, F = 729.96, P < 0.05); the former was the most influential variable (model coefficients: distance to croplands = 0.97, ambient temperature = 0.08). Partial correlation analyses revealed that rainfall, relative humidity, canopy cover, water temperature and size of boulders had the highest influence on the abundance of toads (Table 5).
Observations of tadpoles
We observed a mean of only 11 tadpoles per year in in-stream pools. All tadpoles were found completely submerged in water and were attached to rocky substrates by their oral discs. Their bodies were dark-brown to black in coloration.
Discussion
In our surveys in both wet and dry seasons over 4 years we recorded only c. 44 post-metamorphs in any given year. The overall density was low (< 1 individual per 100 m2) and the area of occupancy did not extend beyond a stream corridor of 0.005 km2. Similar demographic characteristics have been reported for other threatened, narrow-ranging tropical amphibians (Sodhi et al., Reference Sodhi, Bickford, Diesmos, Lee, Koh and Brook2008). We found neither eggs nor gravid females, and only a small number of juveniles and tadpoles. Although no egg masses were observed at Peak Wilderness (the only other known habitat of A. kandianus), gravid females were recorded there (Meegaskumbura et al., Reference Meegaskumbura, Wijayathilaka, Abayalath and Senevirathne2015b). Unlike adults, tadpoles and juveniles are weak swimmers and may not withstand the heightened discharge after torrential precipitation, and therefore they may have been washed downstream (Meegaskumbura et al., Reference Meegaskumbura, Wijayathilaka, Abayalath and Senevirathne2015b). This speculation was substantiated by our opportunistic observations of juveniles in lower altitude paddy fields after heavy rainfall. Adenomus kandianus is a specialist toad adapted to montane, undisturbed forested streams, and therefore tadpoles and juveniles transported into anthropogenic environments are unlikely to survive. As a protective measure (to prevent downstream drift, predation, ambient exposure and radiation damage) adults may oviposit in protected, deep interstices, which are undetectable by surveys (Refsnider & Janzen, Reference Refsnider and Janzen2010). Low abundance of larval and juvenile stages may indicate lower recruitment rates and lower survival of early age classes. During surveys we found dragonfly larvae in the stream; these macroinvertebrates could predate on early life stages of A. kandianus, leading to higher mortality among tadpoles and juveniles (Skelly, Reference Skelly1994).
There was a strong positive correlation between snout–vent length and blotted wet weight, indicating an ontogenic relationship. Distinct size segregation among various age–sex categories indicates that body dimensions are sexually dimorphic features for this species. Larger body size of females compared to males (reversed size-based sexual dimorphism) has been observed in many anurans (Woolbright, Reference Woolbright1983). Among basal vertebrates, large females may produce a greater number of eggs, reproducing early in the season, which may ensure early metamorphosis of large juveniles; larger body size confers higher fecundity, timely reproduction and a high juvenile survival rate, and thus higher reproductive fitness overall (Tejedo, Reference Tejedo1992).
Microhabitat use and behaviour differed substantially between adults and juveniles: adults were mostly observed mating or perching, whereas juveniles were seeking refuge. Niche partitioning within conspecifics, such as microhabitat and resource specialization, can mitigate offspring–adult competition (Meegaskumbura et al., Reference Meegaskumbura, Senevirathne, Wijayathilaka, Jayawardena, Bandara, Manamendra-Arachchi and Pethiyagoda2015a). A closed canopy, high humidity, low temperatures, intermediate-sized boulders, and absence of anthropogenic disturbances were important environmental conditions for A. kandianus. These ecological niche dimensions are peculiar to many rainforest amphibians (Pethiyagoda et al., Reference Pethiyagoda, Manamendra-Arachchi, Bahir, Meegaskumbura and Bambaradeniya2006). The dependence on well-shaded canopy, colder temperatures and high humidity implies that this species is susceptible to desiccation. Medium-sized, moist boulders are likely to be preferred sites for advertisement calls. During surveys we observed a few potential predators of A. kandianus: Tickell's blue flycatcher Cyornis tickelliae, Sri Lanka whistling thrush Myophonus blighi, Indian blackbird Turdus simillimus, Sri Lanka hill myna Gracula ptilogenys and river otter Lutra lutra. Prolonged amplexus may predispose A. kandianus to predation where leaf litter on boulders provides concealment.
Species detectability may have played a significant role in our study. Precipitation and seasonality influenced abundance and breeding of A. kandianus, and may also have influenced the species’ detectability and site occupancy. We are unaware of any refugia used by toads when they are inactive. It is possible that these toads use subterranean burrows or deep crevices in the stream bed that were undetectable by our surveys.
Threats and conservation
In contrast to our findings, surveys at Peak Wilderness revealed that females occurred outside the stream channel (Meegaskumbura et al., Reference Meegaskumbura, Senevirathne, Wijayathilaka, Jayawardena, Bandara, Manamendra-Arachchi and Pethiyagoda2015a,Reference Meegaskumbura, Wijayathilaka, Abayalath and Senevirathneb). It was implied that the species may not be rare, at least at the Peak Wilderness locality, given en masse reproduction and high tadpole densities. There has been relatively less habitat destruction at the Peak Wilderness locality compared to Pidurutalagala Conservation Forest. We recorded the presence of multiple commercial plantations (e.g. banana, tea, cardamom, tomato, cabbage, beans) during our study. In addition, clear-cut forestlands, gem and graphite mining, heavy extraction of non-timber forest products, and canopy dieback were also observed in close proximity to our study site. These anthropogenic stressors may have impaired the reproductive fitness of A. kandianus, and precluded local movements in upland habitats.
Ongoing deforestation and agricultural expansion may lead to higher rates of soil erosion; subsequent siltation and sedimentation in stream channels could smother eggs and destroy larval feeding grounds (Richter et al., Reference Richter, Braun, Mendelson and Master1997). We observed the use of agrochemicals on nearby croplands, which can enter the stream habitat through surface run-off and result in developmental abnormalities, growth retardation, delayed metamorphosis and increased egg and larval mortality (Sampath et al., Reference Sampath, Kennedy and James2002). We also noted that some invasive plant species had colonized the riparian forests and adjoining woodlands. Anthropogenic disturbance such as forest clearing and crop farming may have facilitated the establishment of these species (Didham et al., Reference Didham, Tylianakis, Hutchison, Ewers and Gemmell2005).
Our extensive survey confirmed that at Pidurutalagala Conservation Forest A. kandianus is restricted to a narrow stretch (0.005 km2) of a perennial mountain stream. Fluctuating abundance implies instability of the population. Relatively small, isolated populations are susceptible to both demographic and environmental stochasticity, and human disturbances can cause further decline and result in an extinction vortex (Sodhi et al., Reference Sodhi, Bickford, Diesmos, Lee, Koh and Brook2008). Although ecological niche modelling predicted a potential distribution range of c. 650 km2 for the species, only 20 km2 of suitable habitat was available within the predicted range (Meegaskumbura et al., Reference Meegaskumbura, Wijayathilaka, Abayalath and Senevirathne2015b). Thus, it is possible to infer a population decline of > 80% within the past decade. Like many amphibians A. kandianus faces a high risk of extinction because of ecophysiological constraints, high habitat specialization, poor dispersal capacity and high site fidelity (Blaustein et al., Reference Blaustein, Wake and Sousa1994). Our findings support the species’ current IUCN categorization as Critically Endangered. Despite the narrow area of occupancy, no national-level actions have been implemented to protect this toad. Our study area falls partly within the buffer zone of Pidurutalagala Conservation Forest, where multiple uses and human activities are permitted. We recommend that this vital stream habitat and the surrounding woodlands be incorporated into the core habitat of the reserve, thus shifting the buffer zone to a lower altitude.
It is crucial to protect both the immediate riparian zone and the surrounding woodlands to sustain watershed-scale ecological processes such as provision of allochthonous materials (Olson et al., Reference Olson, Anderson, Frissell, Welsh and Bradford2007). Delineating a core and a terrestrial buffer around streams may negate the adverse effects of agriculture (Semlitsch & Bodie, Reference Semlitsch and Bodie2003). Pidurutalagala is a unique montane rainforest rich in endemic native biodiversity and threatened by human activities (Werner, Reference Werner1988), and we recommend that it be incorporated into Sri Lanka's Central Highlands UNESCO World Heritage Site. Given the presence of a Critically Endangered species, headwater streams and anthropogenic stressors, we recommend upgrading the forest to a Strict Nature Reserve, where actions can be taken to restrict human impacts, implement continuous monitoring and scientific research, and promote ecosystem-wide biodiversity conservation (Dudley, Reference Dudley2008).
Conclusion
Future studies should focus on enumerating the abundance of tadpoles, juveniles and subadults, population viability analyses, and the reproductive biology of A. kandianus, so that scientifically robust inferences can be made about the species’ population dynamics. Given that A. kandianus is now limited to two known populations, both small, we recommend a preliminary attempt at captive breeding and ex situ conservation. Given the species’ cryptic nature and seasonality we recommend that any future surveys use occupancy modelling to account for imperfect detection.
Our research methods could serve as a model for ecological studies on other less-studied, threatened, range-restricted amphibians. The conservation actions we recommend could be adapted for other threatened aquatic species, especially those of tropical montane streams, where there is a need for science-based conservation.
Acknowledgements
We express our sincere gratitude to Anslem de Silva, Thasun Amarasinghe, Kelum Manamendra-Arachchi, Mendis Wickramasinghe, the Forest Department, the Department of Wildlife Conservation, the National Museum of Sri Lanka (Sanuja Kasthuriarachchi, Nanda Wickramasinghe, Lankani Somaratne, Manori Nandasena, Chandrika Munasinghe and Rasika Dasanayake), and the Young Zoologists’ Association for help during this study.
Author contributions
SK and SH designed the field sampling protocols. SK, DG, MB and MM conducted the fieldwork and data entry. SH and TS conducted the statistical analyses and SH, TS, and SK wrote the article. SH and TS produced the maps, figures and tables. All authors contributed equally in finalizing the manuscript.
Biographical sketches
Suranjan Karunarathna is a field biologist, and his research focuses on herpetofaunal ecology, taxonomy and conservation of threatened species. He is an active member of the IUCN Amphibian Specialist Group. Sujan Henkanaththegedara’s research focuses on the ecology, conservation and taxonomy of aquatic species. Dinesh Gabadage is a field biologist. He studies the distribution of birds and mammals, and promotes wildlife conservation programmes in Sri Lankan communities. Madhava Botejue is the Regional Vice Chairman (South Asia & Iran) of the IUCN Crocodile Specialist Group. His research focuses on the ecology, distribution and conservation of herpetofauna, avifauna and mammals. Majintha Madawala is engaged in numerous habitat restoration and snake rescue programmes and is an active member of the IUCN Crocodile Specialist Group. Thilina Surasinghe’s research interests include landscape-scale biodiversity conservation, freshwater ecology, and conservation of threatened species.