Supercapacitors have found a niche in energy efficiency, but reaching a wider market will depend on materials innovation—and lower cost.
Owners of a Toyota Prius C have a right to feel smug. With fuel consumption of up to 53 miles per gallon, it’s rated as the most fuel-efficient compact hybrid car on the market. Part of the credit must go to the 20 or so little cells that supply spurts of power for functions such as electric windows and heating. These clever devices, called electrochemical capacitors or supercapacitors (SCs), also capture kinetic energy during braking, storing it electrically and then releasing it when needed, for example to restart the engine when it switches off as the car idles at a red light.
Energy-storing SCs are becoming ubiquitous in electrical and hybrid transportation, particularly in public trains, trams, and buses (see “Supercapacitors take charge in Germany” in the September 2012 issue of MRS Bulletin, p. 802). Shanghai now has an extensive public bus network that uses SCs in regenerative braking to lower fuel consumption, and they are found in buses and trams throughout Europe. Whereas batteries take hours to charge, SCs need only a few seconds, so buses using them as the sole power source can recharge as they wait at stops. In diesel buses and trucks, SC-based regenerative braking can cut fuel costs by around 30% annually, with a corresponding reduction in carbon-dioxide emissions.
Because they supply short (up to 30-second) bursts of energy, SCs are also well suited to applications such as cordless power tools and heavy-construction machinery. “In the mid-2000s, the use of supercapacitors for opening doors of the A380 Jumbo jet was the first demonstration of the maturity of the technology,” said Patrice Simon, a materials scientist at the Université Paul Sabatier in Toulouse, France. It showed that these devices could perform safely and reliably in a demanding situation.
But SCs are still relatively expensive, partly because the limited demand for them precludes economies of large-scale production. The market could expand substantially if their performance can be improved and their manufacture cheapened. That will depend on clever materials engineering.
At face value, SCs don’t compare favorably with batteries: state-of-the-art SCs have energy densities (energy capacity per unit mass) of around 4–5 watt-hours per kg, whereas lithium-ion batteries achieve over 120 Wh kg–1. But that’s not really the point. Whereas batteries charge and discharge through slowly unfolding electrochemical reactions, SCs can grab and dump all their stored charge in an instant. “The main markets are wherever one needs to deliver current bursts or to consume current surges,” said Donald Sadoway, a materials chemist at the Massachusetts Institute of Technology. So they are complementary to, not competitive with, batteries: SCs for power, batteries for stamina.
Like familiar electrical capacitors, SCs store energy by charging up electrodes electrostatically and then release it during discharge. But they store much more energy than conventional capacitors, because they accumulate charge not as electrons at the electrode surface but as ions adsorbed from an electrolyte into pores within the electrodes.
The most common type of device, an electrical-double-layer (EDL) supercapacitor, consists of two porous electrodes with a high surface area (more than 1000 m2 g–1), usually made of activated carbon, saturated with an electrolyte, and separated by a thin polymer membrane. For the electrolyte, current commercial devices generally use organic salts such as tetraethylammonium tetrafluoroborate in an organic solvent such as acetonitrile or propylene carbonate. During charging, the potential applied to the electrodes draws ions from solution onto their surface. A layer of counterions accumulates right next to the ion-coated electrodes, creating a so-called electrical double layer. It is the intimate proximity of the charged electrode and counterions in this double layer that provides the capacitance: the smaller the separation of charge, the greater the capacitance. When the device is discharged, the ions remix in the electrolyte.
The capacitance can be further enhanced by making use of battery-like electrochemical processes on the porous electrode surface: redox reactions that are fast enough to sustain the rapid charge-discharge behavior. Such devices are called pseudocapacitors. Their electrodes are substances that may undergo redox processes, typically transition-metal oxides such as RuO2, Fe3O4, or MnO2deposited as thin films on porous or nanostructured supports.
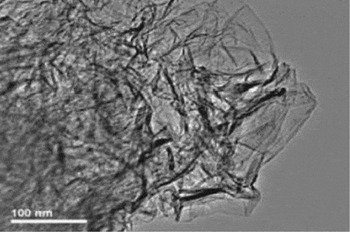
Nanostructured carbon, like these crumpled graphite sheets, can provide electrodes with very large surface area. Image courtesy of Rodney Ruoff, University of Texas at Austin.
Thierry Brousse of the University of Nantes in France, president of the evaluation committee for the French National Research Agency PROGELEC program on energy storage, said that the key aims for improving electrochemical capacitors are to achieve higher energy density while retaining high power density and long lifetime (more than 100,000 cycles), to improve safety, and to achieve coupling with other devices, such as batteries and photovoltaics. “Optimizing today’s products means finding better carbon electrodes and electrolytes,” says Brousse. “New devices definitely need new materials.”
The most obvious way to increase the energy density of SCs is to increase the surface area of their electrodes, packing in more ions during the charging cycle. The advent of nanostructured forms of carbon over the past two decades has attracted great interest from researchers looking to boost SC surface area. Forests of aligned carbon nanotubes are being explored by several groups for SCs, and the start-up company FastCap, a spinoff from research at the Massachusetts Institute of Technology, aims to commercialize such devices.
Another potential source of high-area carbon electrodes is graphene: sheets of graphite-like carbon one-atom thick (although in most devices, the material contains several such layers). Last year, Rodney Ruoff at the University of Texas at Austin and co-workers reported SCs made from graphite oxide treated with an alkali to form graphene sheets interconnected into a three-dimensional network of pores 1–10 nm wide, with a very high surface area. Ruoff’s work has spawned a company called Graphene Energy, based in Austin.
Meanwhile, Simon and colleagues have made tiny SCs just a few micrometers across with electrodes fabricated from carbon “nano-onions”—curved graphene-like shells in a concentric arrangement deposited onto a current-collecting surface. Such devices could open the door to new applications of SCs, for example as power sources in portable electronics and in medical implants used for drug delivery.
Some researchers are using new forms of nanostructured carbon as supports for manganese oxide (MnO2) in pseudocapacitors. MnO2 is relatively cheap and nontoxic and is already used in lithium-ion batteries. It is a poor conductor, but that problem can be overcome by using it as ultrathin films or very small particles. For example, using MnO2 nanoparticles embedded in the walls of an ordered nanoporous form of carbon called CMK3 formed on a template of porous silica, Jianlin Shi and colleagues at the Shanghai Institute of Ceramics have made pseudocapacitors with high capacitance. Such devices could work well with aqueous electrolytes, both keeping costs down and improving the device safety. “People try to avoid using acetonitrile,” said Brousse. He and his co-workers have been exploring the use of aqueous sodium sulfate and lithium nitrate in electrochemical capacitors with carbon/MnO2electrodes.
The performance of SCs might be boosted by entirely new designs. One option is to fully integrate battery-like behavior with capacitive to exploit the best of both worlds: high power and energy densities. Such cells might combine a graphite electrode like those in lithium batteries, which intercalates lithium ions, with a capacitive carbon material. A closely related approach is being pursued by Aquion Energy, a spin-off company from research led by Jay Whitacre of Carnegie Mellon University in Pittsburgh. Whitacre and colleagues have developed a hybrid that works as an EDL cell, a pseudocapacitor and a sodium-ion battery. The key to commercial viability, said Whitacre, is to use cheap materials and established manufacturing methods: for the activated carbon anode, the team forsakes expensive materials developed for SCs in favor of a material made by pyrolysis of caramelized glucose. Aquion’s plant near Pittsburgh should start producing the batteries in 2013.
The voltages of conventional SCs are typically restricted to less than 3 V. This is a nuisance in some applications—vehicle electrics, for example, usually work at 24 V, requiring several of these costly cells to be connected in series. Low voltages also compromise the energy and power densities. But higher voltages decompose the commonly used electrolytes. A promising solution is to replace them with ionic liquids: ionic materials that are liquid at room temperature, which are thermally stable and extremely nonvolatile. SCs using a eutectic (low-melting-point) mixture of ionic liquids can operate over a very wide temperature range of –50°C to 100°C. Some commercial SCs already use ionic liquids to achieve voltages of 3 V, but they could in principle go to 4–5 V.
“New niche markets for electrochemical capacitors will emerge with the extensive use of renewable energy, where they will be used to improve safety or for buffering energy storage and load leveling,” said Brousse. But the biggest challenge, here and elsewhere, is to make the devices cheaper. “Researchers tend to overlook this and instead focus on the electrical performance metrics,” said Sadoway.
While bigger, more powerful and capacious SCs would boost their heavy-duty use in industry and transport, new designs and approaches would open up entirely new applications. “Flexible systems, where the electrodes and the electrolyte are deposited onto flexible current collectors, are getting growing attention,” said Simon. Microscale cells will find uses in microelectronics and medicine, and perhaps also in smart textiles for military and fashion markets. You never know where these power packs might turn up in a few years.


