Background
In England, 886 million National Health Service prescription items were dispensed in 2009 via community pharmacies and dispensing doctors. As compared with the 1999 total, this represented an increase in the number of items supplied per capita of over 60% (The National Health Service (NHS) Information Centre Prescribing Support Unit, 2010). Such growth trends are expected to continue around the world owing to the development of new treatments, new indications for existing medicines and the needs of ageing populations. Today, about one person in six is aged 65 years or above, as against just one in ten when the NHS was first established in England in the late 1940s. Population ageing and an increased use of relatively low-cost medicines for indications such as cardiovascular disease risk reduction and management are postulated to be among the main reasons for this increase. Figures from the United States tell a similar story. The percentage of Americans who took two or more drugs in the past month has increased from 25% to 31% over the last decade (Gu et al., Reference Gu, Dillon and Burt2010).
Problems with managing prescribing volumes and medicine use have been highlighted by agencies such as the National Audit Office (National Audit Office, 2007). They criticised both the prescribing of doctors and the levels of pharmaceutical waste generated by factors such as inappropriate repeat dispensing practices. Primary care organisations (PCOs), supported by the Department of Health (DoH) and independent professional bodies like the Royal Pharmaceutical Society of Great Britain (RPSGB), have in response sought to improve the value for money provided in this area of health-care spending.
In England, the vast majority of prescriptions are supplied to the public through community pharmacies paid through a nationally negotiated contractual framework. Officials from the DoH and representatives of pharmacy contractors from the Pharmaceutical Services Negotiating Committee (PSNC) negotiate the payments made for the supply of medicines and professional services and also decide upon a global sum to be shared among all pharmacy contractors. This is currently in the region of about £2.3 billion. From this amount, pharmacy contractors receive a 90p professional fee for each item they supply (DoH and the Welsh Assembly Government, 2010). In addition, they also receive special fees and allowances for activities such as keeping controlled drug supply records.
On average, these additional payments equate to about 7.1p per item (National Audit Office, 2010). To support overheads, contractors who dispense over 2240 items per month also receive a practice payment equivalent to 70.9p per item (DoH and the Welsh Assembly Government, 2010). Following the supply of an item, pharmacy contractors receive reimbursement for the cost of the medicine provided, according to a government regulated tariff, and a fee for providing a professional service irrespective of the quantity of product supplied. To reflect the advantages associated with purchasing large quantities of medicine, a percentage of this fee is ‘clawed back’ according to a metric based on prescription item volume. Pharmacy contractors also receive other income relating to prescription volume, including establishment payments to support the infrastructure of the pharmacy. (More information on the contractual framework is available at www.psnc.org.uk). It is important to note that while drug reimbursement costs are based on the actual quantity of medicine supplied, the professional fees are paid on a per item basis, meaning that the pharmacist receives the same professional fees for supplying 28, 56 or 84 tablets.
During the 1990s, several studies explored the opportunities available for reducing the drug stocks held by patients at any one time, and where possible preventing the potentially hazardous and wasteful stockpiling of medicines. Reducing the duration of the average prescription emerged as a possible means of achieving these ends. For example, an influential investigation published in 1996 of unused medicine returns made to 30 community pharmacies over a one-month period (Hawksworth, Reference Hawksworth1996) found a positive correlation between prescription lengths and the volume as well as cost of the drugs brought back to the participating pharmacies. The authors of this research claimed that if all prescription supplies could be limited to 28 days then wastage would be reduced by a third, albeit that they did not offset the financial savings implied by the possible cost increases involved in the associated fees to pharmacies.
Following such research, a significant proportion of prescribers were advised to reduce the length of their prescriptions in order to curb medicine wastage. This practice was supported by the PSNC (PSNC, 2007), the DoH (DoH, 2004) and the National Prescribing Centre (National Prescribing Centre, undated). Since their formation in 2002, many Primary Care Trusts (PCTs) sought to restrict prescription lengths while also investing in awareness raising activities such as DUMP (dispose of unused medicines properly) campaigns. Even if the latter do little directly to curb ongoing waste, they may encourage and/or legitimate other interventions (York Health Economics Consortium and School of Pharmacy, University of London, 2010).
Certain medicines, most notably the combined oral contraceptive pill, have been excluded from such restrictions on the grounds that they are relatively inexpensive long-term use products that normally require limited follow-up care. Healthy young women wishing to use ‘the pill’ might be expected to oppose robustly the inconvenient imposition of unduly short supply durations. Older patients might be expected to behave differently.
The provision of levothyroxine for conditions associated with thyroid deficiency offers another example of a medication that might rationally be expected to be supplied via long duration prescriptions. Yet research conducted by the British Thyroid Foundation (Mitchell et al., Reference Mitchell, Hickey, Hickey and Pearce2009a; Reference Mitchell, Hickey, Hickey and Pearce2009b) found that in 2008/09 about a third of PCTs were seeking to apply a 28-day limit on all levothyroxine prescriptions. In other parts of the country, PCTs were ‘allowing’ two-, three- or even six-month prescriptions. However, in more restrictive areas it appears that many GPs accepted that the 28-day prescribing rules should apply to all medicines being taken for long-term conditions (White, Reference White2010).
The extent to which this policy has in fact reduced the cost of medicine waste and other problems is difficult to estimate, not least because of the growing use of relatively low-cost generic medications (The NHS Information Centre Prescribing Support Unit, 2010). The latter has been partly responsible for a fall in the (non-inflation adjusted) net ingredient costFootnote 1 per prescription item to £9.64 in 2009, from £9.99 in 1999 (The NHS Information Centre Prescribing Support Unit, 2010).
A number of studies conducted outside the UK environment highlight the potential importance of such observations. For example, in 2004, US researchers investigating medicine supply via Medicaid concluded that restricting prescriptions to <100 days would not be cost-effective, as the savings made via wastage prevention would be outweighed by increased pharmacy service costs (Domino et al., Reference Domino, Olinick, Sleath, Leinwand, Byrns and Carey2004). Similarly, a New Zealand project that increased the length of prescriptions from around 30 days to 90 days indicated a saving of in excess of NZ $ 100 million. This occurred because the resultant pharmacy cost reductions were greater than the increase in medication wastage observed.
It should not be assumed that such conclusions necessarily apply to the British setting. However, there is a clear case for believing that such a possibility might exist. Furthermore, the British Thyroid Foundation revealed that nearly two-thirds of patients were dissatisfied with 28-day prescriptions for levothyroxine (Mitchell et al., Reference Mitchell, Hickey, Hickey and Pearce2009a; Reference Mitchell, Hickey, Hickey and Pearce2009b). This calls into significant question the desirability of trends observed in this context (White, Reference White2010).
Against this background, the aim of this research is to discover how prescriptions lengths in England have changed over time, with a secondary aim of contributing new evidence relating to the public's interests in prescription durations, and to offer possible policy recommendations.
Design and methods
Each year the DoH publishes a set of Prescription Cost Analysis (PCA) statistics. These data provide details of the number, content and costs of all the prescription items dispensed in the community in England. These data are based on information systems at NHS Prescription Services, part of the NHS Business Services Authority. These data are collected as part of the process of reimbursing for medicines supplied.Footnote 2
Prescriptions written by General Medical Practitioners in England represent the vast majority of prescriptions included. Prescriptions written by dentists and hospital doctors are also included provided that they were dispensed in the community. Also included are prescriptions written in Wales, Scotland, Northern Ireland and the Isle of Man but dispensed in England. Prescriptions written in England but dispensed outside England are not included. The data do not cover items dispensed in hospital or on private prescriptions.
The analysis offered here relates to the period January 1998 to December 2009 inclusive. The information available allows for the calculation of the average quantity of dosage units (tablets or capsules) supplied per prescription item in each year. This serves here as a proxy for prescription duration. Liquid and injectable formulations of medications were excluded, as comparable volume data is not easily accessible.
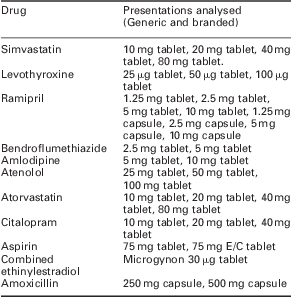
In total, 11 drugs, available as 34 different dosage and strength presentations and in over 60 forms (ie, different brand and generic items) were included in the sample analysed. They were selected because they featured in the ‘top 20’ most frequently prescribed agents in 2009 for which dosage units could be considered an appropriate proxy for prescription length. Nine long-term medications used in chronic conditions (simvastatin, levothyroxine, ramipril, bendroflumethiazide, amlodipine, atenolol, atorvastatin, aspirin and citalopram) were selected, in part, because of the high volume of these products dispensed. Other selection criteria included the characteristic of being taken via a specified dosing schedule (ie, they are not PRN or ‘take as required’ medications), their availability in countable dosage forms and data being available for the period analysed.
Because prior investigations indicated that PCT prescribing advisers usually accept that contraceptive products should be exempted from 28-day supply requirements, Microgynon – the most commonly prescribed combined oral contraceptive – was also included for comparative purposes. Finally, antibiotics are subject to different prescribing restrictions compared with chronic medications as they are usually prescribed for a short defined period of time. Therefore, amoxicillin capsule supply duration was interrogated as a comparator to the chronic medications because it is the most commonly prescribed antibiotic in the community setting.Footnote 3 This would aid the assessment of the a priori hypothesis that prescription durations had decreased in the past decade. A full list of the presentations analysed is shown in Table 1.
In combination they accounted for 194 million of the prescription items dispensed in 2009, representing ∼20% of the prescription items supplied.
Table 1 Presentations analysed
For each drug, all data relating to branded and generic formulations were extracted from the published PCA tables. Data extraction was checked manually to ensure accuracy. Preparation data was then collated to provide values for each drug. This was subsequently analysed using Microsoft Excel 2010 and SPSS V16. Trends in the average number of unit doses (tablets or capsules) supplied per prescription were analysed using Pearson r and linear regression analysis. The complete data set is summarised in Table 2.
Table 2 Prescription lengths comparison

*Rounded to the nearest thousand.
X – product not available in 1999.
Results
All of the long-term use medications analysed showed a clear trend over time towards a reduced number of doses being supplied per prescription. Typically, the mean quantity supplied fell by five doses on each prescription over a 10-year period (Table 2).
To illustrate the nature of the calculations made, the PCA data showed that, in 2009, 7 066 184 prescriptions were written for levothyroxine 50 μg tablets (Figure 1), and that overall 306 141 376 tablets were supplied in that year. This equates to an average of 43.32 tablets per prescription. By contrast, in 1999 3 099 000 prescriptions were written for levothyroxine 50 μg tablets, and 228 594 600 tablets were supplied. This equates to an average of 73.76 tablets per prescription. If all the tablets supplied in 2009 had been supplied via prescriptions written to the 1999 mean, this would have resulted in 4 150 282 prescription items, rather than the 7 066 184 actually dispensed. The close to 3 million item discrepancy between these totals is indicative of the scale of the dispensing workload that could have been avoided if the number of tablets per prescription had not been reduced.

Figure 1 Levothyroxine
Other than in the case of amoxicillin, where the correlation was positive, the majority of the drugs included showed a strong statistically significant (P < 0.001) negative correlation between the year of prescribing and the mean number of doses per prescription. The exceptions to this were citalopram 40 mg tablets (r = 0.631, P = 0.28), ramipril 10 mg tablets (r = 0.546, P = 0.205) and the combined oral contraceptive, microgynon (r = 0.647, P = 0.023; Figure 2). The mean number of doses per prescription decreased by approximately seven tablets over the last decade for atenolol, bendroflumethiazide and amlodipine (Figure 3), which are commonly used to treat hypertension in primary care. Ramipril tablets were not widely available until 2003, which may well explain the non-significant trend observed in this context. The ramipril capsules show an average decrease of nine capsules against a typical initial volume of 50 across all the different strengths in the 11-year period assessed.

Figure 2 Microgynon combined ethinylestradiol 30 μg

Figure 3 Antihypertensives
The mean number of statin doses per prescription reduced by about six tablets against a starting total of just over 40 in the period concerned (Figure 4). The 80 mg atorvastatin presentation did not enter the market until 2000, and is only featured in the data available from 2001. Similarly simvastatin 80 mg does not feature in the data until 2000.

Figure 4 Statins
It was anticipated that there would be no significant change in the mean number of doses for amoxicillin capsules on each prescription. However, these capsules showed a shift towards increased prescription duration. In overall (population level) volume terms the number of 250 mg capsules supplied decreased, whereas the volume of 500 mg capsules prescribed has markedly increased (Table 2).
The reduction in the mean number of doses per prescription observed in the decade 1999–2009 varies considerably between the different medicines analysed. The observed range was from an increase of 4.2% in the case of amoxicillin 500 mg capsules to a fall of 41.3% in that of levothyroxine 50 μg tablets. The prescribing of aspirin 75 mg tablets also reduced markedly (circa 35% in volume per item terms).
The results shown suggest that, overall, if the average prescription length had been kept the same as in 1999, then some 35 million fewer prescription items would have been dispensed in 2009 than was actually the case for the preparations included in this sample.
Discussion
This shift across a range of medications suggests a generalised change in prescribing behaviour, associated with both local and national policies and interventions. Amoxicillin showed an opposite trend to the chronic medications, with longer prescription durations and a greater volume of higher strength capsules. This could in part be explained by concerted national and international campaigns over the last decade to change prescribing behaviour for antibiotics to optimal doses (Finch et al., Reference Finch, Metlay, Davey and Baker2004; Goossens et al., Reference Goossens, Guillemot, Ferech, Schlemmer, Costers, Van Breda, Baker, Cars and Davey2006). However, it is unclear from this data as to why prescription durations for amoxicillin have increased.
One of the most striking differences is observed in aspirin. This may in part be related to changes in the pack sizes available. Aspirin was once commonly supplied in 100 tablet bottles. It is now more often presented in boxes of 28 tablets. The trend shown here may also have been affected by a shift away from 150 mg (2 × 75 mg) daily dosing to 75 mg daily dosing. The move towards the common supply of original pack dispensing, as seen with aspirin, was partly the result of a European Community Directive (92/27/EEC), which requires all dispensed drugs to be accompanied by a Patient information leaflet (PIL). It has been law since 1998 for all UK medicinal products to be supplied with an authorised PIL.
The requirement for a PIL to be provided spurred the development of patient ready packs leading to an increased number of products being packed and supplied in a patient ready format. This suggests one of the limitations of the analysis performed above. In most cases, and certainly for the preparations analysed, items are supplied in patient ready packs. This would suggest that prescription length is not a continuous variable, but instead a dichotomous one (ie, 28 days or 56 days) as dictated by patient pack size. However, evidence from pharmacists suggests that prescribers are often unaware of pack sizes and therefore supply quantities of 28, 30 or 32 days, which causes pharmacists to ‘snip’ packs. This results in additional time consuming processing steps (PSNC, 2007).Footnote 4 Therefore, a limitation of this data is that it does not allow for the evaluation of the spread of data behind each mean. A further limitation is that the drugs and formulations selected may not be fully representative of prescribing as a whole, despite their high prescribing volumes.
The combined oral contraceptive was one of the first products to be supplied in patient ready packs. The view that contraceptives such as microgynon should be excluded from prescribing length restrictions is supported by the data presented. No statistically significant change in prescription length could be observed.
For products where a significant decrease was not observed, several other factors may be important. For example, the discrepancy seen in citalopram may be due to the supply of short-term prescriptions owing to the nature of the indications to which this drug is being used and the associated risk of suicide, coupled with possible prescriber concerns regarding self-harm attempts.
But despite the limitations acknowledged above and the limited number of medicines studied, we conclude that there has been a general trend towards shorter prescription lengths. As prescription durations have reduced, the number of items dispensed has increased. It is not possible to extrapolate with full confidence the findings offered here to the entire range of medicines supplied in the community setting in England. Yet it is probable that if prescription lengths had not decreased over the course of the last decade the total number of items dispensed via community pharmacies and other outlets would be about 10% lower than is currently the case. (A simple extrapolation would suggest a 175 million prescription item variation between 1999 and 2009, although long-term use items subject to prescription volume declines were probably over-represented in the sample selected. If it were assumed that the total number of potentially avoidable items were only 50% of the total implied by simple extrapolation, then it would stand at just under 90 million.)
Assuming that pharmacy fees and other payments remain constant, the increase in prescription numbers due to duration declines cost the NHS in the region of £150 million in 2009. This is ~10% of the cost of the 2009 community pharmacy budget for medicine supply, excluding items such as permitted discounts. A cost of £150 million, the available evidence suggests, is considerably in excess of any possible savings that a blanket rather than selective use of 28-day prescribing periods is likely to generate.
As previously noted, the overall increase in NHS prescription item numbers observed in England during the decade from 1999 to 2009 was over 300 million, a 60–70% rise. The significance of prescription duration reductions alone should not therefore be overstated. The calculation of pharmacy fees is also linked to fixed rather than variable business cost assumptions, and should consequently over time take into account the marginal rather than average costs of activity rate increases. This implies that although community pharmacists will (unlike non-dispensing GPs) in the short-term gain financially through supplying additional prescription items, their longer-term returns will be ratcheted down.
In considering the public policy implications of growing prescription item numbers and their ongoing health and health-care impacts, two initial points deserve emphasis. First, as already highlighted, growth in the overall volume of generic and other medicines being supplied to the population is a multifaceted phenomenon. It is taking place worldwide as relatively rich societies age and relatively poor ones benefit from globalisation and their populations gradually get better access to health care.
One particularly important driver in the last 10 to 20 years has been the mass use of medicines to reduce the risk of, and to treat, vascular diseases. For example, a recent Canadian study showed a 165% increase in the total number of prescriptions for such drugs in the decade 1996–2006 (Jackevicius et al., Reference Jackevicius, Cox, Carreon, Tu, Rinfret, So, Johansen, Kalavrouziotis, Demers, Humphries and Pilote2009). There is substantive evidence that such changes in medical and pharmaceutical care have significantly benefited the communities being served, and have been encouraged by governments as well as by commercial interests. In Britain, for instance, the current general medical services (GMS) contract incorporates a points based Quality and Outcomes Framework (QOF). This incentivises improved chronic disease management (Alabbadi et al., Reference Alabbadi, Crealey, Turner, Rafferty, Keenan, Murray and Mcelnay2010). One of its effects has been to encourage an increased use of medicines such as statins and anti-hypertensives.
A second point regarding the reductions in prescription durations reported here is that the arguments in favour of this strategy in England have related primarily to medicine waste prevention. In addition to evidence such as that published by Hawksworth et al. in the 1990s, more recent studies confirm that restricting periods of chronic/long-term medicine supply to a period of 28 days can reduce the volume of NHS medicines that eventually have to be discarded. For example, a widely cited Bradford University study investigated two groups of 20 elderly patients receiving regular prescriptions for more than three medications. The first had 28-day prescriptions and the second 56-day supplies. It was found that the 56-day group had greater home stocks, and reported disposing of unwanted medicines more often than the 28-day group (Gatley et al., Reference Gatley, Rooney and Chrystyn1995).
This suggests that the rational application of 28-day prescribing polices reduces medicine waste, although this is not a justification for the unselective use of such policies. It would be wrong to assume that the growing volume of NHS prescription items supplied by community pharmacies has necessarily been a negative trend in public health terms, and/or that reducing the length of the average prescription has during the past decade increased dispensing costs without conferring any counterbalancing benefit. Nevertheless, it is important to maximise the efficiency of any intervention. The possibility that therapeutic gain has been lost as a result of policies ostensibly aimed at medicine waste reduction also deserves attention.
For example, evidence from Italy suggests that shortening prescription durations for patients being treated for hypertension reduced adherence rates in those who had previously been taking their medicines appropriately (Atella et al., Reference Atella, Peracchi, Depalo and Rossetti2006). In the American context, Domino et al. (Reference Domino, Olinick, Sleath, Leinwand, Byrns and Carey2004) recognised that reducing duration decreased waste; however, this was not justified because of increases in travel costs to patients and the additional dispensing fees paid to pharmacies. The dissatisfaction and inconvenience that prescribing policies may cause patients should not be ignored in cost benefit evaluations (Mitchell et al., Reference Mitchell, Hickey, Hickey and Pearce2009a; Reference Mitchell, Hickey, Hickey and Pearce2009b).
A recent DoH funded review of medicine waste by York Health Economics Consortium and the (now UCL) School of Pharmacy found that in England alone in 2008/09 some £300 million worth of NHS community supplied medicines were disposed of unused, and that up to £150 million of this inefficiency is cost-effectively avoidable. This last figure is equivalent to about 2% of the cost of all medicines supplied, and a little under 10% of the cost to the NHS of community pharmacy services. The authors also presented evidence that the inappropriate imposition of 28-day prescribing in some circumstances reduces adherence in medicine taking and can lead to other forms of perverse consumer reaction. At the same time some of the GPs and pharmacists interviewed expressed concerns about the quality of personal care being provided to vulnerable NHS patients living in the community and noted the amount of time being spent on activities such as dispensing, as opposed to understanding and meeting patients needs in a flexible and individually tailored manner.
Such research supports the view that the supply of medicines should be organised in ways that maximise customer satisfaction and minimise professional workloads, even if longer average prescriptions durations are on occasions associated with an increased risk of physical waste. The latter's cost might be most successfully offset via greater concentration on identifying and effectively supporting patients at unusually high risk of experiencing problems with medicine taking.
Despite some professionals’ preferences for standardising variables such as prescribing durations, all health service actors should beware incentives and structures that can paradoxically undermine service quality (Jesson et al., Reference Jesson, Pocock and Wilson2005). In this instance, a balance should be struck between standardised prescribing terms and patient desires for individualised care, albeit that in situations where there are unusually high treatment costs or risks of non-consumption, safeguards should be instituted.
The findings reported here do not in themselves define the extent of avoidables waste or the effects of 28-day policies on clinical outcomes. But they do indicate a need for further evaluations. It is possible that a more flexible approach to regulating prescription lengths could increase service efficiency and effectiveness while also creating a more convenient system for some patients. International experience suggests that median community prescription duration periods of up to three months (ie, of around twice the current estimated length) may prove desirable (Domino et al., Reference Domino, Olinick, Sleath, Leinwand, Byrns and Carey2004) especially if community pharmacy and other resources released can be cost-effectively re-deployed towards areas of higher gain.
Conclusion
The data presented in this analysis indicate that in the context of longer-term medicine use the last decade has seen a significant reduction in the duration of prescriptions supplied by the NHS. This may have been due to the encouragement of ‘28-day prescribing’, which has been applied rigidly in some localities. Although such policies may have brought advantages in some contexts, they may nevertheless have imposed additional disadvantages elsewhere.
This study does not provide a definitive answer in favour of prescription duration individualisation as opposed to standardisation. Yet it does suggest this is an area in need of further exploration aimed at discovering if the intelligent, ‘customer needs’ focused, application of informed professional judgement to prescription duration determination should be preferred to blanket prescribing policies.
Acknowledgements
The authors would like to thank Catherine White of the Addison's Disease Self Help Group (ADSHG) for her input and advice during the preparation of this paper.








