INTRODUCTION
Psittacosis is an infectious disease caused by the bacterium Chlamydia psittaci. Human cases of infection can occur via the inhalation of contaminated aerosols originating from urine, faeces, or other excretions from infected birds [Reference Beeckman and Vanrompay1]. Infection with Chlamydia psittaci is mainly described in situations that entail close contact with birds. This includes pet shops, veterinary hospitals, and bird shows [Reference Halsby2–Reference Belchior4]. Furthermore, C. psittaci infections are reported in poultry, with human cases linked to occupational exposure in the poultry industry [Reference Laroucau5–Reference Vorimore7].
Upon successful transmission to humans, C. psittaci mainly presents as a non-specific flu-like illness or ‘community-acquired pneumonia’ (CAP) [Reference Beeckman and Vanrompay1]. However, the proportion of CAP cases caused by C. psittaci is unclear. Diagnostic tests for C. psittaci are rarely done when patients present with CAP [Reference van der Hoek8]. This is in line with prevailing guidelines for general practitioners and medical specialists in countries such as the USA, the UK, and the Netherlands that microbiological investigation is not necessary for adequate treatment of uncomplicated pneumonia [Reference van der Hoek8]. However, this implies that the individual patient with C. psittaci pneumonia might not get the optimal treatment. For example, the common presumptive treatment for CAP in the Netherlands is amoxicillin, which is not effective against C. psittaci. In addition, from a public health point of view it is important to trace the source of any human psittacosis case. Linking to animal sources requires both human and animal or environmental polymerase chain reaction (PCR)-based diagnostics with ensuing genotyping of isolates [Reference Heddema9], as well as veterinary and epidemiological investigation.
The present study was done in the context of an integrated veterinary-human health project entitled Plat4m-2Bt-psittacosis. Two of the aims of this project are to reduce the diagnostic deficit of psittacosis in humans by implementing a harmonised respiratory diagnostic PCR method in medical microbiological laboratories, and to determine the disease burden from psittacosis in humans. A psittacosis disease burden calculation requires information on the incidence of psittacosis, which is currently not available. The objective of the present review is therefore to assess the contribution of C. psittaci in the aetiology of CAP in order to obtain a best possible estimate of the real incidence of psittacosis.
METHODS
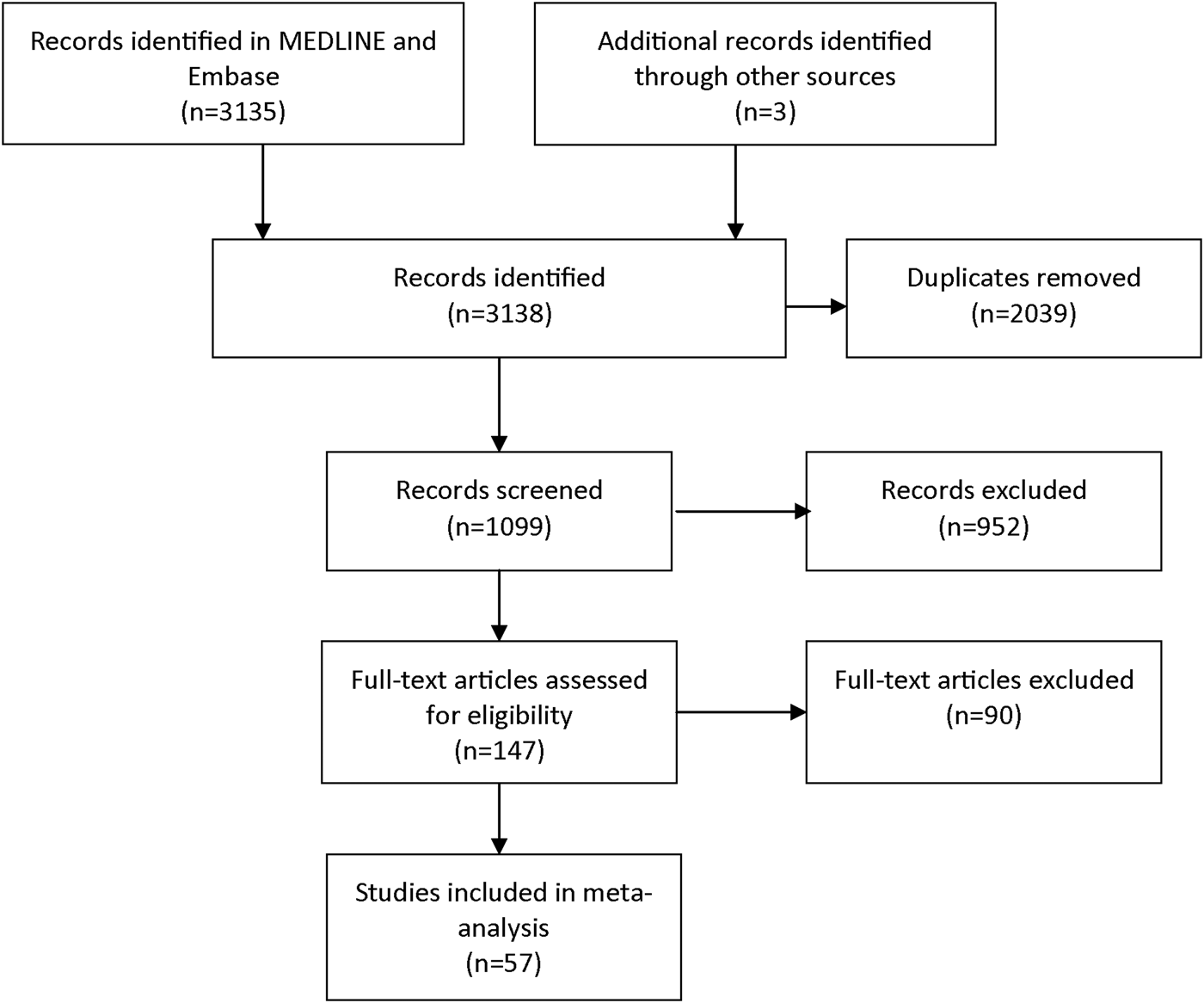
The focus of this systematic review and meta-analysis was on CAP-aetiological studies that included laboratory diagnostics for C. psittaci. We selected articles from MEDLINE and Embase in March 2015. The following key terms, and multiple synonyms hereof, were used to build the search strategy: ‘psittacosis’, ‘Chlamydia psittaci’, ‘Chlamydophila psittaci’, ‘ornithosis’, ‘pneumonia’, ‘community-acquired pneumonia’, ‘incidence’, ‘causative pathogens’. During first screening, studies included were those published from 1986 onwards. In studies before 1986, no distinction was possible between infections caused by C. psittaci and C. pneumoniae, which has a human-to-human transmission route [Reference Grayston10]. A further prerequisite for inclusion was that the research population comprised 100 patients or more. Another prerequisite was that the study had to be written in English, Dutch, German, or Spanish. Exclusion criteria during full text assessment for eligibility were a lack of a full text, not being a CAP-aetiological study, no information on C. psittaci, no specification of the Chlamydia spp., and not presenting original data. Figure 1 shows the search strategy according to PRISMA guidelines [Reference Moher11]. The three additional publications were identified through fellow researchers. Data were extracted about the size of the study population that was tested for C. psittaci, and about the number of C. psittaci detections, the diagnostic test used, the location, and year of study. To estimate the overall proportion of CAP caused by C. psittaci infections, random-effects meta-analysis for proportions was performed using ‘metaprop_one’ package in Stata version 13, with Freeman–Tukey transformation to stabilise variances, weighting by study size, stratified by type of laboratory diagnosis [Reference Nyaga, Arbyn and Aerts12].
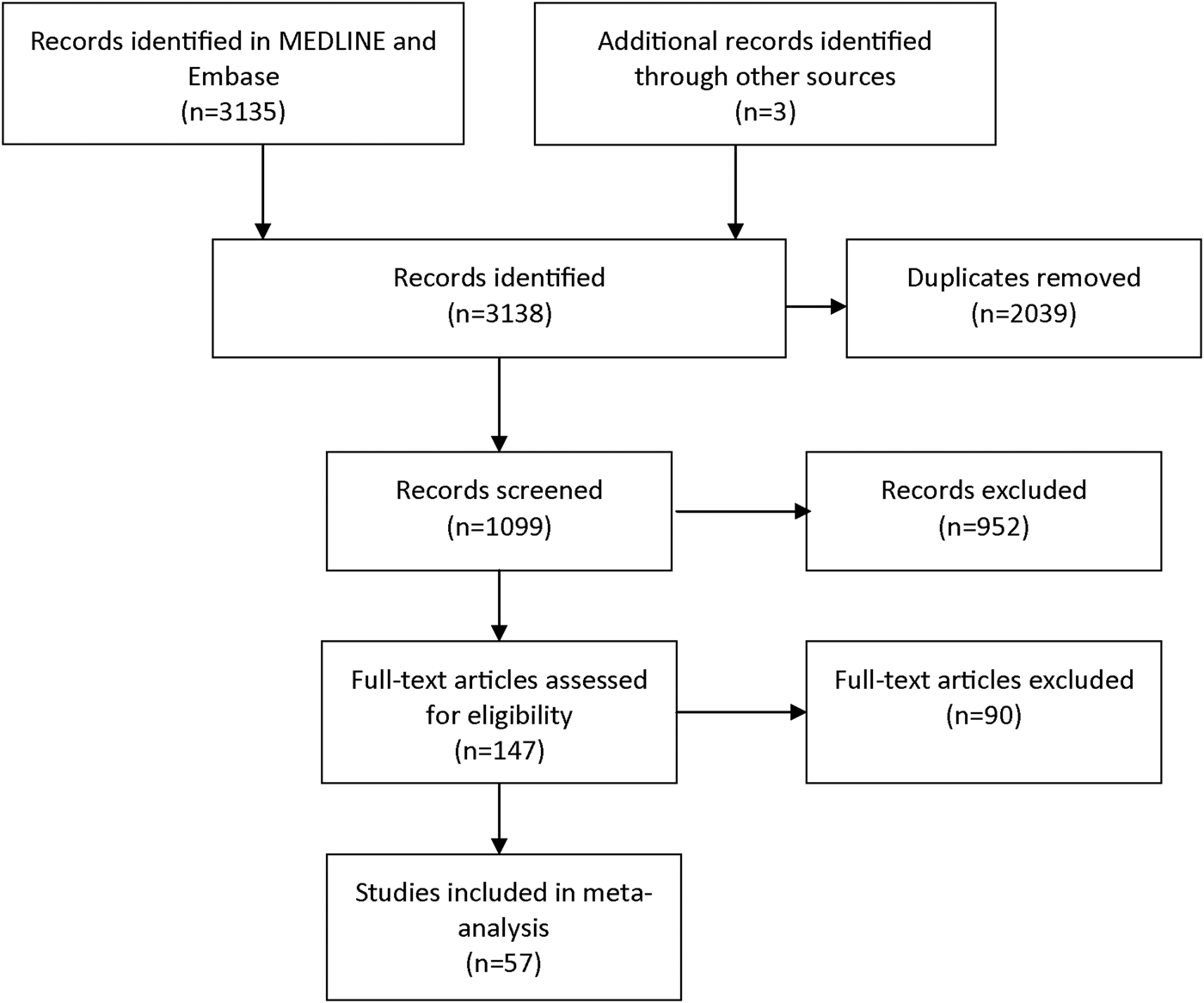
Fig. 1. Selection of publications for the review and meta-analysis.
RESULTS
The literature search yielded 147 studies that seemed eligible for full-text review (Fig. 1). During full text review, a total of 90 articles was excluded because the full text could not be found (n = 10) or provided no information on C. psittaci (n = 49), or was not a CAP-aetiological study (n = 5) or provided no original data (n = 10) or the Chlamydia spp. was not specified (n = 16). This resulted in the inclusion of 57 relevant studies, with a proportion of CAP caused by C. psittaci, ranging from 0 to 6·7% (Table 1). Based on the meta-analysis, C. psittaci was the causative pathogen in 1·03% (95% CI 0·79–1·30) of all cases with CAP that were tested for C. psittaci infection in these 57 studies (Fig. 2).

Fig. 2. Forest plot of meta-analysis of the proportion of CAP caused by Chlamydia psittaci infections, stratified by type of laboratory diagnosis.
Table 1. Details of studies included in the review and meta-analysis

CF, complement fixation test; ELISA, enzyme-linked immunosorbent assay; IF, immunofluorescence test; PCR, polymerase chain reaction.
There are clear changes over time in diagnostic methods used, and in proportion of CAP reported to be caused by C. psittaci. The older studies, including those that were done before 1986, but published from 1986 onwards, were mostly based on complement fixation tests (CF) and reported the highest proportions, with the largest variability between studies (Figs 2 and 3). CF was used in 23 of the included studies but seems to have been replaced by (micro)immunofluorescence (MIF/IF) as the serological test of choice in more recent CAP-aetiological studies. PCR was used in only four of the later studies. Based on PCR results reported in these four studies only, the reported incidence of C. psittaci in CAP is 1·8%. For this PCR-based estimate, only PCR outcomes of the studies were used and CF or IF outcomes that were reported in two of these four studies (classified as ‘mixed or other’ in Figs 2 and 3) were ignored.

Fig. 3. Proportion of CAP caused by Chlamydia psittaci in different studies over time and by type of laboratory diagnosis (top panel), and contribution of each type of laboratory diagnosis to the total over time (bottom panel). In the top panel, each symbol represents a study and the according percentage of CAP patients in which C. psittaci was found. The varying colours indicate the diagnostic methods that were used. CF, complement fixation test; IF, immunofluorescence test; ‘unsp.’, unspecified; PCR, polymerase chain reaction. In the bottom panel, the filled colours represent the contribution of each type of laboratory diagnosis to the total over time, expressed in percentages. ‘Year of study’ represents the year in which the gathering of data commenced. Although studies published before 1986 were not included, the period in which patient data had been gathered usually differed from the year of publication.
DISCUSSION
This review shows that approximately 1% of annual CAP is caused by C. psittaci infection. The estimated proportion of C. psittaci in CAP was remarkably uniform across the wide variety of studies included in this review and meta-analysis. The group of studies using CF formed an exception, with high variability in the reported proportions, and generally higher proportions of positives. This may be explained by cross-reactivity, for instance with C. pneumoniae. Also, some of the included studies were restricted to certain age groups (e.g. children) or patient groups (e.g. intensive care patients), making pooling of the data problematic. Therefore, we repeated the meta-analysis with tighter inclusion criteria, excluding all studies that used only CF (n = 20), all studies in children or intensive care patients (n = 7, of which 1 with CF), and all studies with an onset before 1986 (1 used IF, the others used CF). In this meta-analysis with tighter inclusion criteria, the estimated overall proportion remained approximately 1% (presented in online Supplementary Fig. S1). Another limitation of the present review and meta-analysis is that atypical causative agents in CAP including C. psittaci have been shown to be associated with the non-respiratory season (i.e. late spring, summer, and early autumn in Europe), age <60 years, and male gender [Reference Raeven70], and contact with birds. Unfortunately, there was insufficient information for season-, age-, and gender-specific estimates. The risk of exposure to C. psittaci is likely to differ across geographical areas. Included studies originated from multiple countries, mostly in Europe, and particularly Spain (n = 15). Nevertheless, the heterogeneity across studies was remarkably low, and the estimate of approximately 1% of CAP being caused by C. psittaci was remarkably robust, given the large variation between the studies regarding geographical location, season, diagnostic tests, study population, and the varying (and often not reported) case definitions for CAP and C. psittaci infection.
CAP is a very common condition in all countries of the world. For example, in the Netherlands during the years 2008–2011, the mean annual number of CAP episodes treated in hospitals was 48 843 [Reference Rozenbaum71]. Based on the present review one would expect an annual number of 503 hospitalised CAP patients with psittacosis. If based on the four studies using PCR only, of which three originate from the Netherlands, one would expect an annual number of 879 hospitalised CAP patients with psittacosis. The national infectious diseases surveillance system showed only 93 notified psittacosis patients on average per year over the period 2008–2011, including non-hospitalised cases. The estimation based on the present review therefore entails an incidence of psittacosis that is at least five times higher than the reported figure in the Netherlands.
In many countries, including the Netherlands, most CAP patients are managed in primary care [Reference Snijders72]. However, the CAP-aetiological studies included in the current review were almost entirely done among hospitalised patients. The importance of psittacosis among pneumonia patients in primary care therefore remains elusive, as the proportion of C. psittaci may be different from hospitalised pneumonia patients [Reference Michetti33]. Furthermore, although CAP is likely to be the most important clinical presentation of an infection with C. psittaci, it is not the only one [Reference Beeckman and Vanrompay1, Reference Cunha73]. Other clinical presentations are also possible upon infection with C. psittaci, including severe presentations such as sepsis [Reference Belchior4]. Follow-up studies on the burden of psittacosis, that may use the results of our meta-analysis, would need to take into account other clinical presentations as well.
More frequent testing of CAP patients is recommended to reduce the diagnostic deficit and under-ascertainment. The trend over time in which serological methods are replaced by PCR-based methods is important from a public health point of view as PCR during the acute episode is a very specific method, although less sensitive if the diagnosis is only considered later in the disease episode. Positive samples could be genotyped and matched with animal and environmental samples. Currently, all medical microbiology laboratories in the Netherlands are encouraged to implement PCR-based diagnostics for psittacosis and to send isolates to one laboratory for genotyping [Reference Heddema9]. In time, the increased availability of PCR-based methods and the increased cost-effectiveness of the use of these methods in CAP, particularly in the non-respiratory season, could reduce the diagnostic deficit of CAP, provide better data on the burden of disease from psittacosis, and allow for efficient source detection.
SUPPLEMENTARY MATERIAL
The supplementary material for this article can be found at https://doi.org/10.1017/S0950268817002060
ACKNOWLEDGEMENTS
The study was funded by the Ministry of Health, Welfare and Sports and from a grant by the Netherlands Organisation for Health Research and Development (ZonMw) to the Plat4m-2Bt-psittacosis project, project number: 522001002.
AUTHOR'S CONTRIBUTIONS
BB conducted the review initiated and designed by WH and LH. WH and LH provided BB with oversight and guidance during the project. BG performed the meta-analysis. All authors reviewed the manuscript critically and contributed with revisions.