Introduction
Memory decline is a pervasive complaint of older adults and can exact an enormous toll on individuals, their loved ones, and society (Livingston et al., Reference Livingston, Huntley, Sommerlad, Ames, Ballard, Banerjee and Mukadam2020). Physical exercise is a critical lifestyle intervention for promoting healthy brain aging, particularly preserving the hippocampus and memory function (Voss et al., Reference Voss, Soto, Yoo, Sodoma, Vivar and van Praag2019). Meanwhile, growing evidence suggests a single session of aerobic exercise may provide immediate benefits for hippocampal integrity, function, and memory performance (Callow et al., Reference Callow, Won, Alfini, Purcell, Weiss, Zhan and Smith2021; El-Sayes et al., Reference El-Sayes, Harasym, Turco, Locke and Nelson2019; Hillman, Erickson & Kramer Reference Hillman, Erickson and Kramer2008; Loprinzi et al., Reference Loprinzi, Roig, Etnier, Tomporowski and Voss2021). Specifically, a single session of low to moderate-intensity aerobic exercise upregulates hippocampal BDNF expression in rats (Huang et al., Reference Huang, Jen, Chen, Yu, Kuo and Chen2006; Soya et al., Reference Soya, Nakamura, Deocaris, Kimpara, Iimura, Fujikawa and Nishijima2007; Venezia, Quinlan & Roth, Reference Venezia, Quinlan and Roth2017) and leads to small to moderate improvements in long term episodic memory performance in humans (Labban & Etnier, Reference Labban and Etnier2011; Loprinzi et al., Reference Loprinzi, Roig, Etnier, Tomporowski and Voss2021; Roig et al., Reference Roig, Nordbrandt, Geertsen and Nielsen2013). While a single exercise session may not elicit the same magnitude of benefit for memory performance as long-term exercise training protocols, acute exercise paradigms are ideally suited to understand the temporal interactions between exercise and phases of memory (Loprinzi et al., Reference Loprinzi, Roig, Etnier, Tomporowski and Voss2021). Furthermore, understanding these mechanisms and the timeframe by which a single acute session, or dose, of exercise may promote hippocampal function and integrity is critical to understanding and optimizing long-term brain health interventions in older adults. Nevertheless, previous acute exercise studies have predominantly been conducted on younger adults and utilized nonspecific cognitive tasks that only partially tap into specific hippocampal function or integrity (Griebler et al., Reference Griebler, Schröder, Artifon, Frigotto and Pietta-Dias2022). There is a need to incorporate cognitive tasks that more directly engage and challenge the integrity of important age- susceptible episodic memory circuits to better ascertain the relationship between acute aerobic exercise and hippocampal function in older adults.
One such cognitive task is the Mnemonic Similarity Task (MST), which behaviorally has been shown to engage hippocampal circuits and integrity by placing high demands on pattern separation. During the MST participants are asked to view colored images of everyday objects and then to determine whether the images they view are new, similar, or old compared to previously encoded images. The focus of the task is on quantifying how well participants can accurately distinguish between the previously viewed (old) stimuli and newly presented but visually similar stimuli. The new stimuli, which vary in their degree of similarity to the old items, are termed “lures” (Lacy et al., Reference Lacy, Yassa, Stark, Muftuler and Stark2011). The Lure Discrimination Index (LDI) is a measure from the MST that represents the degree to which participants can successfully discriminate memories of highly similar old and new items. The LDI is the quantitative measure that operationally defines mnemonic discrimination capacity and has been strongly linked to hippocampal function (Stark, Kirwan & Stark, Reference Stark, Kirwan and Stark2019). Episodic memory encompasses the ability to encode, store, and retrieve unique facts or events in our life and is one of the most studied subdomains of memory as it is often the first process to deteriorate in both normal and pathological aging (Harvey, Reference Harvey2019; Tromp et al., Reference Tromp, Dufour, Lithfous, Pebayle and Després2015). Meanwhile, pattern separation, the process of reducing interference among memories, is thought to be a computation that supports episodic memory and is believed to be facilitated by the dentate gyrus (DG), a subfield of the hippocampus (McClelland, McNaughton & O’Reilly, Reference McClelland, McNaughton and O’Reilly1995; Yassa & Stark, Reference Yassa and Stark2011). Furthermore, the ability to accurately perform mnemonic discrimination and distinguish between similar experiences and event related memories is an important aspect of interacting with the world and functioning independently.
Mnemonic discrimination performance may be a useful marker of hippocampal function in older adults because the ability to behaviorally separate similar stimuli often declines earlier and more substantially during the aging process than other cognitive processes (Stark et al., Reference Stark, Stevenson, Wu, Rutledge and Stark2015; Stark & Stark, Reference Stark and Stark2017; Voss et al., Reference Voss, Soto, Yoo, Sodoma, Vivar and van Praag2019). Furthermore, age-related deterioration in mnemonic discrimination performance has been strongly linked to deterioration of hippocampal subfield specific microstructure and function, particularly within the DG (Berron et al., Reference Berron, Schütze, Maass, Cardenas-Blanco, Kuijf, Kumaran and Düzel2016; Lacy et al., Reference Lacy, Yassa, Stark, Muftuler and Stark2011; Yassa et al., Reference Yassa, Mattfeld, Stark and Stark2011). One hypothesis for this age-related deterioration is due to reduced neuroplasticity within the DG (Nakashiba et al., Reference Nakashiba, Cushman, Pelkey, Renaudineau, Buhl, McHugh and Tonegawa2012), as studies that directly stimulate neurogenesis within the DG show specific improvements in pattern separation performance (Sahay et al., Reference Sahay, Scobie, Hill, O’Carroll, Kheirbek, Burghardt and Hen2011).
Voluntary chronic exercise is known to reliably stimulate hippocampal neurogenesis. Animal studies have found regular exercise enhances neural plasticity, particularly within the DG of rats (Van Praag, Kempermann & Gage, Reference Van Praag, Kempermann and Gage1999), and can counteract age-related reductions in neural plasticity (Van Praag et al., Reference Van Praag, Shubert, Zhao and Gage2005; Siette et al., Reference Siette, Westbrook, Cotman, Sidhu, Zhu, Sachdev and Valenzuela2013) and pattern separation performance (Bolz, Heigele & Bischofberger, Reference Bolz, Heigele and Bischofberger2015; Creer et al., Reference Creer, Romberg, Saksida, Van Praag and Bussey2010). In humans, exercise training is associated with increased DG perfusion (Pereira et al., Reference Pereira, Huddleston, Brickman, Sosunov, Hen, McKhann and Small2007), DG volume (Frodl et al., Reference Frodl, Strehl, Carballedo, Tozzi, Doyle, Amico and O’Keane2019; Nauer et al., Reference Nauer, Dunne, Stern, Storer and Schon2019), and better mnemonic discrimination performance (Déry et al., Reference Déry, Pilgrim, Gibala, Gillen, Wojtowicz, Macqueen and Becker2013; Heisz et al., Reference Heisz, Clark, Bonin, Paolucci, Michalski, Becker and Fahnestock2017). While most studies focusing on the relationship between exercise and mnemonic discrimination performance have been conducted in younger college-aged adults, Bullock et al. (Reference Bullock, Mizzi, Kovacevic and Heisz2018) found a relationship between higher cardiorespiratory fitness and LDI across the lifespan and Kovacevic et al. (Reference Kovacevic, Fenesi, Paolucci and Heisz2020) found that a 3-month exercise training program improved LDI scores in older adults. While a growing body of research indicates that maintaining and improving aerobic fitness may benefit mnemonic discrimination and hippocampal function, less is known about the effects of a single session of exercise on hippocampal function in older adults.
Several recent studies suggest a single short bout of light to moderate-intensity aerobic exercise can benefit mnemonic discrimination and DG function in younger adults. Suwabe et al. (Reference Suwabe, Hyodo, Byun, Ochi, Yassa and Soya2017) found that 10 min of moderate-intensity aerobic exercise immediately improved the discrimination of high-interference memories during the MST in 21 younger adults, but there were no benefits for traditional object recognition. Suwabe et al. (Reference Suwabe, Byun, Hyodo, Reagh, Roberts, Matsushita and Soya2018) similarly found 10 min of low to moderate-intensity aerobic exercise led to improvements on moderate- and high-interference memories, but not object recognition, and led to an increase in CA3/DG activity and connectivity with several cortical regions (left angular gyrus, left fusiform gyrus, and left perirhinal cortex) in 16 younger adults. Finally, Bernstein and McNally (Reference Bernstein and McNally2019) found that 30 min of moderate-intensity aerobic exercise improved LDI in young to middle-aged adults with low depressive symptoms. Importantly, all three studies only tested mnemonic discrimination after the exercise intervention, and all three focused on young to middle-aged adults. Far fewer studies have tested the acute effects of exercise on memory in older adults (Roig et al., Reference Roig, Nordbrandt, Geertsen and Nielsen2013), which is surprising given older adults are more likely to experience memory deterioration and, therefore, may experience greater or differential benefits from acute exercise than younger adults (Chang et al., Reference Chang, Labban, Gapin and Etnier2012; Etnier, Vance & Ueno, Reference Etnier, Vance and Ueno2021; Griebler et al., Reference Griebler, Schröder, Artifon, Frigotto and Pietta-Dias2022).
Two studies have explored the relationship between acute exercise and episodic memory in older adults. Segal et al. (Reference Segal, Cotman and Cahill2012) found that 6 min of moderate-intensity aerobic exercise increased the number of words recalled on the Rey Auditory Verbal Learning Task (RAVLT) in healthy older adults (Segal, Cotman & Cahill, Reference Segal, Cotman and Cahill2012). In addition, Etnier et al. (Reference Etnier, Vance and Ueno2021) recently showed that 20 min of moderate-intensity aerobic exercise led to better word recall on the RAVLT in healthy older adults. It is important to note however, that both studies incorporated post-intervention only study designs, which permits comparisons between the exercise and rest conditions, but does not provide measures of change in memory performance from pre- to post-condition. Additionally, these two previous studies measured episodic memory with the RAVLT, which also involves language processing and verbal learning (Schoenberg et al., Reference Schoenberg, Dawson, Duff, Patton, Scott and Adams2006). Meanwhile, the MST is a modified object recognition task and the LDI measure is a more sensitive and specific proxy of hippocampal function and aging than RAVLT scores due to its more robust demand on mnemonic discrimination (Holden & Gilbert, Reference Holden and Gilbert2012; Stark et al., Reference Stark, Yassa, Lacy and Stark2013, Reference Stark, Kirwan and Stark2019). Therefore, understanding the effects of acute exercise on mnemonic discrimination performance via the MST in older adults, while incorporating a pre- and post-exercise study design could better explain the relationship between an acute bout of exercise and hippocampal function and integrity.
The purpose of this study was to determine the effects of an acute bout of moderate-intensity aerobic exercise on mnemonic discrimination performance (measured via LDI on the MST) in healthy older adults. Based on previous studies reporting on the effects of acute aerobic exercise on LDI in younger adults (Bernstein & Mcnally, Reference Bernstein and McNally2019; Suwabe et al., Reference Suwabe, Hyodo, Byun, Ochi, Yassa and Soya2017, Reference Suwabe, Byun, Hyodo, Reagh, Roberts, Matsushita and Soya2018) and previous studies suggesting the benefits of acute exercise for memory may be even more pronounced in older adults (Chang et al., Reference Chang, Labban, Gapin and Etnier2012; Etnier et al., Reference Etnier, Vance and Ueno2021; Roig et al., Reference Roig, Nordbrandt, Geertsen and Nielsen2013), we hypothesized that acute aerobic exercise would lead to higher LDI scores compared to a seated rest control condition. However, given previous studies focusing on MST performance have only employed or reported post-intervention results, we predict that there will be an interactive effect for mnemonic discrimination from pre- to post-intervention between the two conditions, but do not propose a specific directional hypothesis.
Methods
Participants
Forty-one physically active older adults (ages, 60–89 years) were recruited from the local community to participate in the study in accordance with the Helsinki Declaration. Participants were excluded if they reported a history of stroke, diabetes, untreated high blood pressure, neurological disease, major psychiatric disorder, had any contraindications to exercising on a bike, or were less physically active (less than 3 days/week of moderate-intensity physical activity). Six participants dropped out before completing the study, leading to a final sample of 35. All participants completed a baseline session, a rest session, and an exercise session (order of Rest vs. Exercise counterbalanced across participants; see Figure 1 ).
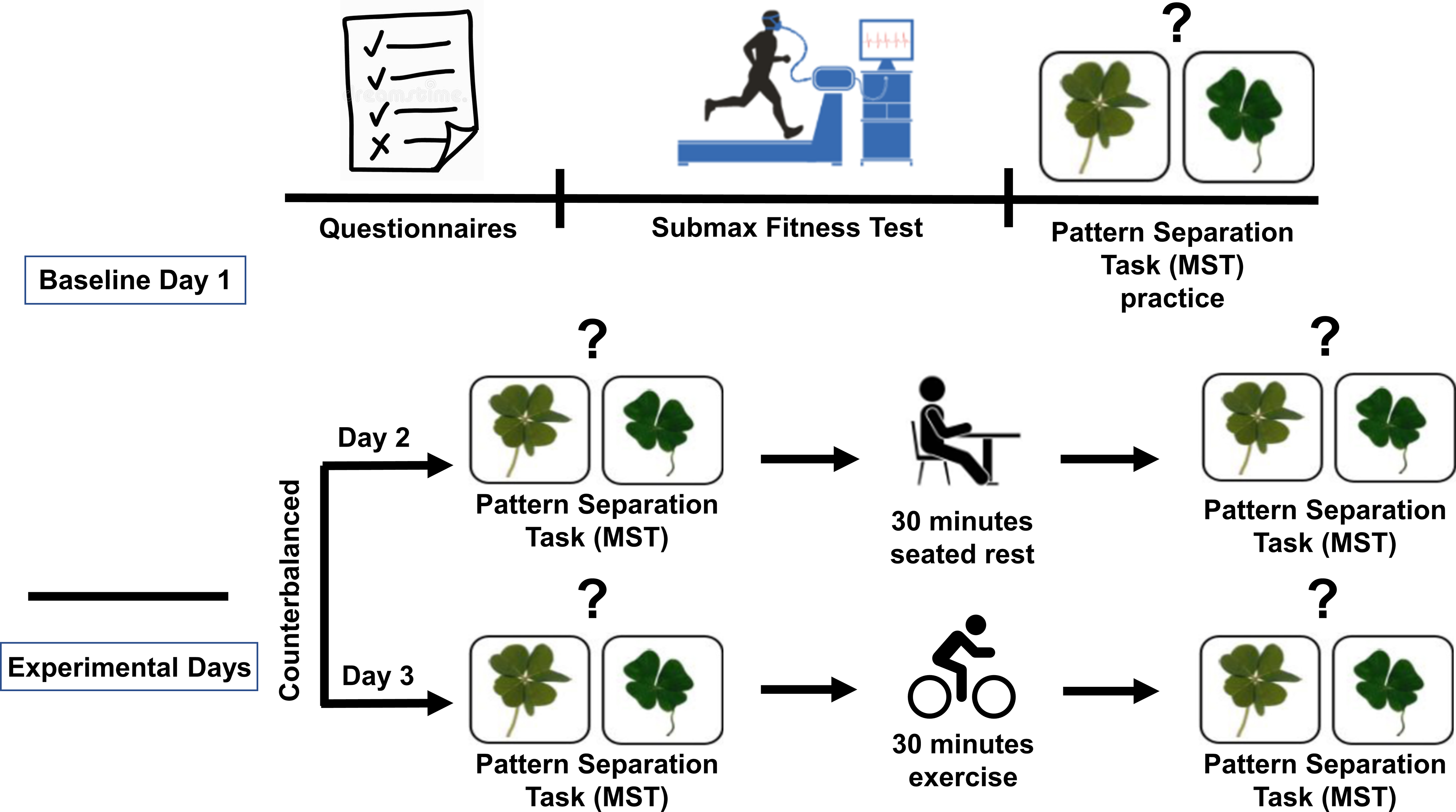
Figure 1. Graphical depiction of study design. Note, all participants completed all 3 days and all conditions with only the order of experimental conditions varying across participants. Comparisons between rest and exercise are therefore within-participant.
Submaximal exercise stress test
Participants performed a submaximal stress test on a cycle ergometer (Corival, Lode, Netherlands) and respiratory gases were monitored via open-circuit spirometry (True One 2400 integrated metabolic system). Briefly, a staged ramp protocol (Cress & Meyer, Reference Cress and Meyer2003) was employed where, following a two-minute warm up at 25 W, an initial 30 W resistance was set and increased by 10 W/min until termination criteria was reached. Throughout the test heart rate (HR) (Polar H9, Polar) and measures of ventilation including rate of oxygen (O2) consumption, rate of carbon dioxide (CO2) production, and the respiratory exchange ratio (RER; CO2 production/O2 consumption) were collected, while the ratings of perceived exertion (RPE; 6–20 scale administered with instructions consistent with (Borg, Reference Borg1982; Cook et al., Reference Cook, O’Connor, Eubanks, Smith and Lee1997)) scale was used to monitor subjective effort every minute. Tests were terminated upon attainment of 85% of participant’s age predicted maximal HR response (220-Age), participant’s request, or observations of exercise contraindications.
Mnemonic similarity task (MST)
The MST (Stark et al., Reference Stark, Yassa, Lacy and Stark2013) was performed on a computer at five different time points and consists of an encoding and retrieval phase. During the encoding phase participants were shown 128 colored images of everyday objects, one at a time, for 2.5 s each (.5 s Interstimulus Interval) and then asked to indicate whether the object was an “indoor” or “outdoor” item. Immediately following the encoding phase, participants were shown a short 2-minute video that provided instructions for the retrieval phase. Participants then immediately performed the retrieval phase in which they were shown 192 colored images one at a time and were asked to identify whether the items were “old,” “similar,” or “new” with a button press. Of the 192 items, 64 were repeats from the encoding phase (targets), 64 were similar but not identical to an image in the encoding phase (lures), and 64 were new images (foils). Trial types were presented randomly and separate stimulus sets were used for each test (Set 1 for the practice condition and Sets 2–5 counterbalanced across conditions). A total of 5 sets were used, with each set being equivalent in terms of the mnemonic similarity of their lures. Specifically, each lure image varied in its degree of similarity and was previously empirically ranked by assessing the false alarm rates (% old response) in a separate population (Lacy et al., Reference Lacy, Yassa, Stark, Muftuler and Stark2011). These lures were then divided into 5 lure bins based on false alarm rates and each set was given an equal number of lures for each bin (Stark et al., Reference Stark, Yassa, Lacy and Stark2013). Given our sample consisted of older adults, we implemented the self-paced version of the MST in which participants were shown an image for 2 s, after which time the screen went blank, and then the program waited for a button response before continuing (Stark et al., Reference Stark, Stevenson, Wu, Rutledge and Stark2015). Two primary measures were obtained from the MST. The first was a traditional object recognition memory measure which was calculated as the rate of “Old” responses to repeats, minus “Old” responses to foils (Old | Target – Old | Foil) to account for response bias. The second measure was LDI, a measure of mnemonic discrimination, which was calculated as the rate of “Similar” responses to Lures minus “Similar” responses to new objects (Similar | Lure – Similar | foil) to again control for response bias.
Baseline testing
Prior to the two experimental day visits, participants attended the baseline testing session. Upon arrival, participants provided written informed consent approved by Institutional Review Board. They then completed the Mini-Mental State Examination (MMSE), a 30-point questionnaire used to screen for global cognitive impairment, and demographic and baseline questionnaires to determine health history, physical activity (Stanford 7-day Physical Activity Recall questionnaire; (Sallis et al., Reference Sallis, Haskell, Wood, Fortmann, Rogers, Blair and Paffenbarger1985)), anxiety symptoms (Geriatric Anxiety Scale; (Segal et al., Reference Segal, June, Payne, Coolidge and Yochim2010)), and depression symptoms (Geriatric Depression Scale; (Yesavage et al., Reference Yesavage, Brink, Rose, Lum, Huang, Adey and Leirer1983)). Finally, participants performed Set 1 of the MST to allow for familiarization with the task and to minimize practice effects during the following two experimental visits (Stark et al., Reference Stark, Stevenson, Wu, Rutledge and Stark2015).
Exercise and rest conditions
At least 48 h after the baseline testing day, all participants underwent counterbalanced control (seated rest) and exercise conditions on separate days, with each participant completing both experimental visits at the same time in the morning each day. Participants were asked to refrain from performing moderate to vigorous physical activities within 24 h of testing, to eat a consistent breakfast, and to refrain from consuming coffee the morning of testing. All participants verbally confirmed that they followed the provided instructions at the beginning of each visit. Upon arrival participants completed the MST (Sets (2–5) and condition order (Exercise or Rest first) were counterbalanced across participants). Then participants completed either 20-minutes of moderate-intensity aerobic exercise or 30 min of seated rest. During both the exercise and rest condition participants’ HR, RPE, and subjective valence (pleasantness) and arousal ratings (Self-Assessment Manikin (SAM) (Bradley & Lang, 1994)) were taken every 5 min. For the moderate-intensity aerobic exercise condition, participants warmed up for the first 5 min and cooled down for the last 5 min of the 30 min session. During the middle 20 min of the moderate-intensity exercise session participants were instructed to exercise at a subjective rating of perceived exertion of 13 to 15 on the Borg 6–20 RPE scale (associated with a verbal anchor of “somewhat hard” to “hard”) and were permitted to adjust the resistance on the cycle ergometer in order to maintain a consistent relative moderate intensity. After a 5-minute seated cooldown period, participants completed a different set of the MST.
Statistical analysis
As a manipulation check, paired t-tests were conducted to determine differences in HR, RPE, valence, and arousal between the exercise and rest conditions. First, we looked at the relationship between age and pre-intervention LDI and object recognition performance using a linear mixed-effects model while controlling for gender. To determine the effects of acute exercise on memory performance, we next compared the change in object recognition and LDI performance between the two intervention conditions with a linear mixed-effects model in which participant ID was a random effect and Condition (exercise vs. rest) by Time (pre vs. post) was modeled as a fixed interaction effect. Main effects of Condition and Time were further reported for both object recognition and LDI scores. To control for any variance related to the age of the participant and order of conditions, Order and Age were included in the model as fixed effects. To compare our findings to previous studies using only post-intervention designs and to further determine the effects on object recognition and LDI performance, we performed a post-intervention only analysis with Order, Age, and Condition modeled as fixed effects and participant ID as a random effect. Finally, to compare our results to previous studies in younger (Suwabe et al., Reference Suwabe, Byun, Hyodo, Reagh, Roberts, Matsushita and Soya2018) and older adults (Segal et al., Reference Segal, Cotman and Cahill2012), we conducted a post hoc analysis to determine whether exercise-induced differences (Exercise minus Rest) in arousal levels were associated with exercise-induced differences (Exercise minus Rest) in post-intervention LDI scores using a Pearson correlation. To accomplish this, we ran a Pearson correlation on differences in arousal scores between the exercise and rest condition with differences in post-condition LDI scores between exercise and rest. Statistical significance was set to an a priori threshold of p < 0.05. All statistical analyses were performed using the R 4.0.1 statistical package.
Results
Participants
Of the 35 participants who completed all study protocols, four participants (2 males and 2 females) were excluded from further analysis due to exceptionally poor performance (<50%) on MST’s traditional object recognition memory component and one additional (male) participant was excluded due to a failure to use the “similar” response button at least ten times. These criteria have been similarly employed to remove participants that were not following task instructions (Kolarik, Stark & Stark, Reference Kolarik, Stark and Stark2020). A final sample size of 30 participants were included in the analyses (see Table 1).
Table 1. Participant Demographic information (n = 31)

Notes: bpm = beats per minute; RHR = resting heart rate; HR max = maximum age predicted heart rate; MMSE = Mini-Mental State Examination. kg/ml/min = kilogram per milliliter per minute. MET = ratio of working metabolic rate relative to energy at rest. 7-day Energy Expenditure = the total MET-hours completed in the last 7 day period. MET is a unit of energy expenditure relative to the resting metabolic rate. VO2peak = Peak oxygen consumption estimated from submaximal exercise stress test.
a American College of Sports Medicine 50th percentile for peak oxygen consumption of older adults aged 60+ is approximately 30 (male) & 27 (female).
b American Heart Association physical activity guidelines suggest 8.3–16.66 MET-hours/week for significant health benefits.
c MMSE scores below 27 indicate potential mild cognitive impairment.
d Geriatric Depression Scores between 9 and 15 indicate moderate to severe depression symptoms.
e Geriatric Anxiety Scores between 16 and 63 indicate moderate to severe anxiety symptoms.
“Participants were physically active, with an average 7 day metabolic equivalent (MET) of 56.5 h and with all participants getting at least 8.3 MET-hours (consistent with physical activity guidelines of 150 min of moderate-intensity physical activity per week (Kaminsky & Montoye, Reference Kaminsky and Montoye2014)). Additionally, participants were cognitively healthy (MMSE > 26) and did not have major depressive (GDS < 12) or anxiety (GAS < 22) symptoms.” (Page 12/13)
Age and baseline behavioral performance
While controlling for gender, we found that pre-intervention LDI scores (F(1,30)=5.08, p = .032), but not object recognition scores (F(1,30) = 0.684, p = .415), were negatively associated with age.
Experimental manipulation check
As expected, HR (t(59) = 22.21, p < .001), RPE (t(59) = 39.20, p < .001), and arousal (t(59) = −5.82, p < .001) were significantly higher during the exercise condition compared to the seated rest condition, see Table 2. There were no significant differences in valence (t(59) = −0.37, p = .709) values between conditions.
Table 2. Experimental condition outcomes and manipulation check

Notes: SD = standard deviation. Measures of HR = heart rate; BPM = beats per minute; RPE = rating of perceived exertion. Valence = subjective measure of valence; Arousal = subjective measure of arousal; All measures were averaged and compared over the final 10 min of the moderate-intensity exercise session (minutes 15–25 of the experimental conditions). Reaction Time = the average response time (in milliseconds) that participants took for each response during the test phase of the MST. Average participant heart rate in the final 10 min of the exercise condition was approximately 78% (SD 11%) of age predicted maximal heart rate. This is consistent with a moderate to hard intensity rating based on ACSM guidelines (American College of Sports Medicine, 2013).
Time by condition analysis
While controlling for the Order and participant Age, the interaction effect of Time by Condition on object recognition performance was not significant (F(1,90) = 1.32, p = .254, η2 = .01). Furthermore, there was no significant main effect of condition (F(90,1) = 537, p = .770), but there was a significant main effect of Time with participants on average performing better post-intervention (F(1,90) = 1.32, p = .004, η2 = .09). Additionally, while there was no difference in pre-exercise versus rest (t(93) = 0.23, p = .992) or for post-exercise versus rest object recognition performance (t(93) = −.13, p = .561), there was a significant increase in object recognition performance from pre- to post-exercise (t(93) = 2.88, p = .025) (Figure 2). With respect to LDI performance, there was a significant main effect of Time (F(1,90) = 4.20, p = .04, η2 = .04), but not Condition (F(1,90) = 2.17, p = .144), with participants on average performing worse post-intervention compared to pre-intervention. Furthermore, there was a significant interaction effect of Time by Condition on LDI performance (F(1,90) = 6.65, p = .012, η2=.07). Specifically, while pre-rest LDI was not significantly different from pre-exercise LDI (t(93) = 0.77, p = .868), there was a significant decline in LDI from pre-rest to post-rest (t(93) = −3.22, p = .010). Additionally, there was no significant decline in LDI from pre-exercise to post-exercise (t(93) = 0.37., p = .983) (Figure 2).

Figure 2. Panel (a) depicts raw LDI (Lure Discrimination Index) score before and following both the exercise and rest condition (error bars = 1 SEM). * Indicates a significant interactive effect of Time (pre vs. post) by Cond (exercise vs. rest) on LDI performance while controlling for condition Order and participant Age. ** Indicates a significant decrease in LDI from pre- to post-rest while NS indicates a non significant difference in LDI scores from pre- to post-exercise. Panel b) depicts raw Object Recognition performance before and following both the exercise and rest condition (error bars = 1 SEM). * Indicates a significant increase in object recognition from pre- to post-exercise while NS indicates a non significant difference in object recognition scores from pre- to post-rest and a nonsignificant interactive effect. *p < .05; **p < .01; NS (p > .05).
Post-intervention analysis
While controlling for condition Order and participant Age, the post-intervention analysis showed no significant effect of Condition on post-condition object recognition performance (F(1,30) = 1.83, p = .186, η2 = .06). Meanwhile, LDI was significantly higher following exercise compared to rest (F(1,30) = 8.29, p < .001, η2 = .22), see Figure 3.
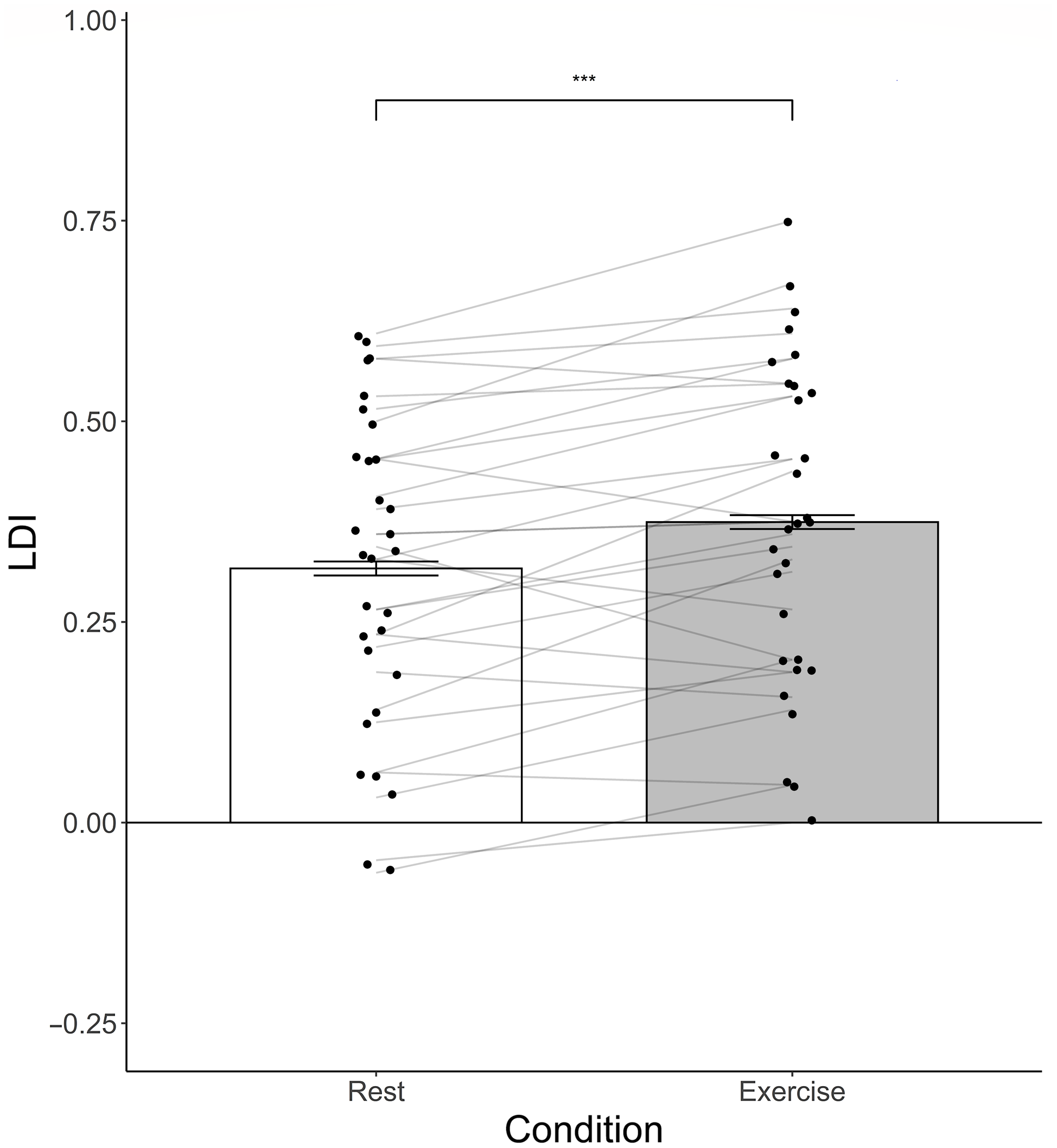
Figure 3. Bar graph of raw LDI (Lure Discrimination Index) performance following both the exercise and rest condition (error bars depict standard errors). *** Indicates a main effect of Condition (Exercise vs. Rest) on LDI performance while controlling for condition Order and participant Age. *** p < .001.
Arousal and post-condition behavioral performance
We found a significant increase in self-rated arousal in the last 10 min of the exercise condition compared to the rest condition (t(59) = −5.88, p-value <.001), see Table 2. However, exercise-induced differences in post-intervention LDI scores were not associated with exercise-induced differences in self-rated arousal levels (r = .044, p = .817).
Discussion
The primary purpose of this study was to determine if moderate-intensity aerobic exercise alters mnemonic discrimination performance in healthy older adults. In a group of 30 healthy older adults, we found that a 30-minute session of moderate-intensity aerobic exercise did not have a significant interactive effect or main effect of Condition on object recognition. However, acute exercise led to better maintenance of LDI scores from pre- to post-condition performance compared to the seated rest condition. Exercise also resulted in a significantly higher post-exercise LDI score compared to after seated rest. Additionally, we found that pre-intervention LDI scores were negatively related to age. These results suggest moderate-intensity aerobic exercise may promote better hippocampal-specific function via reduced interference and better mnemonic discrimination of similar objects in healthy older adults.
Age and mnemonic discrimination
LDI scores on the two pre-intervention tasks were negatively related to age which is consistent with numerous previous studies (Stark et al., Reference Stark, Yassa, Lacy and Stark2013, Reference Stark, Stevenson, Wu, Rutledge and Stark2015; Stark & Stark, Reference Stark and Stark2017), showing that aging is associated with poorer discrimination of similar objects on the MST task, but not with object recognition. We further explored this relationship and found that age was negatively associated with correctly identifying lure images and was positively associated with incorrectly identifying a lure image as a repeated image, suggesting a worsening ability to discriminate images with increased age and indicating a shift in older age from behavioral pattern separation to pattern completion (Nauer, Schon & Stern, Reference Nauer, Schon and Stern2020). Critically, there was no significant relationship between age and object recognition performance (i.e., identifying a repeated image correctly). This is consistent with widely reported age-specific effects on mnemonic discrimination that cannot be attributed to age-related differences in object recognition memory (Holden et al., Reference Holden, Toner, Pirogovsky, Kirwan and Gilbert2013; Stark et al., Reference Stark, Yassa, Lacy and Stark2013).
These behavioral findings corroborate previous computational and animal findings. Specifically, computational and animal models of pattern separation propose that newborn neurons within the DG of the hippocampus are critical for distinguishing between highly similar information and storing the information as separate and distinct representations (Kesner & Rolls, Reference Kesner and Rolls2015; Marr, Reference Marr1971; Rolls & Kesner, Reference Rolls and Kesner2006). Furthermore, aging rodents (Kuhn, Dickinson-Anson & Gage, Reference Kuhn, Dickinson-Anson and Gage1996) and potentially humans (Kempermann et al., Reference Kempermann, Gage, Aigner, Song, Curtis, Thuret and Frisén2018; Moreno-Jiménez et al., Reference Moreno-Jiménez, Flor-García, Terreros-Roncal, Rábano, Cafini, Pallas-Bazarra and Llorens-Martín2019) appear to exhibit fewer newborn neurons within the DG. Meanwhile, reductions in newborn DG neurons are directly associated with poorer pattern separation performance in rodents (Creer et al., Reference Creer, Romberg, Saksida, Van Praag and Bussey2010; Sahay et al., Reference Sahay, Scobie, Hill, O’Carroll, Kheirbek, Burghardt and Hen2011). In humans, it is not possible to directly measure DG neurogenesis, and thus, we can not definitively determine the underlying mechanisms for this relationship. Additionally, a recent study suggests that LDI deficits are significantly associated with deficits in visual perception (Davidson et al., Reference Davidson, Vidjen, Trincao-Batra, Collin and Gutchess2019) and thus, age related deficits in visual perception could be partially contributing to our finding of an age and behavior relationship.
Acute exercise and object recognition performance
Interestingly, while we found an improvement in object recognition from pre- to post-intervention, we found no interactive effect or main effect of condition for object recognition performance. This suggests that acute exercise does not significantly affect simple object recognition performance for healthy older adults when compared to seated rest. Notably, the MST’s measure of object recognition provides a valuable comparison to mnemonic descrimination, as object recognition is considered to be less heavily implicated in hippocampal function (Stark et al., Reference Stark, Kirwan and Stark2019), given it is relatively robust to aging and in those who have sustained hippocampal damage (Kirwan et al., Reference Kirwan, Hartshorn, Stark, Goodrich-Hunsaker, Hopkins and Stark2012; Stark et al., Reference Stark, Yassa, Lacy and Stark2013). This finding aligns with previous acute exercise studies that have examined mnemonic discrimination performance in younger adults. For example, Suwabe et al. (Reference Suwabe, Hyodo, Byun, Ochi, Yassa and Soya2017, Reference Suwabe, Byun, Hyodo, Reagh, Roberts, Matsushita and Soya2018) reported improvements in mnemonic discrimination performance but not object recognition. Furthermore, Bernstein and McNally (Reference Bernstein and McNally2019) did not report a significant effect of acute moderate-intensity aerobic exercise on object recognition performance compared to a stretching condition. Taken together, this suggests the effects of acute aerobic exercise on memory and hippocampal function, at least for visual objects, may be somewhat specific to mnemonic discrimination performance.
Mnemonic discrimination interaction effect
We found LDI scores were better maintained following the exercise condition but decreased after rest. Furthermore, we found a main effect of time in which participants had worse LDI scores during the post-intervention test than the pre-intervention test, likely predominantly driven by the rest condition. While decreased or maintained performance may seem counterintuitive to the expected increase in performance after exercise (or due to practice), this finding suggests participants were further challenged when performing the task for a second time in the post-intervention task, which required disentangling similar images in the context of extra interference induced by the previously viewed images from the pre-intervention task. Notably, participants performed different versions of the task at each time point with entirely different images that did not overlap with each other (Stark et al., Reference Stark, Kirwan and Stark2019). Previous work by Stark and colleagues (Reference Stark, Stevenson, Wu, Rutledge and Stark2015) has shown that the MST can be used repeatedly in the same person to determine changes in performance over time. However, these previous studies performed repeat tests using different temporal spacings (either immediately or days between tests) and often compared standard versus overt instructions during the encoding phase, to try to determine the effects of repeated testing on performance (Kolarik et al., Reference Kolarik, Stark and Stark2020; Stark et al., Reference Stark, Stevenson, Wu, Rutledge and Stark2015). However, previous work has not compared repeated task performance within the moderate time window we employed (approximately 35 min). Interestingly, however, we had numerous cases in which participants provided unsolicited feedback after the experimental session had ended that they found the post-condition version of the task more challenging than the pre-condition version due to interfering memories of the pre-condition task images. While the main effect of reduced LDI scores over time might suggest that reductions in post-condition task performance could be due to some level of interference, future studies will need to specifically test whether repeated testing over short time intervals negatively affects LDI performance.
Nevertheless, we did find an interaction effect in which participants’ LDI scores were maintained from pre- to post-test for the exercise but not the rest condition. Previous acute exercise studies focusing on the MST and mnemonic discrimination have thus far only performed post-intervention study designs (Suwabe et al., Reference Suwabe, Hyodo, Byun, Ochi, Yassa and Soya2017, Reference Suwabe, Byun, Hyodo, Reagh, Roberts, Matsushita and Soya2018) or failed to report pre- to post-intervention results (Bernstein & Mcnally Reference Bernstein and McNally2019). Additionally, while several previous studies have found that the effects of acute exercise were dependent on the degree of lure similarity in healthy younger adults (Suwabe et al., Reference Suwabe, Hyodo, Byun, Ochi, Yassa and Soya2017, Reference Suwabe, Byun, Hyodo, Reagh, Roberts, Matsushita and Soya2018), we did not find that the degree of lure similarity interacted significantly with treatment condition in our older adult sample (see Supplemental Table 1 and Supplementary Figure 1). Interestingly, previous work by Segal et al. (Reference Segal, Cotman and Cahill2012) found 6 min of walking after viewing a set of emotional images led to elevated endogenous norepinephrine and better recall of emotional image details in older adults, suggesting exercise may retrogradely enhance memory consolidation of images. Concerning our current findings, this could mean participants who performed exercise between the two sets of MST tasks may remember more details of previously depicted images, and thus, be more resistant to interference from the previously viewed images during the post-intervention test phase.
In support of this hypothesis, Etnier et al. (Reference Etnier, Vance and Ueno2021) recently tested the effects of acute exercise timing on RAVLT performance in healthy older adults. They found 20 min of moderate-intensity aerobic exercise improved the number of words recalled in healthy middle-aged and older adults. Specifically, they found that an exercise prior condition led to the greatest improvement in words recalled and that this improvement was specific to the short-term (Trial 1), learning portion (Trials 1–5), and the retroactive interference portion (Trial 7) of the RAVLT, but not for the proactive interference trial (Trial 6) (Etnier et al., Reference Etnier, Vance and Ueno2021). This suggests that while acute aerobic exercise may not affect verbal recall on an interference list, it did lead to significantly better post-interference recall and suggests that aerobic exercise might help overcome interference issues when performing memory tasks. However, it is also important to note that there was not a significant improvement in LDI from pre- to post-exercise condition. Given our control condition consisted of 30 min of quite seated rest, which has the potential to elicit negative emotional and cognitive effects, could be leading to a reduction in LDI. Yet, there were no differences in subjective measures of pleasantness (valence) between the exercise and rest condition and differences in arousal between the two conditions were not related to differences in LDI scores. Thus, our data do not support the view that unpleasant emotion or boredom of the rest condition explains the memory performance differences between the conditions. Nevertheless, future studies should consider implementing more active or engaging control conditions to better determine if benefits in MST performance are specific to a single session of exercise.
Post-exercise improvements
Previous studies looking at the effects of acute aerobic exercise on mnemonic discrimination in younger adults have only reported post-condition effects. We took the same approach and found that following the 20 min of moderate-intensity aerobic exercise, older adults had significantly higher LDI scores compared to following the seated rest condition. This finding is in line with three previous studies conducted in healthy younger adults, all of which found 10–30 min of light to moderate-intensity aerobic exercise on a cycle ergometer led to better LDI scores via improvements in the discrimination of mnemonically similar objects (Bernstein & Mcnally, Reference Bernstein and McNally2019; Suwabe et al., Reference Suwabe, Hyodo, Byun, Ochi, Yassa and Soya2017, Reference Suwabe, Byun, Hyodo, Reagh, Roberts, Matsushita and Soya2018). Furthermore, previous work has shown an inverted-U-shaped dose–response relationship, where moderate-intensity aerobic exercise between 15 and 30 min appears to be optimal for eliciting benefits in complex cognitive processes, including memory (Chang et al., Reference Chang, Labban, Gapin and Etnier2012; Hacker et al., Reference Hacker, Banzer, Vogt and Engeroff2020). It is important to note that acute exercise paradigms are ideally suited to understand the temporal interactions between exercise and phases of memory. In particular, looking at the effects of exercise before the encoding phase and the incorporation of retrieval shortly after suggests these effects may be more specific to encoding mechanisms (Loprinzi et al., Reference Loprinzi, Roig, Etnier, Tomporowski and Voss2021). However, to truly disentangle these effects from storage/consolidation, a study that specifically compares pre-encoding versus post-encoding interventions (between the encoding and test phase) would be needed. Nevertheless, these potential benefits are relevant given that numerous previous studies conducted in college-aged adults have shown that exercising shortly before, but not during or after a memory task leads to improvements in both short and long-term memory (Firth et al., Reference Firth, Stubbs, Vancampfort, Schuch, Lagopoulos, Rosenbaum and Ward2018; Labban & Etnier, Reference Labban and Etnier2011; Sng, Frith & Loprinzi, Reference Sng, Frith and Loprinzi2018). Etnier et al. (Reference Etnier, Vance and Ueno2021) similarly found improvements in short and long-term memory performance on the RAVLT in healthy older adults when employing a similar length (20 min) and intensity (moderate) of acute aerobic exercise in middle and older-aged adults. Interestingly, we found a relatively large positive effect of exercise on post-condition LDI scores (η2 = .22), which is consistent with effects reported in older adults by Etnier et al. (Reference Etnier, Vance and Ueno2021). Unfortunately, previous acute exercise studies on mnemonic discrimination in younger adults did not report effect sizes for direct comparisons (Bernstein & Mcnally, Reference Bernstein and McNally2019; Suwabe et al., Reference Suwabe, Hyodo, Byun, Ochi, Yassa and Soya2017, Reference Suwabe, Byun, Hyodo, Reagh, Roberts, Matsushita and Soya2018). However, our finding of a relatively large effect size supports our previous hypothesis, which may relate the premise that older adults have more room to benefit from behavioral interventions given they are theorized to have less cognitive reserve, and younger adults have greater potential for performance ceiling effects (Chang et al., Reference Chang, Labban, Gapin and Etnier2012; Etnier et al., Reference Etnier, Vance and Ueno2021; Loprinzi et al., Reference Loprinzi, Roig, Etnier, Tomporowski and Voss2021; Reuter-Lorenz & Park, Reference Reuter-Lorenz and Park2014). However, since our study includes older adults from a relatively narrow age range, future studies are needed to determine if age moderates this effect.
Strengths and limitations
Our study is the first to show, in healthy older adults, effects of moderate-intensity aerobic exercise on a highly hippocampal-specific and age susceptible mnemonic discrimination task. Furthermore, this is the first study to measure and compare MST performance before and after an exercise and control intervention, providing more rigorous insight into the effects of aerobic exercise on mnemonic discrimination. By accounting for baseline LDI scores, we were able to determine how participants’ performance changed over time. However, our study sample was predominantly White, well-educated, and female, making it challenging to generalize at the population level. Furthermore, our volunteers were all physically active individuals, who may respond differently to a single exercise session compared to more sedentary individuals.
Conclusions
In conclusion, this is the first study to show effects of a single 30-minute session of moderate-intensity aerobic exercise on mnemonic discrimination in healthy older adults relative to rest. We found exercise led to better discrimination of similar objects and better maintenance of mnemonic discrimination capacity than following the seated rest control condition. Furthermore, and consistent with the literature, we found that baseline mnemonic discrimination was negatively related to age in our sample. Our results suggest a single session of moderate-intensity aerobic exercise may provide benefits for hippocampal-specific memory function and thus, provide evidence for exercise as a safe and easy-to-implement intervention to maintain healthy cognitive function.
Conflicts of interest
This work was supported by the Department of Kinesiology at the University of Maryland. None of the authors have conflict of interests to report. DC contributions to this research were supported in part by NSF award DGE-1632976 and NIH award F31 AG074670-01.







