The corpus callosum has been strongly implicated as an anatomical mediator of dysfunctional inter-hemispheric transfer in schizophrenia, Reference David1 and anomalies of the corpus callosum are found more commonly in people with schizophrenia than the general population. Reference Swayze, Andreasen, Erhardt, Yuth, Alliger and Cohen2 The first magnetic resonance imaging (MRI) study suggested alterations in its size and shape in schizophrenia. Reference Nasrallah, Andreasen, Coffman, Olson, Dunn, Ehrhardt and Chapman3 Subsequent analyses have found conflicting results, including global increases, Reference Nasrallah, Andreasen, Coffman, Olson, Dunn, Ehrhardt and Chapman3,Reference Jacobsen, Giedd, Rajapakse, Hamburger, Vaituzis, Frazier, Lenane and Rapoport4 reductions Reference Bachmann, Pantel, Flender, Bottmer, Essig and Schroder5,Reference Woodruff, Pearlson, Geer, Barta and Chilcoat6 or no differences Reference Gunther, Petsch, Steinberg, Moser, Streck, Heller, Kurtz and Hippius7,Reference Meisenzahl, Frodl, Greiner, Leinsinger, Maag, Heiss, Hahn, Hegerl and Möller8 compared with healthy individuals, although a meta-analysis of the first decade of studies suggests this structure is smaller in patients than in controls. Reference Woodruff, McManus and David9 Data from people experiencing their first episode of schizophrenia suggest that the corpus callosum may also be smaller than in healthy individuals. Reference Bachmann, Pantel, Flender, Bottmer, Essig and Schroder5,Reference Keshavan, Diwadkar, Harenski, Rosenberg, Sweeney and Pettegrew10
The aim of the present MRI study was to determine whether the measures of callosal size and shape in a large cohort (n=217) of individuals across differing illness stages (first-episode schizophrenia-spectrum disorders and established illness) differed from that of controls. Based on previous work we hypothesised that patients with established illness would show a globally smaller corpus callosum and reductions in regions connecting frontal and temporal regions, with patients with first-episode schizophrenia-spectrum disorders exhibiting similar reductions.
Method
Participants
All participants with first-episode schizophrenia-spectrum disorders, aged between 16 and 30 years, were recruited from the Early Psychosis Prevention and Intervention Centre (EPPIC; n=76) and participants with chronic schizophrenia from inpatient and community mental health settings (n=86) within the North Western Mental Health Program, Melbourne, Australia. The DSM–III–R 11 diagnoses were based on chart review and structured diagnostic interviews. Reference McGorry, Singh, Copolov, Kaplan, Dossetor and Van Riel12,Reference First, Spitzer, Gibbon and Williams13 Control participants (n=55) from similar socio-demographic areas as the patients were recruited from ancillary hospital staff and through advertisements. Demographic data were obtained for all participants (see online data supplement Table DS1). Inclusion criteria for participants in the first-episode schizophrenia-spectrum group (‘first-episode group’, which included patients with schizophrenia (n=30), schizophreniform disorder (n=31) and schizoaffective disorder (n=15)), established illness group and for controls, recruited from 1994 to 1999, have been previously described. Reference Velakoulis, Wood, Wong, McGorry, Yung, Phillips, Smith, Brewer, Proffitt, Desmond and Pantelis14 Patients in the established illness and first-episode groups were rated on the total positive and negative symptoms scales on the Positive and Negative Syndrome Scale (PANSS) Reference Kay, Opler and Lindenmayer15 by trained raters, and medication at the time of scanning was converted to chlorpromazine equivalents.
Participants were screened for comorbid medical and psychiatric conditions by clinical assessment, and physical and neurological examination. Written informed consent was obtained from all participants. The study was approved by the local Research and Ethics Committee. Exclusion criteria for patients were: a history of significant head injury, seizures, neurological diseases, impaired thyroid function, steroid use or DSM–III–R criteria of alcohol or substance dependence. Controls with a personal history of psychiatric illness or family history of psychosis were excluded.
Magnetic resonance scanning acquisition and analysis
All participants were scanned on a 1.5 T GE Signa MRI machine. A three-dimensional volumetric spoiled gradient recalled echo in the steady state sequence generated 124 contiguous, 1.5 mm coronal slices. Imaging parameters were: time-to-echo (TE), 3.3 ms; time-to-repetition (TR), 14.3 ms; flip angle, 30°; matrix size, 256 × 256; field of view (FOV), 24 × 24 cm matrix; voxel dimensions, 0.938 × 0.938 × 1.5 mm. Head movement was minimised by foam padding and straps across the forehead and chin. This scanner was calibrated fortnightly using the same proprietary phantom to ensure stability and accuracy of measurements. A numerical code was used to ensure masked analysis of data.
The brain was automatically segmented from the rest of the head. Reference Smith16 Using the software package Automated Image Registration (Red Hat Linux V9), Reference Woods, Grafton, Holmes, Cherry and Mazziotta17 images were registered to a template image comprising the average of 152 normal T1-weighted MRI scans previously placed in stereotaxic coordinate space. A nine-parameter linear transformation was used which allowed translation, rotation and scaling along each of the three principal axes. The midsagittal slice was identified and interpolated to a voxel dimension of 0.5 mm × 0.5 mm in the y- and z-planes. White matter voxels in the midsagittal slice were identified using a histogram segmentation procedure. Reference Otsu18 Non-callosal voxels were then removed manually. A measure of callosal area in total mm2 was then generated. To measure regional callosal thickness, voxels at the edge of the callosum were identified, and upper and lower edges were defined according to anterior and posterior endpoints. An iterative search for optimum endpoints which maximised the length of a line segment traversing the centre of the callosum was then performed (Fig. 1). The line segment was defined by dividing the upper and lower surfaces of the callosum into 40 equidistant portions by 39 nodes. The midpoints between corresponding nodes on the upper and lower surfaces were identified. The line segment was created by joining endpoints and successive midpoints. Once the optimum endpoints and corresponding midpoints were identified, a smooth curve joining them was obtained with cubic spline interpolation. This curve was divided into 40 segments of equal lengths by 39 nodes. At each node, the line orthogonal to the curve was calculated. The distance between its intersection with the dorsal and ventral surfaces of the callosum represented regional callosal thickness at these 39 points; the average of these thicknesses represented mean callosal thickness (Fig. 1).
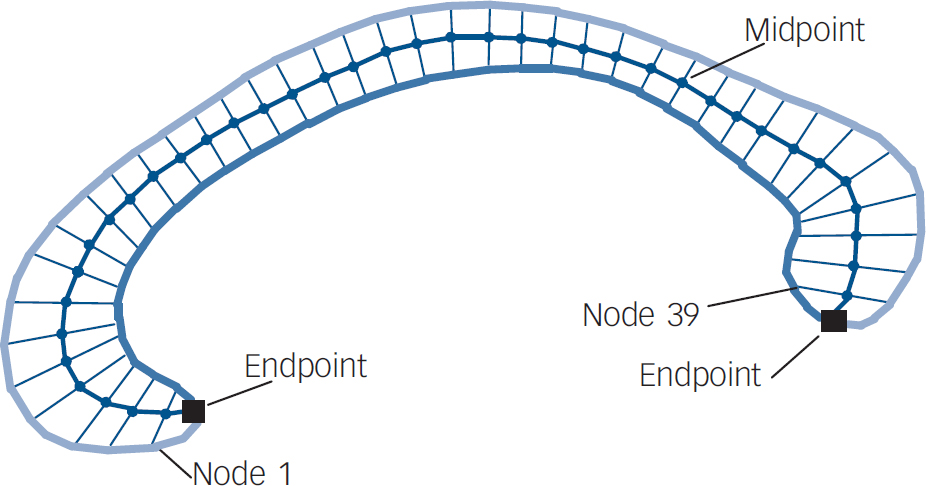
Fig. 1 Endpoints, midpoint and dividing nodes used to measure callosal thickness.
Statistical analysis
Five analyses were undertaken on the four callosal measures of total callosal area and regional callosal thickness:
-
(a) comparison of main patient groups: first-episode, established illness and controls
-
(b) comparison of first-episode psychosis subgroups and controls
-
(c) analysis of effect of age on patients with established illness and controls, and duration of illness on patients with established illness
-
(d) analysis of the relationship between medication and callosal measures.
-
(e) analysis of the relationship between psychotic symptoms and callosal measures.
Determination of the effects of demographic variables was undertaken with chi-square analyses for gender and one-way analysis of variance (ANOVA) with Tukey's post hoc comparison for age in years, height in centimetres and premorbid IQ measured using the National Adult Reading Test. Reference Nelson19 Positive and negative symptom scales on the PANSS were compared using ANOVA for three-group comparisons and t-tests for two-group comparisons. Callosal area was compared between groups using ANOVA. For regional callosal thickness, a non-parametric permutation method Reference Holmes, Blair, Watson and Ford20 of 10 000 randomisations was used for group comparisons to account for non-independence between adjacent thickness measurements and for multiple comparisons; step-down tests were used to localise at which slices the thickness differed significantly. Two-group comparisons were undertaken with t-tests, and localisation for individual slice differences again using step-down testing. Non-parametric regression analyses using multiple dependent variables were undertaken to determine the effect of medication dose and positive and negative symptoms on callosal thickness measures. Statistical inference was based on the family-wise error rate method to correct for multiple comparisons. Reference Holm21
Results
Demographic and illness data
Comparison of the established illness, first-episode and control groups (see online Table DS1) demonstrated significant differences in gender (χ2=9.68, P<0.01) with an excess of males with established illness compared with other groups, and age (F (2,216)=53.80, P<0.001) with first-episode<controls<established illness. Premorbid IQ differed significantly across groups (F (2,216)=6.48, P<0.005) with controls>first-episode=established illness. There were no significant differences across groups in handedness (χ2=5.13, P=0.274) or height (F (2,216)=0.188, P=0.829). Mean duration of illness prior to first scan in the established illness group was 13.30 years (s.d.=8.95), and in the first-episode group the duration of their index psychotic episode prior to first scan was 64.20 days (s.d.=112.22). The age at onset of psychosis did not differ between the two broad patient groups (t=1.264, P=0.208). The PANSS negative symptom total score was higher in the established illness group at a trend level (t=1.741, P=0.084), although positive symptoms did not differ (t=–1.634, P=0.105). Medication dosage was significantly higher in the established illness group (t=7.346, P<0.0001). When the three first-episode groups were compared, there were no significant differences on any demographic, medication, illness onset/duration variables or symptom variables, other than PANSS positive symptoms which were lower in the first-episode schizophrenia group (F=(2,73)=6.683, P=0.002).
Analysis 1: main groups
Total area of the corpus callosum did not differ significantly between the three main groups (F (2,216)=1.094, P=0.337), nor when analyses were limited to males and right-handers. Illness duration was not associated with area in the established illness group (r=–0.052, P=0.638). Females across the sample had larger callosal area (675.94 mm2) than males (648.76 mm2), although this did not reach significance (P=0.076). A significant group × gender effect was found (F (6,216)=3.238, P=0.041) with females (715.44 mm2) having a significantly larger total area than males (640.70 mm2) only in the control group (F (1,54)=6.028, P=<0.05). When left-, right- and mixed-handed groups were examined, corpus callosum area did not differ overall (F 2,216)=0.710, P=0.493), although a significant group × handedness effect was found (F (6,216)=3.335, P=0.038). Although in the control group left-handed people had a larger callosal area than right-handed people, this difference was not seen in the patient groups. There was no gender × handedness effect (by group) on area. Group differences were apparent in the length (F (2,216)=3.844, P=0.023), curvature (F (2,216)=6.871, P=0.001) and mean width (F (2,216)=3.685, P=0.026) of the callosum however, with patients with established illness having longer, thinner and more angulated callosi than the other two groups, which were comparable.
When comparing regional callosal thickness across groups, a number of differences were found. Across the three main groups (Fig. 2), a main effect of group was found (P<0.0001) at slices 1–5 (anterior genu) and 29–30 (isthmus) which remained significant when age was controlled for (P<0.05) or when only males were analysed (P<0.001). In step-down comparisons, significantly smaller widths were seen in the established illness group compared with the control group at slices 1–5, 11–21 and 28–30 (P=0.0005); and in the first-episode group as a whole at slices 1–3 (P<0.005). Identical regional changes were seen in the male only cohort.
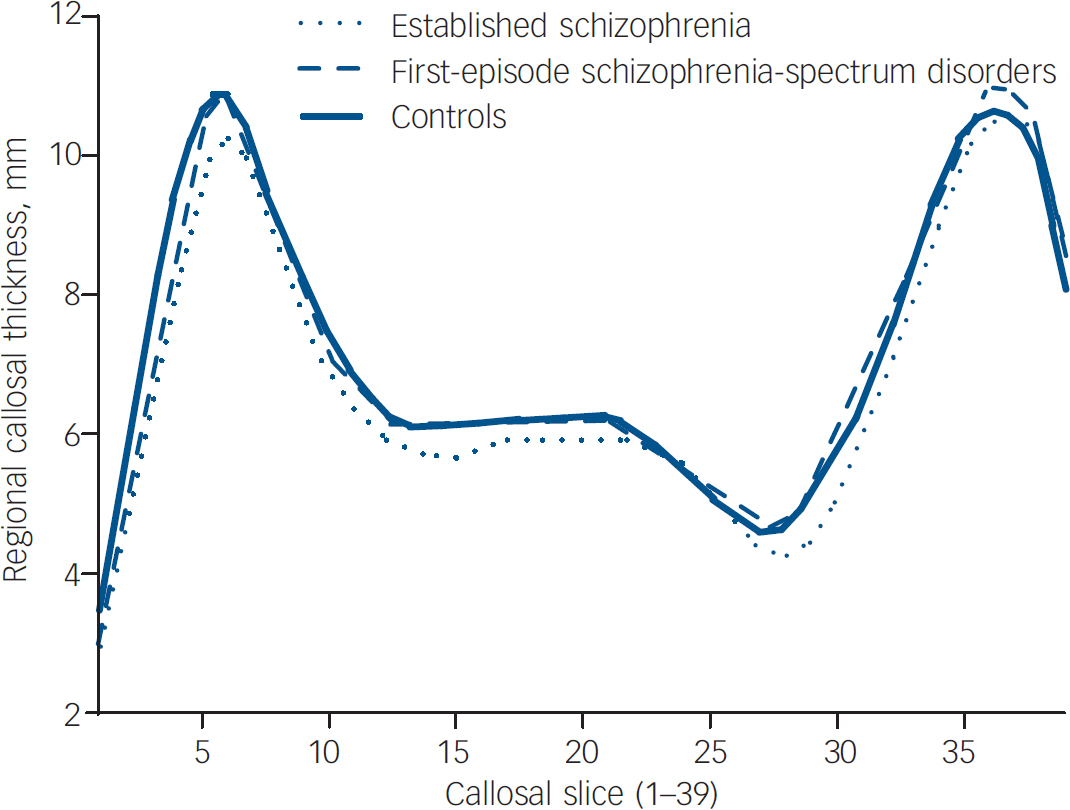
Fig. 2 Regional callosal thickness by main diagnostic group.
To examine differences in corpus callosum thickness across illness stages, the two patient groups (first-episode and established illness) were compared. When the established illness group was compared with the first-episode group, the established illness group had significantly smaller widths in slices 13–17 and 27–32 (P<0.05).
Analysis 2: first-episode subgroups
The three first-episode subgroups did not differ significantly on measures of callosal area (F (1,75)=1.429, P=0.246), curvature (F (1,75)=1.927, P=0.153), length (F (1,75)=0.103, P=0.902) and mean thickness (F (1,75)=1.690, P=0.192). When regional callosal thickness was compared between groups, there was no overall effect of group (Fig. 3, P=0.145). When first-episode subgroups were compared with the control group, only when the schizophrenia group was compared was an effect of group found (P<0.05), with significant reductions seen in slices 3 and 4, located in the genu of the callosum; the schizophreniform v. control comparison, equally well-powered, showed no overall effect of group (P=0.638). Interestingly, the schizoaffective disorder group showed a trend (P=0.079) towards a group effect with a reduction seen in slice 3, and exploratory post hoc analyses suggested significant (P<0.05) increases in slices 25–27 and 36–39, when compared with the control group.
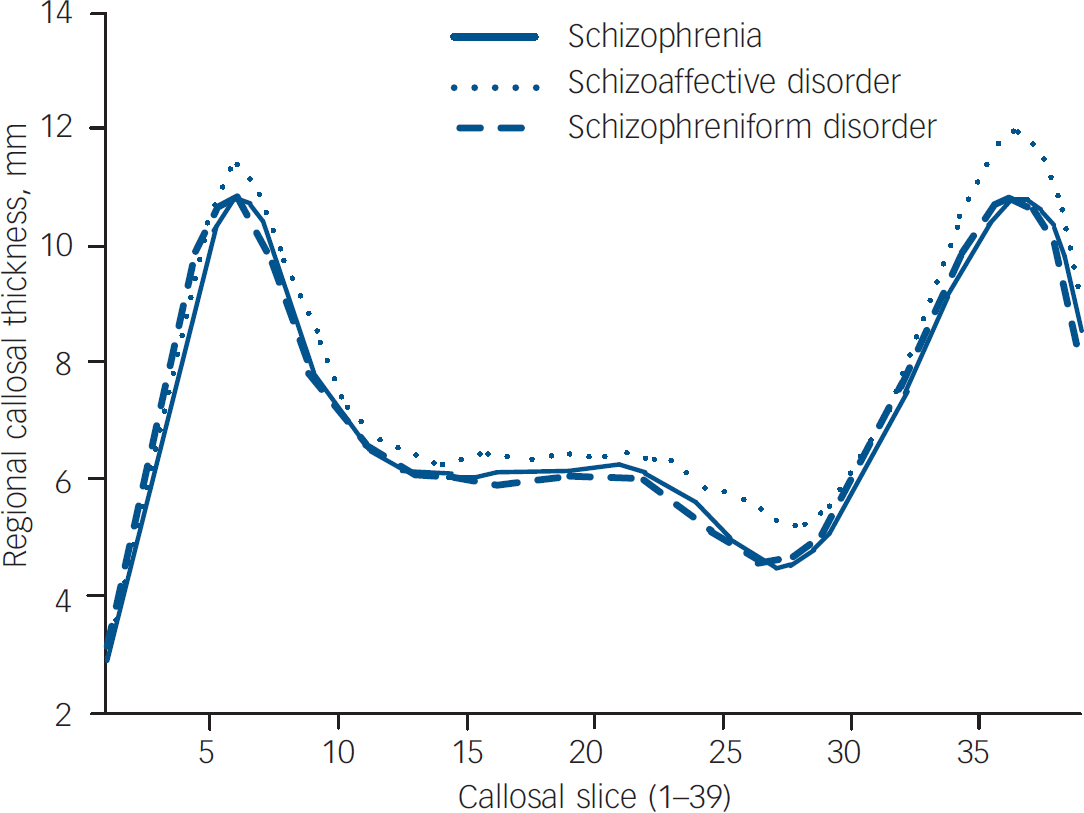
Fig. 3 Regional callosal thickness in first-episode subgroups.
Analysis 3: age and duration of illness variables
As previous authors had found a loss of age-related expansion of callosal area in people with first-episode schizophrenia, Reference Keshavan, Diwadkar, Harenski, Rosenberg, Sweeney and Pettegrew10 the relationship of age to callosal area was examined separately in each group (Fig. 4). This relationship was seen in the control group (r=0.297, P<0.05), but not in the established illness group (r=–0.050, P=0.648), and this difference was significant (P<0.05); the first-episode group's narrow age range prevented meaningful comparisons.
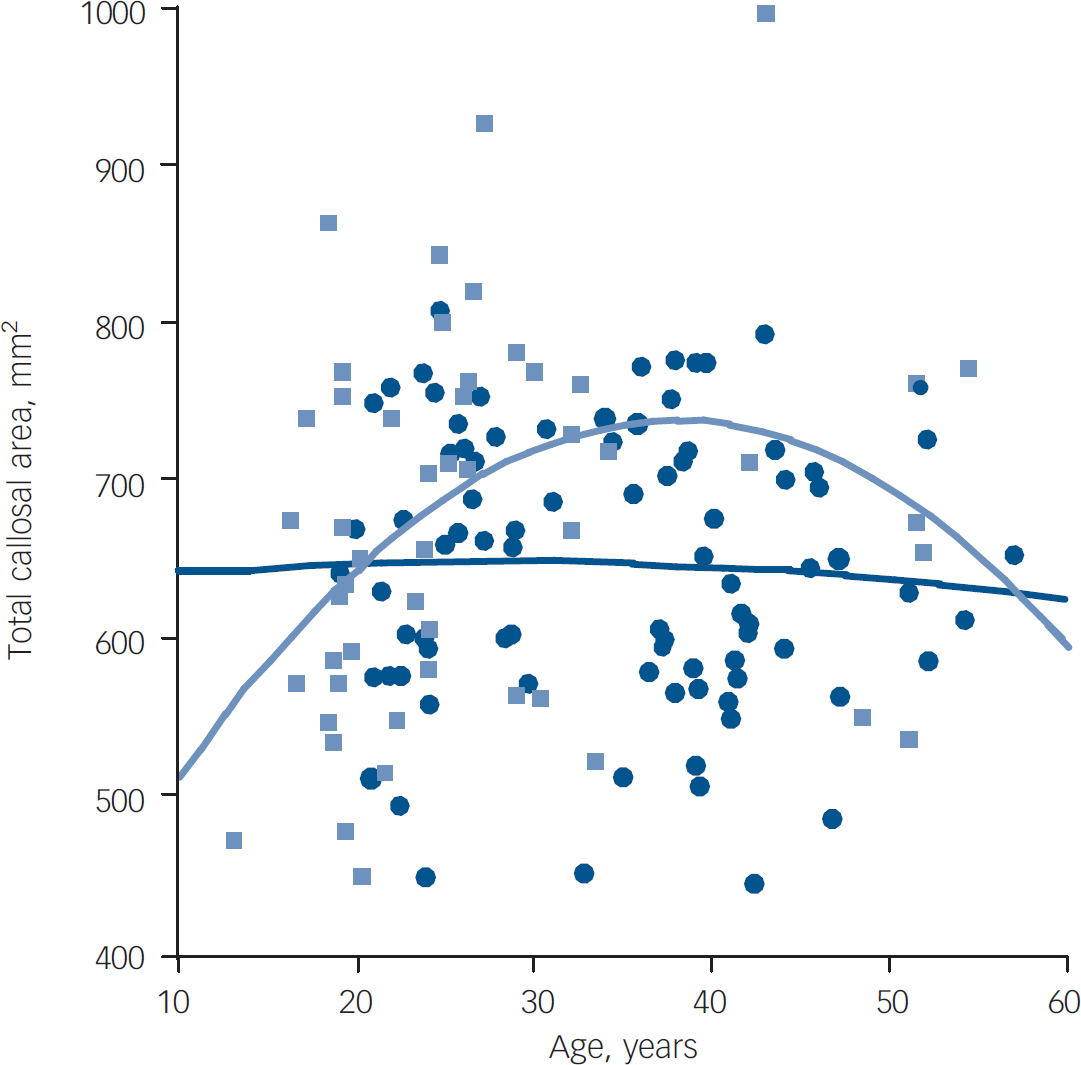
Fig. 4 Callosal area between groups according to age. Light blue, control group; dark blue, established illness group.
When callosal shape was investigated through examination of callosal slice thickness, no age × group interaction was found (P=0.232). When examining the effect of age on regional callosal thickness, in the healthy control sample, an expansion was seen in nodes 38 and 39 with age (P<0.05), not seen in the established illness group. However, the established illness group showed a reduction with age in nodes 30–32 (P<0.01); of note, a trend towards an effect of duration of illness was seen in this region, in nodes 30–31 (P=0.078).
Analysis 4: medication dosage
Complete medication data at scanning time were available for 66/86 patients with established illness (28 on typical and 36 on atypical antipsychotics and 2 on no medication) and for 72/76 patients with first-episode schizophrenia-spectrum disorders (27 on typicals, 43 on atypicals and 2 on no medication). There was no difference in any demographic measure (age/gender/height/premorbid IQ) between those on typical v. atypical antipsychotics, and no difference in the main callosal measures (area, length, curvature and thickness) across and within the established illness and first-episode groups. Removing patients on lithium treatment (n=3) did not affect the results.
Medication dosage in chlorpromazine equivalents did not affect any of the main callosal measures, although it negatively correlated with curvature alone (r=–0.357, P=0.007) in the first-episode group. In the regression analysis, medication dose was not significantly related to regional slice thickness in the first-episode or established illness groups.
Analysis 5: symptoms
In the patient group (established illness and first-episode schizophrenia-spectrum disorders) as a whole, positive symptoms on the PANSS did not correlate with any callosal variable whereas negative symptoms significantly negatively correlated with callosal bending angle (r=–0.215, P=0.013). In the established illness group alone, neither positive nor negative symptoms correlated with any callosal variable; in the first-episode group, a positive correlation was demonstrated between negative symptoms and callosal area (r=0.307, P=0.018) and thickness (r=0.288, P=0.027). Regression analysis showed no relationship between positive or negative symptoms in either patient group.
Discussion
We used a novel method to examine the size and shape of the corpus callosum in a large sample of patients with psychosis and schizophrenia across varying stages of illness. Additionally, this study is unique in comparing individuals at two illness stages (first psychotic episode and established psychosis) with healthy controls. This study has identified that callosal morphological changes are greatest in those patients with chronic schizophrenia. The anterior corpus callosum changes in patients with first-episode schizophrenia-spectrum psychosis suggest that changes seen in patients with chronic schizophrenia are present in the early stages of illness, as the reductions present in the genu in both patient groups were not seen in controls. These findings suggest that anatomical changes in the anterior callosum are present at the time of the first episode, and further changes develop in non-genu regions with progression of illness – or that the subgroup of patients with first-episode psychosis who develop chronic illness may have additional changes not seen in those who do not progress. These changes appear to be independent of medication, and independent of positive symptoms, although negative symptoms at first episode did show an unexpected positive relationship with total callosal size and mean thickness that was not present in established illness.
Regional thickness changes
Regional thickness of the corpus callosum has not been examined in other cohorts using a method like that described in this study. The most similar method of analysis was a study by Downhill et al Reference Downhill, Buchsbaum, Wei, Spiegel-Cohen, Hazlett, Haznedar, Silverman and Siever22 comparing controls, patients with schizophrenia and patients with schizotypal personality disorder, dividing the callosum into 30 segmental areas (not widths) based on equidistant nodes along a mid-callosal spline and using repeated-measures ANOVA. This study found a smaller area in both the genu and splenium of patients with schizophrenia; findings in the genu are consistent with our findings. Their research did not assess a first-episode group but did include a schizotypy comparator group. The advantage of using computational morphometry is that it avoids the arbitrary distinction of the corpus callosum into defined subregions, such as the method defined by Witelson, Reference Witelson23 and ensures that the methodology is free of arbitrary anatomical-assumptions that may alter the results. Additionally, a methodology that uses a large number of subdivisions or slices increases the potential of detecting subtle regional changes, and rigorous non-parametric methods for statistical inference allow correction for multiple comparisons and dependence of adjacent slice measurements.
Changes at illness stage
In addition, no other study has examined callosal morphology using patients with first-episode psychosis/schizophrenia or chronic schizophrenia using the same methodology. As the findings in patients with first-episode schizophrenia-spectrum disorders and with established illness were homologous in anterior regions, a similar process may be involved in causing the regional reductions in the genu of the corpus callosum in both groups, indicating that anterior pathology is present at first episode of illness. Chronic schizophrenia shows similar reductions in the anterior genu (slices 2–5, carrying ventral/medial prefrontal fibres), with additional reductions in posterior genu/anterior body (slices 13–17, carrying cingulate, premotor and supplementary motor area fibres) and isthmus (slices 27–32, carrying cingulate, superior temporal and posterior parietal fibres). Reference Pandya, Seltzer, Lepore, Pitto and Jasper24 It may be these changes outside the anterior genu that are related to a subgroup who develop chronic illness, whereas changes in the genu that occur with or prior to first episode of psychosis may reflect an earlier neurodevelopmental insult present across subgroups, or changes that occur during first psychosis. A second possibility is that these changes represent illness progression; however, longitudinal analysis in the same individuals is needed to demonstrate the presence of ‘neuroprogressive’ change conclusively. Reference Pantelis, Yücel, Wood, Velakoulis, Sun, Berger, Stuart, Yung, Phillips and McGorry25 Examining individuals pre-psychosis who later progress to psychosis would allow a determination of the timing of the onset of these changes.
The subgroup analysis of the first-episode group identified anterior reductions in the schizophrenia and schizoaffective disorder subgroups, but not the schizophreniform subgroup. Assuming that the schizophreniform subgroup differs from the schizophrenia subgroup only in duration of symptoms, this raises the possibility that the genual changes seen actually occur during the first psychotic episode rather than prior to it. Furthermore, since only the schizoaffective disorder subgroup showed expansions in other callosal regions, the results may suggest that the affective axis of this illness is associated with changes in other brain regions. We also mirrored the results of Keshavan et al in showing a loss of the normal age-related expansion of corpus callosum area in schizophrenia, Reference Keshavan, Diwadkar, Harenski, Rosenberg, Sweeney and Pettegrew10 although this finding contrasts with that of Woodruff et al, who showed a loss of the negative correlation between age and corpus callosum area in patients. Reference Woodruff, Phillips, Rushe, Wright, Murray and David26
The corpus callosum is topographically organised, with anterior segments connecting anterior cortical regions and posterior segments connecting posterior cortical regions. Reference Pandya, Seltzer, Lepore, Pitto and Jasper24 In Alzheimer's disease, patients with dementia and in the pre-dementia phase show reductions in callosal regions associated with cortical hypometabolism and atrophy; Reference Teipel, Hampel, Pietrini, Alexander, Horwitz, Daley, Möller, Schapiro and Rapoport27 it could be expected that alterations in cortical regions seen in schizophrenia in prefrontal, temporal and inferior parietal cortex Reference Pearlson28 would be associated with regional changes in the callosal genu, isthmus and anterior splenium. Shape analysis of the corpus callosum has implicated these regions in patients with first-episode schizophrenia. Reference DeQuardo, Keshavan, Bookstein, Bagwell, Green, Sweeney, Haas, Tandon, Schooler and Pettegrew29 This suggests that not only are these changes present at the first episode of schizophrenia and thus potentially representative of neurodevelopmental changes, but that they may relate to grey matter changes – although studies examining both cortical regions and callosal subregions are lacking. Previous studies of the corpus callosum in schizophrenia have been limited by small sample sizes (the mean number of patients was 25 and of controls was 17 in studies prior to Woodruff's 1995 meta-analysis) Reference Woodruff, McManus and David9 and many studies have not controlled for factors known to affect corpus callosum size and shape, including gender, handedness and age. Reference Witelson23 In addition, as antipsychotic medication has been shown to produce increases and decreases in regional white matter volume Reference Christensen, Holcomb and Garver30 and may confound longitudinal studies or those that aim to compare individuals at different illness stages, Reference Dorph-Petersen, Pierri, Perel, Sun, Sampson and Lewis31 the possible effect of medication is an important potential confounder on corpus callosum structure, not yet examined in morphometry studies. Examining patients across the life-cycle of schizophrenia may shed light on the neurodevelopmental timing of a potential neuropathological process Reference Pantelis, Yücel, Wood, Velakoulis, Sun, Berger, Stuart, Yung, Phillips and McGorry25 and may provide insights about the relationship of brain changes to prognosis.
Relevance of white matter changes
This study leaves a number of unanswered questions. The first of these is whether callosal changes are primary or secondary to grey matter changes that have been reported in studies of individuals who are pre- or peri-psychotic. A compelling body of neuroimaging evidence exists implicating white matter structures, including the corpus callosum, in schizophrenia, Reference Walterfang, Wood, Velakoulis and Pantelis32–Reference Kubicki, McCarley and Shenton34 but most of these studies have not examined related grey matter structures in unison in the way that studies of other neurodegenerative disorders, such as Alzheimer's disease, have. Reference Teipel, Hampel, Pietrini, Alexander, Horwitz, Daley, Möller, Schapiro and Rapoport27 Alterations in either compartment may produce changes in the other; for example, loss or reduction of cortical neurons will result in a reduced number of inter-hemispheric axons, whereas impaired myelination and thus conduction can result in changes in neuronal size and local connectivity. Reference Walterfang, Wood, Velakoulis and Pantelis32 We cannot comment on the diagnostic specificity of these changes, as patients with bipolar disorder have also been described as showing reductions in the genu and isthmus, Reference Brambilla, Nicoletti, Sassi, Mallinger, Frank, Kupfer, Keshavan and Soares35 – more work is needed, including direct comparison between patients with chronic schizophrenia and bipolar disorder using the same methodology.
Owing to the limitations of volumetric MRI analysis, the alterations in regional size of the corpus callosum in schizophrenia in our study do not allow the determination of the underlying neuropathological changes. A reduction in volume may represent a reduction in number of axons, size of axons or a reduction in their myelin sheaths (thus increasing the density of axons). Aboitiz et al first demonstrated in healthy controls that variance in area was generally the result of alterations to number rather than density of interhemispheric fibres, but only those small-diameter fibres that connect association cortices; Reference Aboitiz, Scheibel, Fisher and Zaidel36 thus, it may be that regional reductions in genu and isthmus in our study represent a decreased number of inter-hemispheric fibres connecting association cortices. In addition, a negative correlation exists between lateralisation and total fibre number, such that greater hemispheric asymmetry results in a reduced number of callosal fibres; Reference Aboitiz, Scheibel, Fisher and Zaidel37 the greater loss of callosal area in female than in male patients with psychosis in our study could suggest a reduction of gender-specific lateralisation, consistent with studies positing a loss of asymmetry in both grey matter Reference Yücel, Stuart, Maruff, Wood, Savage, Smith, Crowe, Copolov, Velakoulis and Pantelis38 and white matter Reference Kubicki, Westin, Maier, Frumin, Nestor, Salisbury, Kikinis, Jolesz, McCarley and Shenton39 structures in schizophrenia. Fibre number also decreases with age, Reference Highley, Esiri, McDonald, Cortina-Borja, Herron and Crow40 and age × gender interactions, already described for regional callosal volume, have been reported for fibre numbers in the corpus callosum in healthy individuals Reference Aboitiz, Rodriguez, Olivares and Zaidel41 and in people with schizophrenia. Reference Highley, Esiri, McDonald, Cortina-Borja, Herron and Crow40 Some evidence for an alternative explanation (that reduced callosal size is secondary to reduced myelination) comes from studies examining signal intensity in callosal white matter, an index of myelination; reduced signal intensity has been shown in schizophrenia and bipolar disorder, but not in major depression or other psychiatric disorders. Reference Brambilla, Nicoletti, Sassi, Mallinger, Frank, Keshavan and Soares42,Reference Diwadkar, DaBellis, Sweeney, Pettegrew and Keshavan43 Thus, one explanation for our findings is an interaction between the development of psychotic illness and normal neurodevelopment on corpus callosum fibre number and/or myelination.
Conclusions
Our findings suggest that the corpus callosum in schizophrenia differs significantly in shape from that in healthy individuals, and that some of these changes are present or occur during the first episode of psychosis. The relationship between findings of changes in white matter regions that connect grey matter structures previously demonstrated to show neuropathological and volumetric change in schizophrenia is intriguing, but these findings alone do not allow elucidation of which of these pathologies may be primary, or whether they occur in concert. Longitudinal studies that elucidate the temporal relationship between white and grey matter change are necessary to shed light on causal relationships, if any, between changes in these two compartments of the central nervous system in schizophrenia.
Acknowledgements
This research was supported by project grants from the National Health and Medical Research Council (NHMRC; grant ID numbers: 970598 and 981112), Ian Potter Foundation, Woods Family Trust, and a program grant from the Victorian Health Promotion Foundation. M.W. was supported by a Stanley Research Centre Grant. A.W. was supported by an NHMRC Clinical Research Training Fellowship (251755). S.W. was supported by an NHMRC Clinical Career Development Award. S.W., C.P., D.V. and P.McG. were supported by an NHMRC Programme Grant (350241). P.McG. was supported by a National Alliance for Research on Schizophrenia and Depression Distinguished Investigator Award. M.W. takes responsibility for the integrity of the data and the accuracy of the data analysis. All authors had full access to all the data in the study.







eLetters
No eLetters have been published for this article.