Obesity and its co-morbidities are commonly related to over-indulgence in food and a sedentary lifestyle. The worldwide increase in the incidence of obesity has been so rapid that it cannot be explained by genetic factors alone, and suggests a central role for environmental factors and lifestyle choices that promote energy storage(Reference Damcott, Sack and Shuldiner1, Reference Jequier2). It has been suggested that early-life environmental influences can have long-lasting effects on health(Reference Gluckman and Hanson3, Reference Gluckman, Hanson, Spencer and Bateson4). Nutrition during early life is one such factor that may determine developmental pathways and health outcomes in adulthood. Experimentally, prenatal undernutrition has been widely used to investigate biological mechanisms that link prenatal events with long-term health consequences in later life, such as metabolic dysregulation, obesity and CVD(Reference Gluckman and Hanson3, Reference Breier, Krechowec, Vickers and Langley-Evans5).
Much less is known about effects of the early-life nutrition on behaviour, cognitive function and lifestyle choices during later life (for a review, see Landon et al. (Reference Landon, Davison, Breier, Gluckman and Hanson6)). Epidemiological data suggest a positive relationship between birth weight and cognitive function until early adulthood(Reference Richards, Hardy, Kuh and Wadsworth7, Reference Richards, Hardy, Kuh and Wadsworth8). Additionally, animal studies have confirmed that early-life events influence behavioural and cognitive development, although there have been conflicting reports on the nature and extent of such differences(Reference Landon, Davison, Breier, Gluckman and Hanson6).
Because obesity results from an imbalance between food consumption and energy expenditure, an understanding of its aetiology requires research into the factors that lead to different lifestyle choices. To date, research on the effects of prenatal nutrition on physical activity levels has been equivocal. Using an open-field test with rats, there have been reports that maternal undernutrition both increases(Reference Wolf, Almli, Finger, Ryan and Morgane9) and decreases(Reference Vickers, Breier, McCarthy and Gluckman10) adult levels of apparent locomotor activity. Procedures that arrange concurrently available physical activity and food may be a more precise way of measuring the choice between these commodities. Measures of preference can be obtained by arranging different alternatives and calculating the ratio of the number of responses emitted at each alternative(Reference Baum11).
The aim of the present study was to investigate whether the level of prenatal nutrition influences choice between eating and running in adult life using an operant choice procedure. To reach our goal we employed a well-established experimental approach in the rat which has shown that offspring of mothers which were undernourished during pregnancy develop obesity and altered metabolic regulation in adulthood when food supply is unlimited(Reference Breier, Krechowec, Vickers and Langley-Evans5, Reference Vickers, Breier, Cutfield, Hofman and Gluckman12–Reference Thompson, Norman, Donkin, Shankar, Vickers, Miles and Breier15). Using this experimental approach, two studies were conducted during which offspring of ad libitum-fed dams and of dams undernourished throughout pregnancy were given the choice between wheel running and pressing a response lever for food.
Methods
Animals
Offspring were bred in accordance with previously published experimental procedures(Reference Krechowec, Vickers, Gertler and Breier14–Reference Vickers, Reddy, Ikenasio and Breier16). Virgin Wistar rats were mated at a mature age of 100 ± 5 d using a rat oestrous cycle monitor (Fine Science Tools, Foster City, CA, USA) to assess the stage of oestrous of the female rats before introducing the male(Reference Ramos, Lee and Peuler17). The colony of Wistar rats and the breeding pairs for the present study were generated by using the ‘maximum avoidance of inbreeding’ method(Reference Wright18), and the genetic variance of the animals in the present study was maintained by using a male:female ratio of 2:3. After confirmation of mating, the dams were housed individually in standard rat cages containing wood shavings as bedding with free access to water. Dams were randomly assigned to receive standard rat chow (Teklad 18 % protein diet; Harland Tekland, Bicester, Oxon, UK) either ad libitum (AD group), or 30 % of ad libitum intake throughout pregnancy (undernourished (UN) group). To prevent a carry-over effect of prenatal undernutrition to the immediate neonatal period, pups from undernourished mothers were cross-fostered onto dams that had received ad libitum feeding throughout pregnancy. Thus, UN offspring were provided with the same nutrient-rich environment after birth as the control offspring. This experimental approach generates UN offspring of a significantly reduced birth weight without affecting litter size(Reference Vickers, Breier, Cutfield, Hofman and Gluckman12, Reference Woodall, Breier, Johnston and Gluckman13, Reference Thompson, Norman, Donkin, Shankar, Vickers, Miles and Breier15, Reference Vickers, Reddy, Ikenasio and Breier16, Reference Woodall, Breier, Johnston, Bassett, Barnard and Gluckman19). To allow a direct comparison with our previous studies investigating physiological, metabolic and endocrine consequences of maternal undernutrition during gestation, we generated identical conditions to those reported by our laboratory before(Reference Woodall, Bassett, Gluckman and Breier20–Reference Vickers, Gluckman, Coveny, Hofman, Cutfield, Gertler, Breier and Harris23).
Litter size was adjusted to eight pups per litter in all groups to standardise and optimise nutrition of the neonates until weaning. After weaning at 22 d, rats were housed in pairs with ad libitum access to water and chow. All rats were exposed to a 12 h light–dark cycle with lights on at 06.00 hours. The present study was approved by the University of Auckland Animal Ethics Committee.
Apparatus
Ten conventional operant chambers for rats (model ENV-007; Med Associates Inc., St Albans, VT, USA) with dimensions 305 × 241 × 292 mm were situated in sound- and light-attenuating shells. The chambers were fitted on the right wall with one retractable response lever and a pellet dispenser. Access to a 356 mm diameter running wheel was provided throughout the session through a raised guillotine door on the back wall. The apparatus arrangement is shown in Fig. 1. The distance run in the wheel was measured in terms of cm run in either direction. A house light provided ambient light, and a fan provided ventilation. All experimental events were controlled remotely by an IBM-compatible personal computer running MED-PC© software (Med Associates Inc., St Albans, VT, USA). The time that each experimental event occurred was recorded at a resolution of 10 ms.
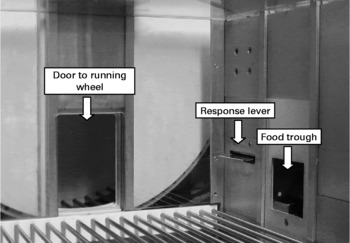
Fig. 1 Photograph of the experimental apparatus. The right chamber wall features a retractable lever and food pellet delivery trough. Access to the running wheel is through the raised guillotine door on the rear wall.
Study design
For the purpose of replication, two studies were conducted, each using identical methods but differing in the timing of key experimental events and group size (study 1, n 6 per group, representing two litters and three litters for AD and UN, respectively; study 2, n 10 per group, representing five litters and six litters for AD and UN, respectively). The timeline of each study is given in Fig. 2. Scheduled feeding was established in all offspring whereby ad libitum food access was limited to 2 h/d to ensure food pellets were effective reinforcers during the behaviour tests. Behavioural sessions were conducted daily between 09.00 and 12.00 hours, immediately followed by 2 h ad libitum feeding.
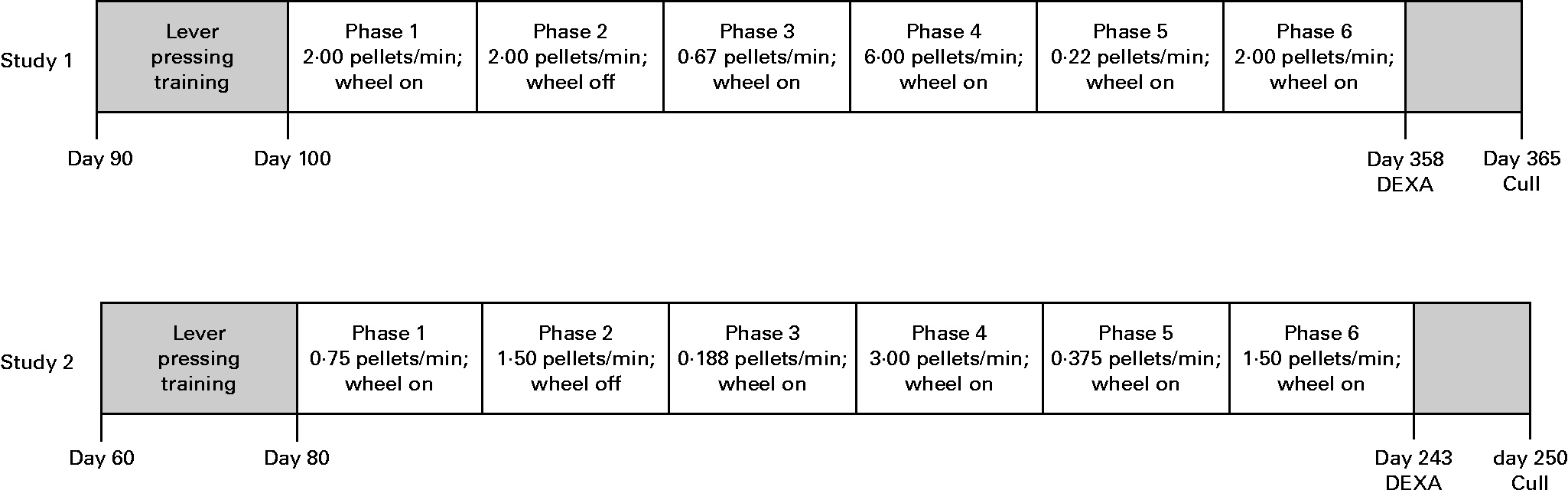
Fig. 2 Experimental timelines and experimental phases for each study. Time point units are age of animals (d). Food delivery rate (average number of 45 mg pellets delivered per min) and wheel running availability are also presented. DEXA, dual-energy X-ray absorptiometry.
Procedure
Pre-training
Rats were trained to press a response lever using an autoshaping procedure (see experimental timeline; Fig. 2)(Reference Brown and Jenkins24). An autoshaping trial consisted of the presentation of the response lever for 20 s followed by its retraction and the delivery of a 45 mg Noyes food pellet (Research Diets, Inc., New Brunswick, NJ, USA). When a response occurred, the lever was immediately retracted, and a food pellet was delivered. Trials were spaced on average 60 s apart. Daily sessions were 60 min in length. Once a rat responded on at least 95 % of trials in a session, it was considered autoshaped, and it was then trained on a variable-interval 1 s schedule, according to which a response was reinforced if it occurred a variable amount of time (1 s on average) after the last reinforcer. Across sessions, the average time between food availability was gradually increased to variable-interval 30 s (i.e. on average two food reinforcers delivered per min).
Experimental procedure
The experimental procedure consisted of six phases, summarised in Fig. 2. Preference between exercise and food was measured across a range of conditions by allowing unlimited access to the running wheel and varying the rate at which food was delivered for lever pressing. Each phase differed in the rate of food delivery, with the rate arranged in an irregular order across phases. All phases continued until at least twenty sessions had been completed, and/or session ratios of running to lever pressing had stabilised. In addition, replicate conditions were used to determine consistency of responding across phases. Daily behavioural sessions lasted until 45 min had elapsed or until the animal had received the maximum of 60 food pellets, whichever occurred first.
Body composition and endocrine analysis
Upon completion of the final experimental phase (see Fig. 2), all animals were placed under halothane anaesthesia to enable body-length measurement, and body composition analysis using dual-energy X-ray absorptiometry scanning (Lunar Prodigy; GE Medical Systems, Madison, WI, USA). Rats were then culled by decapitation under halothane anaesthesia. Blood was collected into heparinised vacutainers and stored on ice (4°C) until centrifugation and removal of supernatant fractions for analysis. Fasting insulin and leptin levels in plasma were measured by ELISA and rat-specific RIA kits L10-1124 (Mercodia AB, Uppsala, Sweden; sensitivity 0·07 μg/l; intra-assay variation 1·2 %; inter-assay variation 3·6 %) and RL-83K (Linco, St Charles, MO, USA; sensitivity 0·04 ng/ml; intra-assay variation 1·88 %, inter-assay variation 3·31 %), respectively.
Statistical analyses
In order to determine the effect of prenatal undernutrition on preference between running and responding for food, taking food reinforcer rate as a continuous variable, we conducted regression analyses for each individual animal between preference (measured by the log10 of the ratio of cm run to number of lever presses for food) and the log10 of the obtained food reinforcers rates per min for that animal. We fitted a mixed model with prenatal nutrition level (AD, UN) and the log10 of the obtained food rate as fixed terms, and rat within treatment and study as a random term using the REML algorithm in the package GENSTAT® (VSN International Ltd, Hemel Hempstead, UK)(Reference Gelman and Hill25, 26). This model assumes a different regression line for each animal. The slopes and intercepts for the regression lines for individual rats within a treatment group are assumed to vary randomly about the parameters for the group regression lines. The value of R 2 of each group regression line was calculated to determine goodness-of-fit. Physiological data were analysed by one-way ANOVA with post hoc analysis using Fisher's protected least significant difference test using Stat-View software (Abacus Concepts Inc., Berkeley, CA, USA). All data are presented as mean values with their standard errors unless otherwise stated. Values of P < 0·05 were considered statistically significant.
Results
Body weight, food intake and plasma analyses
Physiological parameters from both studies are presented in Table 1. Consistent with previous studies using this model of fetal undernutrition(Reference Vickers, Breier, Cutfield, Hofman and Gluckman12, Reference Woodall, Breier, Johnston and Gluckman13), UN offspring from both studies were significantly shorter and lighter than AD offspring at birth (P < 0·001). Furthermore, UN offspring remained shorter than their AD counterparts throughout life.
Table 1 Physiological parameters and plasma endocrine analyses*
(Mean values with their standard errors)
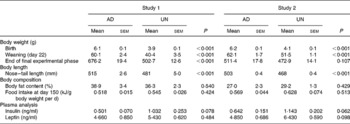
AD, offspring of dams fed ad libitum throughout pregnancy; UN, offspring of dams undernourished during pregnancy.
* Body weight, nose–tail length and body fat content (by dual-energy X-ray absorptiometry) were measured at the completion of the final experimental phase (study 1, 358 d of age; study 2, 243 d of age). Plasma collection for endocrine analysis took place at cull (study 1, 365 d of age; study 2, 250 d of age).
No differences in body fat mass before cull, as assessed by dual-energy X-ray absorptiometry, were found (Table 1). Food intake per g body weight during adult life did not differ between the two prenatal nutrition groups (representative food intake data are presented at day 150 in Table 1).
Although plasma insulin concentrations appeared to be slightly elevated in UN offspring from both studies (see Table 1), no statistically significant differences were observed in either plasma insulin or leptin levels when analysing the two studies independently. However, when the two studies were combined and analysed by two-way ANOVA, prenatal undernutrition was associated with hyperinsulinaemia (P < 0·05). In addition, when using the combined analysis, there was no ‘between-study effect’ on plasma insulin (P = 0·54). No significant difference in plasma leptin between the combined AD and UN groups was observed (P = 0·17).
Wheel running
Fig. 3 shows cm run per min in each phase of the experiment. Running rates in the UN groups were consistently higher than those for the AD groups in both study 1 (Fig. 3 (a); P < 0·005) and study 2 (Fig. 3 (b); P < 0·0005). Running rates during phases with identical food delivery rates (i.e. phases 1 and 6 of study 1, and phases 2 and 6 of study 2) did not differ significantly from each other, thus providing repeatability of the behavioural measures. There was no systematic relationship between body weight and running, and there was no effect of running rate on food intake at any time during either study (data not presented).
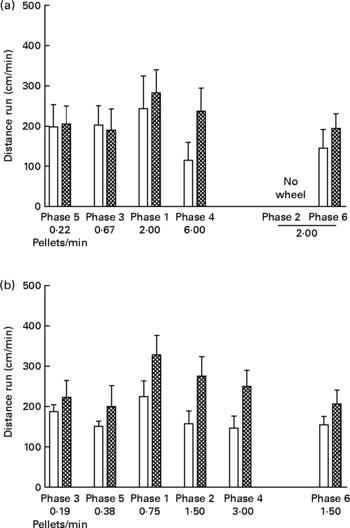
Fig. 3 Distance run (cm/min) in each phase (food delivery rate) of study 1 (a) and study 2 (b) for offspring of control ad libitum-fed dams (AD; □) and offspring of dams undernourished during pregnancy (UN; ![]() ). Data are shown from the last ten sessions of each phase. Phases (phase 1, phase 2, etc) are ordered by increasing food delivery rate for lever pressing, with replicated phases and the phase during which no wheel was available (phase 2 of study 1) displayed on the right side of the axis. Values are means, with standard errors represented by vertical bars. Running rates for the UN groups were consistently higher than those for the AD groups in both study 1 (P < 0·005) and study 2 (P < 0·0005).
). Data are shown from the last ten sessions of each phase. Phases (phase 1, phase 2, etc) are ordered by increasing food delivery rate for lever pressing, with replicated phases and the phase during which no wheel was available (phase 2 of study 1) displayed on the right side of the axis. Values are means, with standard errors represented by vertical bars. Running rates for the UN groups were consistently higher than those for the AD groups in both study 1 (P < 0·005) and study 2 (P < 0·0005).
Lever pressing for food
Fig. 4 shows lever presses per min in each phase of the experiment. Rates of lever pressing increased with increasing rates of food delivery during both study 1 (Fig. 4 (a); P < 0·0001) and study 2 (Fig. 4 (b); P < 0·001). In addition, the lever pressing rates of the UN groups were consistently higher than those for the AD groups in both studies (study 1, P < 0·05; study 2, P < 0·05).
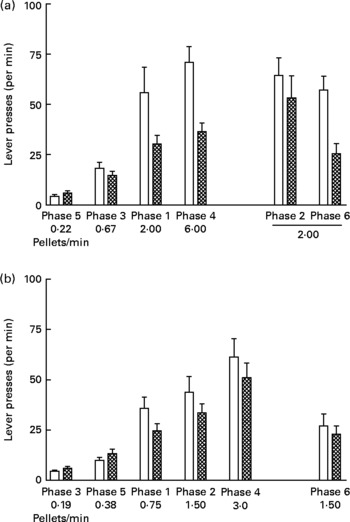
Fig. 4 Lever presses (per min) in each phase (food delivery rate) of study 1 (a) and study 2 (b) for offspring of control ad libitum-fed dams (AD; □) and offspring of dams undernourished during pregnancy (UN; ![]() ). Data are shown from the last ten sessions of each phase. Phases (phase 1, phase 2, etc) are ordered by increasing food delivery rate for lever pressing, with replicated phases and the phase during which no wheel was available (phase 2 of study 1) displayed on the right side of the axis. Values are means, with standard errors represented by vertical bars. Lever pressing rates for the UN groups were consistently higher than those for the AD groups in both study 1 (P < 0·05) and study 2 (P < 0·05).
). Data are shown from the last ten sessions of each phase. Phases (phase 1, phase 2, etc) are ordered by increasing food delivery rate for lever pressing, with replicated phases and the phase during which no wheel was available (phase 2 of study 1) displayed on the right side of the axis. Values are means, with standard errors represented by vertical bars. Lever pressing rates for the UN groups were consistently higher than those for the AD groups in both study 1 (P < 0·05) and study 2 (P < 0·05).
Preference between wheel running and lever pressing for food
The mean data and mean fitted regression lines for each group of animals are shown in Fig. 5. Because we fitted a mixed model linear regression, each rat had its own regression line, and within each treatment group, the regression lines are clustered about a common mean and intercept. The use of a mixed model with random slopes and intercepts for each individual within a treatment group results in the mean slopes and intercepts experiencing shrinkage toward zero. Consequently, the mean slopes (plotted as continuous lines on Fig. 5) will appear flatter than a line fitted to the plotted points. The fitted regression lines maximised R 2, with 88·28 and 99·04 % of the variance explained by the regression for the AD and UN groups, respectively. The degree of preference for running v. lever pressing for food decreased with increasing food rates for both groups of animals, shown by the regression slopes being significantly less than 0 (P < 0·001). The way in which these preferences changed across food reinforcer rates did not differ significantly between AD and UN offspring (AD slope − 0·99; UN slope − 0·91; P = 0·383). Importantly, preference for wheel running over lever pressing for food was significantly higher for the UN offspring compared with AD offspring across the entire range of food reinforcer rates (P = 0·002). This is illustrated by the vertical shift between the fitted regression lines shown in Fig. 5. Results from study 1 and study 2 were similar in these respects. The obtained reinforcer rates (the rate at which food pellets were delivered) did not differ between the AD and UN groups (P = 0·79).
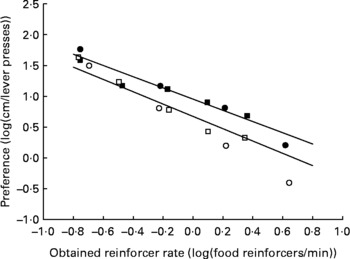
Fig. 5 Average preference (log10 (cm run/lever presses)) for offspring of ad libitum-fed (AD; study 1, ○; study 2, □) and undernourished (UN; study 1, ●; study 2, ■) dams as a function of the average obtained food delivery rate (log10 (food reinforcers/min)) for both studies combined. For AD, the mixed model linear regression is given by y = −0.99x +0.673 (R 2 = 88·28 % of variance). For UN, the mixed model linear regression is given by y = − 0·91x+0·953 (R 2 = 99·04 % of variance). The fitted regression lines are the average of regression lines for each individual rat of each group. The slopes of the regression lines did not differ between AD and UN offspring (P = 0·383), and the results from study 1 and study 2 were the same. Mean preference for wheel running v. lever pressing for food was consistently higher for the UN offspring compared with AD offspring across all food reinforcer rates (P = 0·002).
Discussion
We previously established a robust animal model in which maternal undernutrition leads to low-birth-weight offspring that develop obesity and metabolic dysregulation during adult life(Reference Breier, Krechowec, Vickers and Langley-Evans5, Reference Krechowec, Vickers, Gertler and Breier14–Reference Vickers, Reddy, Ikenasio and Breier16, Reference Breier, Vickers, Ikenasio, Chan and Wong27). However, in these studies animals were maintained under laboratory conditions where exercise was limited to movement around a standard laboratory cage. The present results show that, when rats were offered a choice between exercise (wheel running) and lever pressing for food, the offspring of undernourished mothers exhibited a greater preference for wheel running compared with offspring of normally nourished mothers. The expression of choice between wheel running and lever pressing for food was assessed over an extended period of time and under a broad range of parametric manipulations, spanning a near 30-fold range of food reinforcement rates. Furthermore, behaviour was assessed at stability and excludes the possibility of novelty effects commonly seen in brief assessments(Reference Landon, Davison, Breier, Gluckman and Hanson6). The long-lasting and stable preference for exercise observed in the present studies provides the first direct experimental evidence that prenatal nutrition may influence later lifestyle choices throughout adult life.
The results of the present study indicate that, despite an apparent predisposition to develop obesity(Reference Thompson, Norman, Donkin, Shankar, Vickers, Miles and Breier15) when environmental conditions during postnatal life do not encourage exercise, prenatally undernourished offspring have an increased preference for exercise over feeding when the opportunity to express this choice is available. Furthermore, with the ability to express choice, UN offspring exercised more than controls during the daily 1 h sessions and did not develop the obesity and hyperleptinaemia that is consistently observed under standard (sedentary) laboratory conditions(Reference Breier, Krechowec, Vickers and Langley-Evans5, Reference Krechowec, Vickers, Gertler and Breier14–Reference Vickers, Reddy, Ikenasio and Breier16, Reference Breier, Vickers, Ikenasio, Chan and Wong27). We speculate that this shift in lifestyle choice, if encouraged, is likely to have major health benefits and may prevent the onset of apparently pre-programmed obesity. However, it is not clear from the data of the present study whether the lack of obesity development and its associated metabolic dysregulation were directly related to the increase in physical activity. Future behavioural nutrition research should address this possibility by using standardised exercise protocols. Combining the results of the two present studies, the UN offspring maintained hyperinsulinaemia irrespective of exercise. We have made similar observations in a previous study where elevated insulin secretion was maintained despite an energy-restricted diet, and this was associated with enhanced insulin action(Reference Thompson, Norman, Donkin, Shankar, Vickers, Miles and Breier15). It is tempting to speculate that this prenatally induced hyper-insulin secretion and enhanced insulin action in UN offspring may increase their ability to sustain exercise, although more work is required to investigate muscle metabolic function in this setting(Reference Huber, Miles, Thompson, Norman and Breier28).
The change in choice behaviour observed in the present study is in agreement with a recent study conducted in our laboratory, which found offspring of undernourished mothers to be less adaptive to change in relative (food) reinforcer rates than the offspring of ad libitum-fed mothers(Reference Landon, Davison, Krägeloh, Thompson, Miles, Vickers, Fraser and Breier29). Whether this difference in learning reflects a learning impairment in these offspring, or an altered learning strategy that depends on the specific current environment, remains to be elucidated, as does the extent to which this result is generalisable to reinforcers other than food in other situations. The neurophysiological mechanisms that underpin these phenotypic and choice changes are yet to be determined, but they may affect cortico-striatal activity(Reference Tanji and Hoshi30–Reference Wickens, Budd, Hyland and Arbuthnott32) and/or changes in neuroendocrine regulation that are commonly observed after early-life malnutrition(Reference Ikenasio-Thorpe, Breier, Vickers and Fraser33). We have recently shown in a companion study that offspring of undernourished mothers have decreased hypothalamic agouti-related protein gene expression(Reference Ikenasio-Thorpe, Breier, Vickers and Fraser33). Agouti-related protein is an orexigenic hormone and recent research indicates it plays a role in the suppression of spontaneous physical activity(Reference Tang-Christensen, Vrang, Ortmann, Bidlingmaier, Horvath and Tschöp34). We speculate that a decrease in hypothalamic agouti-related protein expression by prenatal undernutrition(Reference Ikenasio-Thorpe, Breier, Vickers and Fraser33) may be responsible, at least in part, for the increased preference for exercise v. eating observed in the present experiment. Further studies are required to investigate the neurodevelopmental and neuroendocrine mechanisms that control the nutritional programming of lifestyle choices.
While the experimental approach of the present study models approximately the first two trimesters of human pregnancy(Reference Bayer, Altman, Russo and Zhang35), future studies, investigating nutritional or dietary influences during early life on brain development, should identify the developmental ‘windows’ that are most sensitive to dietary influences on brain development. Similarly, the influence of contemporaneous food deprivation on running activity could be investigated in this experimental setting, as acute food deprivation has been shown to enhance locomotor activity(Reference Mistlberger, Webb, Simon, Tse and Su36). Conversely, the possible consequence of an increased choice for exercise on long-term potentiation requires further exploration(Reference Bronzino, Austin La France, Morgane and Galler37).
While it is generally accepted that maternal nutrition during gestation can influence metabolic regulation in offspring throughout later life(Reference Gluckman and Hanson3, Reference Breier, Vickers, Ikenasio, Chan and Wong27), the present study provides experimental evidence that maternal nutrition during pregnancy can also influence choice behaviour that is relevant to obesity prevention. When given the choice, offspring of dams undernourished during pregnancy showed consistently higher preference for wheel running over lever pressing for food compared with controls, even though they resembled controls in body fat composition. These results suggest that nutritional or dietary factors during early development may result in a permanent shift in the nature of lifestyle choices. Such a shift will have substantial and wide-ranging health consequences throughout the lifespan. The present study also highlights that the availability and endorsement of exercise is a vital factor for the prevention of obesity and metabolic dysregulation. Therefore, the present study generally suggests the need for careful consideration of the availability of exercise for the interpretation of experimental studies that investigate dietary or pharmacological treatment or prevention of obesity. The present study also suggests that public health initiatives that intend to curb the global obesity epidemic should pay close attention to increasing the availability and the endorsement of exercise.
Acknowledgements
This research was supported by the National Research Centre for Growth and Development and the Health Research Council of New Zealand. B. H. B., M. D. and J. L. were involved in securing funding for the study. J. L., M. D., B. H. B., J. L. M. and N. M. T. designed the experiment. The experimental procedures were carried out by J. L. M., J. L., C. U. K., M. D. and N. M. T.; J. L. M., C. M. T., J. L., M. D., B. H. B. and C. U. K. were involved with the data analysis. In addition, all authors contributed to the interpretation of results and the writing of the manuscript. The authors have no conflicts of interest to declare.








