The nutritional transition in Brazil is composed of changes in the population’s dietary habits from a traditional dietary pattern, composed mostly of culinary preparations, to a Western pattern rich in high-energy foods. As a consequence, a significant increase in the prevalence of overweight people and associated diseases has been observed( Reference Sichieri 1 , Reference Marchioni, Claro and Levy 2 ).
In nutritional epidemiology, population dietary intake is traditionally evaluated from macro- and micronutrient counts and energy intake( Reference Neumann, Martins and Marcopito 3 , Reference Rodrigues, Pereira and Cunha 4 ). However, more recent studies have evaluated population food consumption based on nutrients and foods alongside identification of dietary patterns. Among these, authors have described ones defined according to locality, such as ‘Mediterranean’, ‘Western’ and ‘Traditional’ patterns( Reference Rodrigues, Pereira and Cunha 4 – Reference Silva, Lyra and Lima 6 ). This new approach has enabled a better understanding of the association between population dietary intake and health outcomes( Reference Rodrigues, Pereira and Cunha 4 , Reference Moreira, Corrente and Boas 5 , Reference Silva, Pereira and Segheto 7 , Reference Hoffmann, Mendes and Canuto 8 ) since each of these patterns has a different nutritional profile and, therefore, relates differently to lifestyle and human health( Reference Silva, Lyra and Lima 6 ).
Metabolic phenotypes, in turn, are metabolism variations in individuals with similar BMI( Reference Wildman, Muntner and Reynolds 9 ). Metabolic changes such as hypertension, dyslipidaemia, insulin resistance and inflammation can be identified in both overweight and normal-weight individuals. Thus, four phenotypic groups have been described: metabolically healthy and normal weight (MHNW); metabolically unhealthy but normal weight (MUNW); metabolically healthy but overweight (MHOW); and metabolically unhealthy and overweight (MUOW)( Reference Wildman, Muntner and Reynolds 9 , Reference Phillips 10 ).
The prevalence of metabolic phenotypes in Brazilian studies has varied according to the population studied and the classification criteria adopted: 18·8–20·7 % for MUNW, 13·1–18·5 % for MHOW and 48·1–55·8 % for MUOW( Reference Roberson, Shaharyar and Aneni 11 , Reference Diniz, Beleigoli and Ribeiro 12 ). The high prevalence of these phenotypes in the population is worrisome, especially in relation to MUNW and MHOW, which are considered transitional phenotypes( Reference Rodrigues, Pereira and Cunha 4 ). Thus, they should be the target of interventions aimed at lifestyle changes to prevent the future development of adverse metabolic and cardiovascular outcomes( Reference Sichieri 1 , Reference Marchioni, Claro and Levy 2 , Reference Wildman, Muntner and Reynolds 9 ).
Other authors have identified the influence of sociodemographic and behavioural factors on metabolic phenotypes( Reference Xia, Dong and Gong 13 , Reference Goday, Calvo and Vázquez 14 ) as well as the associations of dietary patterns with excessive weight( Reference Sichieri 1 , Reference Perozzo, Olinto and Dias-da-Costa 15 , Reference Kimokoti, Judd and Shikany 16 ) and isolated metabolic alterations( Reference Neumann, Martins and Marcopito 3 , Reference Silva, Lyra and Lima 6 , Reference Fung, Rimm and Spiegelman 17 ). Nevertheless, research on the dietary intake–metabolic phenotype relationship is scarce. We identified only two studies evaluating the association of food intake with metabolic phenotypes among Americans( Reference Kimokoti, Judd and Shikany 16 , Reference Kimokoti, Judd and Shikany 18 ) and we found no studies investigating dietary patterns.
Thus, the objective of the present study was to investigate the relationship between dietary patterns and metabolic phenotypes in a population-based study conducted with Brazilian adults.
Methodology
Design and sampling procedure
The present study is part of the research project ‘Metabolic syndrome and associated factors: a population-based study in adults in Viçosa-MG’, which was approved by the Human Research Ethics Committee of the Federal University of Viçosa (opinion number 008/2012). All participants signed the free and informed consent form.
This is a cross-sectional study carried out with the adult population (20–59 years old) of both sexes living in the urban area of Viçosa, Minas Gerais, Brazil. The sample size was calculated using the OpenEpi program considering the following parameters: reference population of 43 431( Reference Kimokoti, Judd and Shikany 16 ), expected prevalence of 50 % for the phenomenon (multiple outcomes), 95 % CI, 4·5 % sample error and design effect of 1·7. In addition, 10 % was added to compensate for refusals and losses and 10 % to control for confounding factors, obtaining a final sample size of 957 individuals. We used probabilistic sampling to select in two stages.
The sampling process was carried out through conglomerates. The first-stage units were the census tracts (census units defined by the Brazilian Institute of Geography and Statistics) and the second-stage units were households. We selected thirty sectors out of the ninety-nine existing in the urban area of the city of Viçosa, Minas Gerais, through a random procedure, without replacement. The blocks were identified and drawn for each census sector based on their maps. After this procedure, a block corner was drawn for each field in which the research would take place, adopting clockwise direction. As the calculated size of the sample required was 957 individuals, approximately thirty-two individuals were investigated per census tract.
A detailed description of the methodology for our study can be found in Segheto et al.( Reference Segheto, Cristina Guimarães da Silva and Araújo Coelho 19 ).
Collection of clinical and anthropometric data
Data collection took place in the period from 2012 to 2014. Interviewers and surveyors were trained by the research coordination team to carry out the data collection. Laboratory and clinical tests and anthropometry were performed. Blood samples were collected by intravenous puncture using a Vacutainer system (Becton Dickinson, Plymouth, UK), after a 12 h fast, to determine levels of glucose, total cholesterol, HDL-cholesterol, TAG and high-sensitivity C-reactive protein. Serum samples were separated from whole blood by centrifugation at 3000 rpm (2000 g ) for 15 min. Glucose was determined by the enzymatic glucose-oxidase method; total cholesterol, HDL-cholesterol and TAG by the calorimetric enzymatic method using commercial Bioclin kits (São Paulo, SP, Brazil); insulin by ELISA (Linco Research®, St. Charles, MO, USA); and high-sensitivity C-reactive protein by immunoturbidimetric assay. The homeostasis model assessment of insulin resistance index was used as an indicator of insulin resistance, calculated from the following formula: [fasting insulin (μU/ml) × fasting glucose (mmol/l)]/22·5.
Blood pressure was measured in duplicate on the same upper limb while the individual was sitting. The first measure was obtained after a 5 min rest and the second one 15 min afterwards. The mean of the two measurements was calculated and considered as the final value. For the measurements, we used an automatic blood-pressure monitor with clamp (model Omron HEM 629, Weymouth, UK).
Body weight was measured on a digital scale (model Ironman™ BC-554; Tanita Corporation, London, UK) with a capacity of 200 kg and accuracy of 100 g, and height was measured to the nearest 0·1 cm using a metal stadiometer (Welmy In Wall, Santa Barbara d’Oeste, São Paulo, Brazil). BMI was calculated using weight and height measurements and classified as normal weight (BMI ≤ 24·99 kg/m2) or overweight (BMI ≥ 25·00 kg/m2)( 20 ).
The National Health and Nutrition Examination Survey (NHANES)( Reference Wildman, Muntner and Reynolds 9 ) was used for the definition of metabolic phenotypes. The following metabolic changes were considered to determine the phenotypes: high blood pressure (≥130/85 mmHg or hypertensive medication use); hypertriacylglycerolaemia (fasting TAG ≥ 150 mg/dl); low HDL-cholesterol (≤40 mg/dl for men and ≤50 mg/dl for women or lipid-lowering medication use); hyperglycaemia (fasting glucose ≥ 100 mg/dl or antidiabetic agent use); insulin resistance (homeostasis model assessment of insulin resistance index > 3·22, regarding the 90th percentile of the sample); and systemic inflammation (high-sensitivity C-reactive protein > 6·07 mg/l, regarding the 90th percentile of the sample)( Reference Wildman, Muntner and Reynolds 9 ). Participants who presented two or more of these metabolic alterations were considered metabolically unhealthy. As mentioned above, participants were also classified according BMI as normal weight (BMI ≤ 24·99 kg/m2) or overweight (BMI ≥ 25·00 kg/m2). Finally, the participants were classified into four phenotypes based on their metabolic status and the presence or absence of overweight: MHNW, MUNW, MHOW and MUOW.
Collection of food consumption data and covariables
Food consumption data were collected through the FFQ developed and validated for the study population( Reference da Silva, Segheto and de Lima 21 ). The FFQ included questions regarding the habitual consumption of ninety-five food items. The reference period was the 12 months prior to the interview and the frequency of consumption was 0–12 times per day, week, month or year. An FFQ item, soya milk, was excluded because its consumption frequency was less than 15 %( Reference Silva, Pereira and Segheto 7 ).
Additionally, we used a structured questionnaire composed of sociodemographic and behavioural questions. The covariables considered as potential confounders were: sex (male/female); age (continuous, in years); education (≤4, 5–8, 9–11 or ≥12 years); and physical activity level assessed by application of the International Physical Activity Questionnaire (IPAQ), long version( Reference Pardini 22 ) (defined as physically active (≥150 min/week) or inactive <150 min/week))( Reference Wildman, Muntner and Reynolds 9 , Reference Haskell 23 , Reference Pinho, Silveira and Botelho 24 ).
Data analysis
In the analysis of dietary patterns, the ninety-five items of the FFQ were grouped into eighteen food groups (Table 1) and consumption was considered in grams, as a continuous variable. Dietary patterns were evaluated using the exploratory factor analysis methodology. The Kaiser–Mayer–Olkin coefficient, Bartlett’s test of sphericity and communalites were calculated before the factor analysis to verify the applicability of the analysis. Subsequently, factors were excluded by using principal component analysis and orthogonal varimax rotation. Factors with eigenvalue > 1·5 were retained, as defined by the scree plot of the variance v. the number of components. The exploratory factor structure was obtained from the indicators with an absolute factor loading greater than 0·20 and the dietary patterns were labelled according to the nutritional composition of the foods. Factor scores were categorized into quartiles and the first quartile was used as reference in all analyses( Reference Olinto, Kag and Sichieri 25 ).
Table 1 Food groups extracted, for factor analysis, from the food consumption frequency questionnaire completed by adults (n 896) aged 20–59 years of both sexes, Viçosa, Minas Gerais, Brazil, 2012–2014

Multinomial logistic regression models were constructed to verify the association between dietary patterns (exposure variable) and metabolic phenotypes (outcome variable). The OR and their respective 95 % CI were estimated for each dietary pattern analysed. All models were adjusted for age, sex, education and physical activity level, considered as potential confounders in the studied relationship( Reference Rodrigues, Pereira and Cunha 4 , Reference Wildman, Muntner and Reynolds 9 ). The MHNW phenotype was used as a reference in all analyses. Additionally, the χ 2 test for trend was used to assess the linear trend over the quartiles of food consumption. All analyses were conducted with the statistical software package Stata version 13.0.
Results
Data from 896 individuals were evaluated. Over half the sample were women (55 %) and the mean age was 34 years (first quartile, 24 years; third quartile, 45 years). The sample consisted predominantly of individuals with more than 12 years of education (54·1 %), non-white (57·3 %), without a partner (54·9 %) and of intermediate socio-economic level (66·5 %). The majority reported no alcoholic beverage consumption (60·2 %), no smoking (69·5 %) and physical inactivity (69·3 %). The MHNW metabolic phenotype was the most prevalent (44·3 %), followed by the MUOW and MHOW phenotypes, which presented similar prevalence (23·0 and 22·7 %, respectively). The MUNW phenotype was identified in 10·0 % of the sample.
The food items of the FFQ were grouped into food groups, as shown in Table 2.
Table 2 Dietary patterns derived from exploratory factor analysis of foods consumed by adults (n 896) aged 20–59 years of both sexes, Viçosa, Minas Gerais, Brazil, 2012–2014
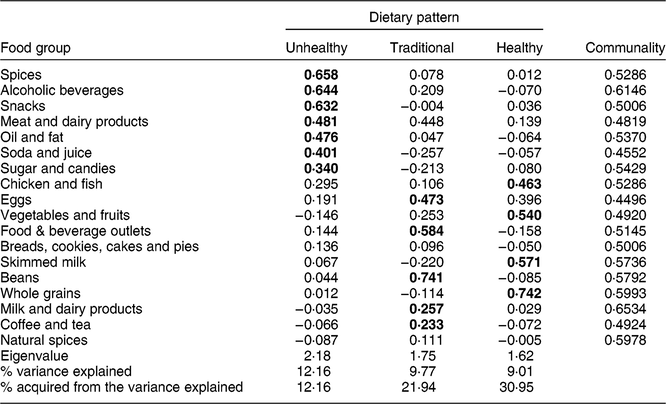
Bold values represent food groups kept in their related eating pattern.
The use of exploratory factor analysis to evaluate the data was satisfactory (Kaiser–Mayer–Olkin coefficient = 0·73 and Bartlett’s test of sphericity = 0·40). It was possible to identify three eating patterns in the study population, with eigenvalues above 1·5, which explained 30·95 % of the total variance in food intake. Food groups with an absolute factor loading greater than 0·20 were considered valid in the dietary patterns, with each pattern being composed of the food groups with the highest factor loadings (Table 2).
The patterns identified were: (i) Unhealthy pattern, composed of alcoholic beverages, oils and fats, condiments, soda and juice, sugars and sweets, snacks, and meats and derivatives; (ii) Traditional pattern, composed of food groups of culinary preparations, beans, milk and dairy products, and coffee and tea; and (ii) Healthy pattern, composed of vegetables and fruits, whole grains, chicken and fish, and skimmed milk (Table 2).
In Table 3, the OR and 95 % CI for the association of the three identified dietary patterns with the metabolic phenotypes can be observed with reference to the first quartile of consumption. The fourth quartile of consumption of the Unhealthy pattern was associated with an 84 % increase in the chance of occurrence of MHOW phenotype (OR = 1·84; 95 % CI 1·06, 3·22), and the third and fourth quartiles were associated with an increase of 94 % (OR = 1·94; 95 % CI 1·11, 3·39) and 156 % (OR = 2·56; 95 % CI 1·41, 4·64), respectively, in the chance MUOW phenotype occurrence. The Healthy pattern was also associated with the MHOW phenotype in a positive way, with a 70 % increase in the chance of occurrence of this phenotype in the fourth quartile of consumption (OR = 1·70; 95 % CI 1·01, 2·86). This pattern was also associated with the MUOW phenotype in all quartiles of consumption, with an increase in the phenotype’s chance of occurrence of 115 % (OR = 2·15; 95 % CI 1·23, 3·76), 191 % (OR = 2·91; 95 % CI 1·68, 5·04) and 198 % (OR = 2·98; 95 % CI 1·65, 5·37) for the second, third and fourth quartile of consumption, respectively. The Traditional pattern, on the other hand, was not associated with metabolic phenotypes.
Table 3 Association between dietary patterns and metabolic phenotypes among adults (n 896) aged 20–59 years of both sexes, Viçosa, Minas Gerais, Brazil, 2012–2014
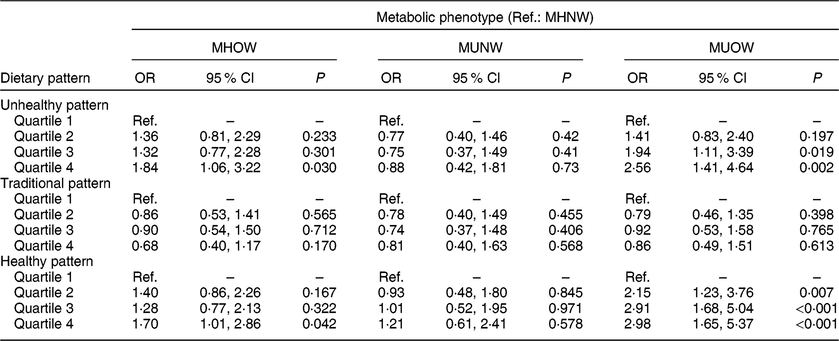
Ref., reference category; MHNW, metabolically healthy normal weight; MHOW, metabolically healthy overweight; MUNW, metabolically unhealthy normal weight; MUOW, metabolically unhealthy overweight.
Models are adjusted for age, sex, education and physical activity.
A significant linear trend was observed only for the relationship between the Healthy pattern and MUOW (P trend < 0·01).
Discussion
To the best of our knowledge, the present study is the first to investigate the relationship of dietary patterns with metabolic phenotypes in Brazilian adults. The results indicated a positive association between the identified Unhealthy and Healthy dietary patterns and the overweight phenotypes (MHOW and MUOW). On the other hand, there were no significant associations of these patterns with the MUNW phenotype nor of the Traditional pattern with any of the evaluated phenotypes.
In studies on the association between dietary patterns and metabolic phenotypes, results similar to our study were observed. In investigations carried out with the American population, individuals with MUOW showed significantly higher consumption of red meat, processed meat and fried foods when compared with those with MHNW( Reference Kimokoti, Judd and Shikany 16 , Reference Kimokoti, Judd and Shikany 18 ). These foods compose the Unhealthy pattern identified in our study, which was also positively associated with the MUOW and MHOW phenotypes. Likewise, other authors have associated patterns with high energy content to increased risk of metabolic disorders and weight gain( Reference Neumann, Martins and Marcopito 3 , Reference Rodrigues, Pereira and Cunha 4 , Reference Silva, Pereira and Segheto 7 ).
Among the biological mechanisms that may explain this relationship, high consumption of high-energy foods influences the profile of adipokine secretion, insulin sensitivity and endothelial function, besides promoting changes in the intestinal microbiota affecting energetic homeostasis and lipid accumulation( Reference Fardet 26 , Reference de Moraes, Adami and Falcão 27 ). These alterations are associated with increased risk of metabolic disturbances and weight gain( Reference Rodrigues, Pereira and Cunha 4 , Reference Salvatti, Escrivão and Taddei 28 ). On the other hand, the high consumption of healthy foods has been pointed out as a protective behaviour because of their high fibre content and low energy density( Reference Pomerleau, Østbye and Bright-See 29 ). Moreover, diets based on unprocessed and minimally processed foods have been associated with lower glycaemic response and higher satiety( Reference Fardet 26 ).
The Healthy pattern identified in the current study presents characteristics of a healthy dietary pattern. This pattern is similar to those found in other studies which include white meat, whole grains, fruits and vegetables in their composition( Reference Pinho, Silveira and Botelho 24 , Reference Fardet 26 ). However, this pattern was positively associated with MUOW in the current study, as observed in other studies assessing dietary patterns’ association with body adiposity( Reference Silva, Pereira and Segheto 7 , Reference Perozzo, Olinto and Dias-da-Costa 15 ). These results can be explained, at least in part, by the under-reporting of high-energy foods and the over-reporting of healthy foods by overweight individuals( Reference Pomerleau, Østbye and Bright-See 29 , Reference Lissner, Heitmann and Bengtsson 30 ). In addition, given the cross-sectional nature of our study, reverse causality is possible since overweight people may have changed their eating habits in order to control body weight.
Our study presents strengths such as the use of different quality assurance and quality control strategies and the use of an FFQ developed and validated for the population assessed. Among the limitations, information bias cannot be ruled out since, as already pointed out, the under- or over-reporting of foods composing the dietary patterns, especially among obese individuals, may have contributed to the positive associations observed.
Conclusion
In conclusion, in the present study, the more energy-dense dietary pattern was related to excess weight phenotypes. In addition, the healthier dietary pattern was also related to these phenotypes, probably due to under- and over-reporting of foods by overweight individuals and reverse causality. More studies are necessary to evaluate the relationship between dietary patterns and metabolic phenotypes for a more targeted nutritional recommendation.
Acknowledgements
Acknowledgements: The authors are grateful to the collaborators for the development of this study, the volunteers who participated in the research and the funding sources. Financial support: During the 24-month period of research data analysis, author D.L.M.P. received financial support from the Coordination for the Improvement of Higher Education Personnel (CAPES). CAPES had no additional role in the design, analysis or writing of this article. Conflict of interest: The authors declare to have no conflict of interest with the topic addressed. Authorship: D.L.M.P. and L.L.J. participated in the conception and design of the manuscript, statistical analysis, data interpretation and manuscript drafting. D.C.G.S. participated in the conception and design of the manuscript, data interpretation and helped write the manuscript. L.L.J. and D.C.G.S. participated in statistical analysis, data interpretation and contributed intellectual content to the paper. G.Z.L. conceived the study, participated in its design and coordination, and contributed intellectual content to the paper. All authors have read and approved the final manuscript. Ethics of human subject participation: This study was carried out in accordance with the guidelines established in the Declaration of Helsinki and all procedures involving research participants were approved by the Ethics and Research Committee of the Federal University of Viçosa (opinion number 008/2012). Written informed consent was obtained from all participants.





