INTRODUCTION
Chromosome 22q11.2 deletion syndrome (22q11DS) is a genetic condition affecting multiple systems, including the brain, and is associated with a specific neuropsychological profile involving deficits in multiple cognitive domains (McDonald-McGinn et al., Reference McDonald-McGinn, Sullivan, Marino, Philip, Swillen, Vorstman and Bassett2015). Among these domains, executive functions (EF) are part of the key cognitive abilities affected by the deletion. Given that EF play a critical role in formulating goals, planning, and carrying out successful goal-directed behaviors, EF inherently contribute to academic and professional success, as well as autonomy in daily life (Anderson & Reidy, Reference Anderson and Reidy2012; Diamond, Reference Diamond2013). More specifically, performance of individuals with 22q11DS on EF measures in childhood predict adaptive behavior and social adjustment in young adulthood (Albert, Abu-Ramadan, Kates, Fremont, & Antshel, Reference Albert, Abu-Ramadan, Kates, Fremont and Antshel2018).
For over two decades, deficits in EF and attention have been studied in 22q11DS. A recent meta-analysis reported a moderate to large EF impairment in 22q11DS (Moberg et al., Reference Moberg, Richman, Roalf, Morse, Graefe, Brennan and Gur2018). Similarly, deficits in EF are supported by neuroimaging studies showing structural and functional alterations of frontal regions (known to underlie EF) that correlate with task performance (Da Silva Alves et al., Reference Da Silva Alves, Schmitz, Bloemen, van der Meer, Meijer, Boot and van Amelsvoort2011; Harrell et al., Reference Harrell, Zou, Englander, Hooper, Keshavan, Song and Shashi2017; Padula et al., Reference Padula, Schaer, Scariati, Maeder, Schneider and Eliez2017; Rogdaki et al., Reference Rogdaki, Gudbrandsen, McCutcheon, Blackmore, Brugger, Ecker and Howes2020; Scariati, Padula, Schaer, & Eliez, Reference Scariati, Padula, Schaer and Eliez2016; Shashi et al., Reference Shashi, Kwapil, Kaczorowski, Berry, Santos, Howard and Keshavan2010). Yet, previous studies have used a wide range of different methodologies and samples, yielding sometimes contradictory findings and an inconsistent overall profile. Furthermore, the current literature is inconclusive as to whether 22q11DS is associated with an overall EF impairment, or whether impairments may be more pronounced for specific subdomains of EF. This is mainly due to methodological shortcomings regarding task selection and developmental trajectories of EF.
More specifically, despite the recognized diversity of EF models (Karr et al., Reference Karr, Areshenkoff, Rast, Hofer, Iverson and Garcia-Barrera2018; Miyake et al., Reference Miyake, Friedman, Emerson, Witzki, Howerter and Wager2000), early studies aiming to describe the neuropsychological profile of 22q11DS have considered EF as a unitary construct and thus have assessed the participants’ overall executive functioning with only one or two global EF measures such as Wisconsin Card Sorting Test or Trail-Making Test (Lewandowski, Shashi, Berry, & Kwapil, Reference Lewandowski, Shashi, Berry and Kwapil2007; Woodin et al., Reference Woodin, Wang, Aleman, McDonald-McGinn, Zackai and Moss2001). In contrast to examining overall executive functioning, later studies focused on one specific executive domain at a time, such as inhibition, working memory, or multitasking (Kates et al., Reference Kates, Krauss, AbdulSabur, Colgan, Antshel, Higgins and Shprintzen2007; Majerus, Van der Linden, Braissand, & Eliez, Reference Majerus, Van der Linden, Braissand and Eliez2007; McCabe et al., Reference McCabe, Atkinson, Cooper, Melville, Harris, Schall and Campbell2014; Montojo et al., Reference Montojo, Ibrahim, Karlsgodt, Chow, Hilton, Jonas and Bearden2014; Schneider et al., Reference Schneider, Eliez, Birr, Menghetti, Debbané and Van Der Linden2016; Shapiro, Wong, & Simon, Reference Shapiro, Wong and Simon2013). These studies contributed important information on specific EF impairments in 22q11DS. However, in order to achieve a more fine-grained understanding of EF and attentional deficits in the syndrome, multiple executive domains need to be assessed simulatenously in the same sample. In addressing this goal, a major challenge is task impurity, as tests designed to measure EF recruit both executive and nonexecutive abilities. The use of different measures across studies to assess the same construct could contribute to explain the observed differences, and this issue could be solved by the use of several tasks assessing the same executive domain in the same sample, which so far has never been done in this population.
Moreover, the role of age in the development of EF has not always been considered for 22q11DS, as most previous studies separately focused either on children/adolescent populations or on adults (Campbell et al., Reference Campbell, Azuma, Ambery, Stevens, Smith, Morris and Murphy2010; Chow, Watson, Young, & Bassett, Reference Chow, Watson, Young and Bassett2006; Henry et al., Reference Henry, Amelsvoort, Morris, Murphy, Murphy, Owen and Murphy2002). Yet, studies conducted in the general population show that both EF and attention strongly rely on the frontal brain regions, which reach maturation only during early adulthood (Sousa, Amaro, Crego, Gonçalves, & Sampaio, Reference Sousa, Amaro, Crego, Gonçalves and Sampaio2018). In 22q11DS, pre-frontal regions undergo excessive cortical thinning during adolescence (Ramanathan et al., Reference Ramanathan, Mattiaccio, Coman, Botti, Fremont, Faraone and Kates2017; Schaer et al., Reference Schaer, Debbané, Bach Cuadra, Ottet, Glaser, Thiran and Eliez2009), suggesting abnormal maturation processes which could, on a behavioral level, be associated with atypical developmental trajectories of EF. Thus, to fully apprehend EF development in 22q11DS, the full age range from childhood to (early) adulthood should be examined. Unfortunately, as highlighted in Morrison et al. (Reference Morrison, Chawner, van Amelsvoort, Swillen, Vingerhoets, Vergaelen and van den Bree2020), the literature on the cognitive trajectories from childhood to adulthood in 22q11DS is scarce and inconsistent.
Different study designs have been used for investigating developmental trajectories of EF and attention in 22q11DS. Using a cross-sectional design, a large multisite study examined the cognitive performance of 236 participants with 22q11DS aged between 6 and 60 years (Morrison et al., Reference Morrison, Chawner, van Amelsvoort, Swillen, Vingerhoets, Vergaelen and van den Bree2020). They showed that the magnitude of impairment not only differed by developmental stage (i.e., how old patients are) but also differed across distinct cognitive domains. More specifically, processing speed seemed to be more impaired in children, whereas working memory was more impaired in adults, and sustained attention was altered across age groups. Although this study provides important insights into developmental differences between age groups, age was modeled as a categorical variable based on the definitions of “childhood,” “adolescence,” and “adulthood” provided by the World Health Organization guidelines (https://www.who.int). However, EF and attention mature in a nonlinear dynamic way, with different domains showing different trajectories over time (Akshoomoff et al., Reference Akshoomoff, Newman, Thompson, McCabe, Bloss, Chang and Jernigan2014; Anderson, Reference Anderson2002; Romine & Reynolds, Reference Romine and Reynolds2005; Waber et al., Reference Waber, De Moor, Forbes, Almli, Botteron, Leonarf and Rumsey2007). Therefore, to fully grasp the complex dynamic of EF trajectories, age should preferably be considered as a continuous variable. To our knowledge, only a few of studies on 22q11DS have examined continuous age-related trajectories of EF and attention while also assessing multiple executive domains. One study showed a lack of improvement of inhibition and cognitive flexibility performance with age (Shapiro, Tassone, Choudhary, & Simon, Reference Shapiro, Tassone, Choudhary and Simon2014). However, there was a significant effect of age on working memory (verbal and nonverbal) performance, with older participants exhibiting a higher working memory span. Another study found that executive control of attention is affected by age, with younger children having more pronounced impairments and more variable scores (Stoddard, Beckett, & Simon, Reference Stoddard, Beckett and Simon2011). However, both studies that were limited by a cross-sectional design preclude the assessment of individual variability. In addition, the ages range from 7 to 14 years, thereby yielding only a partial view of the full childhood-to-adulthood trajectory.
Using longitudinal data (several assessment per participants), one study examined neurocognitive changes over a 3.5-year interval in children and adolescents (Hooper et al., Reference Hooper, Curtiss, Schoch, Keshavan, Allen and Shashi2013). They reported significantly lower performance in the 22q11DS group compared to healthy controls for intellectual functioning, attention, cognitive flexibility, working memory, and processing speed at first and second evaluation. When controlled for chronological age, changes in raw scores over time between evaluations were significantly different only for one measure of sustained attention, with slower gain for 22q11DS participants. Furthermore, from a developmental perspective (for a visualization, see Figure 1), a study discussing the use of raw scores found that most measures of reasoning (verbal and nonverbal), EF (planning, set-shifting, and spatial working memory), and attention follow a developmental deficit model (i.e., static cognitive impairments that emerged early in development and remain stable) (Chawner et al., Reference Chawner, Doherty, Moss, Niarchou, Walters, Owen and van den Bree2017). Only one measure of nonverbal reasoning (block design) showed a development lag pattern (i.e., syndromic individuals showed absolute growth in cognitive ability but were lagging behind compared to typical developing individuals) and one measure of processing speed yielded a developmental maturation pattern (i.e., individuals with 22q11DS showed initial cognitive impairment but caught up with the control group at later stages of development). No developmental deterioration (i.e., decline in absolute ability) was observed. Thus, encouraging new insights have emanated from longitudinal approaches to EF maturation. However, the age range of the study (mean age visit 1 = 9.9, standard deviation = 2.4; mean age visit 2 = 12.5, standard deviation = 2.3) again prevented a characterization of the full developmental trajectory of EF.
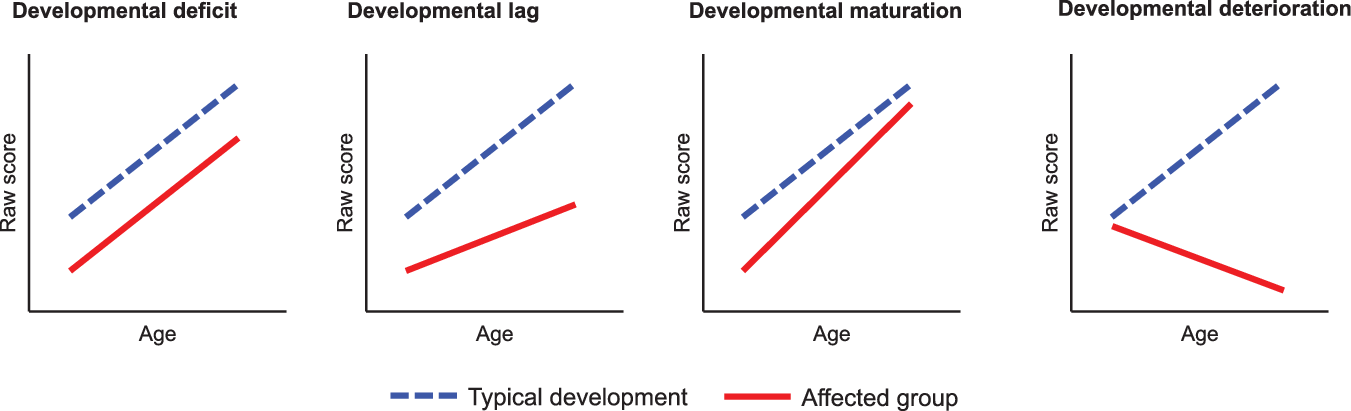
Fig. 1. Visualisation of four developmental patterns of raw scores with age (Adapted from Chawner et al., Reference Chawner, Doherty, Moss, Niarchou, Walters, Owen and van den Bree2017).
Finally, in the only prior study assessing a larger age range (individuals aged 6–26 years), findings revealed deviant trajectories of updating (small improvement with age in the 22q11DS with individuals reaching a developmental plateau much faster than controls) and verbal fluency (very modest improvement with age in the 22q11DS group compared to controls) with age (Maeder et al., Reference Maeder, Schneider, Bostelmann, Debbané, Glaser, Menghetti and Eliez2016). In contrast, inhibition followed the same trajectory as controls, even though performances were overall significantly weaker in 22q11DS. However, only a modest number of EF domains were examined in this study, yielding again only a partial view of the overall developmental profile of EF and attention in 22q11DS.
Taken together, the available literature has provided evidence for distinct patterns of development in different cognitive domains or tasks but has thus far been limited by the age range examined and the number of cognitive domains examined in the same sample of participants. Identifying developmental patterns in specific domains (developmental deficit, lag, deterioration or maturation) is however crucial for the development of age-appropriate guidelines for evaluation, as well as for the selection of relevant intervention strategies (such as compensation or remediation).
The present study aimed to confirm and further extend previous findings on the developmental trajectories of EF and attention in 22q11DS through two major aspects. First, a wider range of cognitive domains was examined to determine whether 22q11DS patients perform worse than controls on all EF and attention domains or whether some domains are less affected, yielding no group difference. To address the issue of impurity, each domain was examined using at least two different tasks. For a domain to be considered as truly impaired, we expected that multiple tasks in the same domain would yield converging results. Otherwise, group differences could be related to specific aspects to the task (e.g., speed, visual, or motor skills).
Second, participants were examined using a wide age range (8-35 years), considering age as a continuous variable and using raw scores to fully observe developmental patterns. We hypothesized that overall, we would observe an effect of age on all variables showing improvement in raw performance with age. We expected results to show either a developmental deficit or a lag, demonstrated by linear or quadratic trajectories. More specifically, based on previous literature, we expected to find a developmental lag for verbal and nonverbal updating and initiation processes (Maeder et al., Reference Maeder, Schneider, Bostelmann, Debbané, Glaser, Menghetti and Eliez2016; Morrison et al., Reference Morrison, Chawner, van Amelsvoort, Swillen, Vingerhoets, Vergaelen and van den Bree2020; Shapiro et al., Reference Shapiro, Tassone, Choudhary and Simon2014, Reference Shapiro, Wong and Simon2013), a developmental deficit for inhibition and visual attention (Chawner et al., Reference Chawner, Doherty, Moss, Niarchou, Walters, Owen and van den Bree2017; Hooper et al., Reference Hooper, Curtiss, Schoch, Keshavan, Allen and Shashi2013; Stoddard et al., Reference Stoddard, Beckett and Simon2011), and finally, either a developmental lag or a deficit in cognitive flexibility (Chawner et al., Reference Chawner, Doherty, Moss, Niarchou, Walters, Owen and van den Bree2017; Hooper et al., Reference Hooper, Curtiss, Schoch, Keshavan, Allen and Shashi2013; Shapiro et al., Reference Shapiro, Tassone, Choudhary and Simon2014).
METHOD
Participants
One hundred and eighty-three participants (103 with 22q11DS and 80 controls) were recruited as part of a longitudinal cohort of 22q11DS patients (Geneva cohort, e.g., Maeder et al., Reference Maeder, Schneider, Bostelmann, Debbané, Glaser, Menghetti and Eliez2016; Schaer et al., Reference Schaer, Debbané, Bach Cuadra, Ottet, Glaser, Thiran and Eliez2009). The control group was mainly composed of participants’ siblings (80%) and community controls. The age ranged from 8 to 35 years. Participants of the two groups did not differ in terms of age or gender distribution (see Table 1). The presence of the deletion was confirmed using quantitative fluorescent polymerase chain reaction. All participants were recruited through advertisement in patient association reunions, newsletters, and word of mouth. Written informed consent, based on protocols approved by the Swiss Ethical Committee of Geneva (CCER, Switzerland), was obtained for all participants and their parents (if the participant was younger than 18 years of age).
Table 1. Participant characteristics
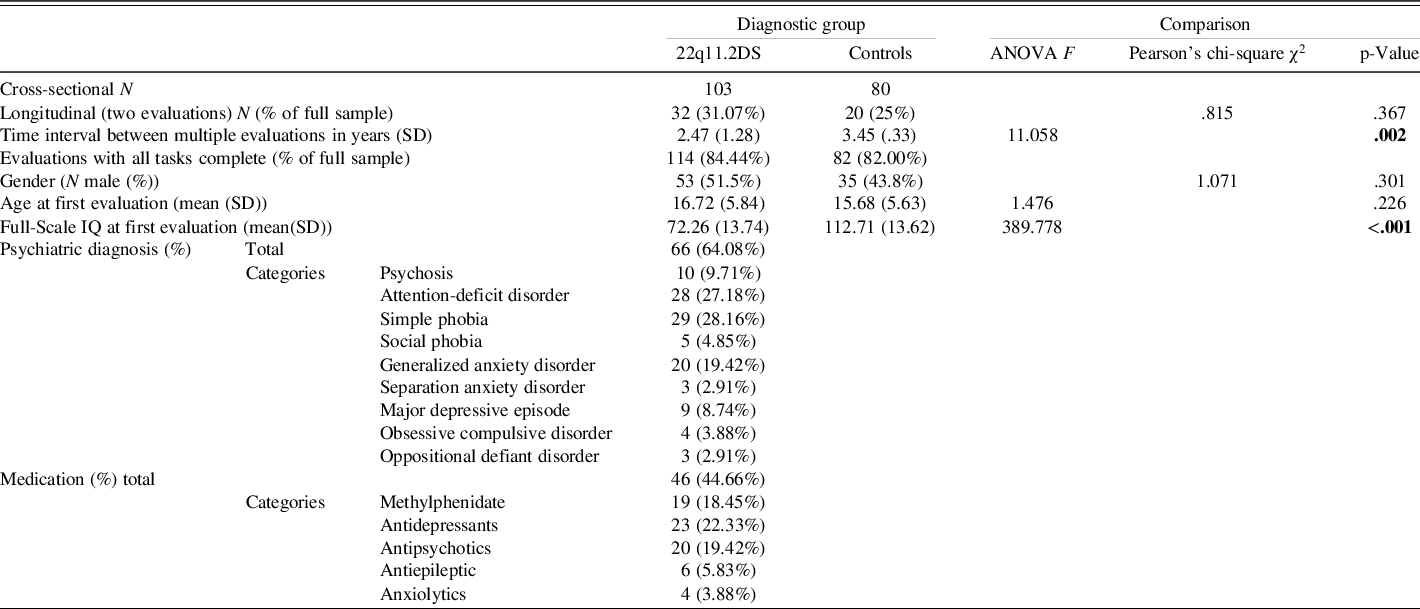
Significant values at the .05 level are displayed in bold.
NB: Participants who had the same diagnosis or received the same medication at several evaluations were only counted once.
Longitudinal data were collected at 2 different time points for a subset of 52 participants (28.42%) with a mean time interval of 2.88 years. As shown in Table 1, a similar proportion of longitudinal data (two evaluations) was available for 22q11DS and control participants. Mean time interval between visits was significantly smaller in 22q11DS due to the presence of additional assessments with slightly shorter delays in a subset of 22q11DS participants (N = 13) obtained through a supplementary longitudinal project.
A trained psychiatrist (SE) interviewed all participants with 22q11DS and their caregivers using the computerized Diagnostic Interview for Children and Adolescents-Revised (DICA-R; Reich, Reference Reich2000) or the Structured Clinical Interview for DSM-IV Axis I (SCID-I; First, Spitzer, & Williams, Reference First, Spitzer and Williams1996). Psychotic disorders and psychotic symptoms were assessed with the supplement of the Schedule for Affective Disorders and Schizophrenia for School-age children Present and Lifetime (K-SADS-PL; Kaufman et al., Reference Kaufman, Birmaher, Brent, Rao, Flynn, Moreci and Ryan1997). At the time of testing, 66 (64.08%) of the participants with 22q11DS had at least 1 psychiatric diagnosis and 46 (44.66%) were taking medication that can affect cognitive performance (for details, see Table 1). Typically developing controls were screened for psychiatric illnesses and medication prior to inclusion in the study.
Due to the longitudinal design of the cohort, half of the individuals (49.18%) were included in a previous study (Maeder et al., Reference Maeder, Schneider, Bostelmann, Debbané, Glaser, Menghetti and Eliez2016), although assessed at an older age and with a broader task set (only four similar tasks).
Materials
Assessment of EF and attention
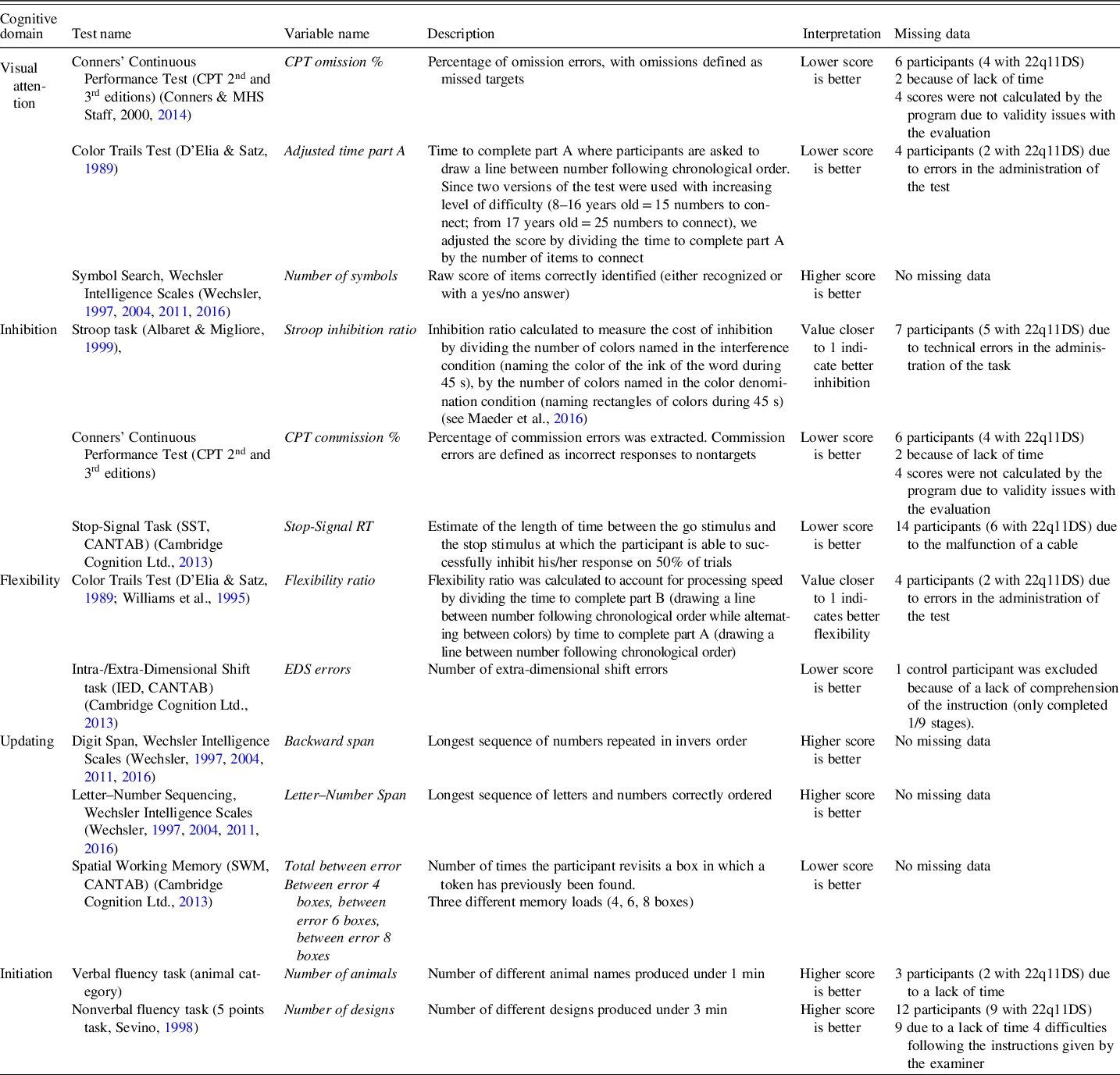
All participants completed an extensive assessment with several tasks of EF and attention, including visual focal attention, inhibition, flexibility, updating, and initiation (see description in Table 2). For visual focal attention, the omission errors from Conners’ Continuous Performance Test (CPT-II and CPT-III; Conners & MHS Staff, Reference Conners2000, Reference Conners2014), the first part of the Color Trails Test (D’Elia & Satz, Reference D’Elia and Satz1989; Williams et al., Reference Williams, Rickert, Hogan, Zolten, Satz, D’Elia and Light1995), and Symbol search from age-appropriate Wechsler Intelligence Scales (WISC-IV, WISC-V, WAIS-III, and WAIS-IV; Wechsler, Reference Wechsler1997, Reference Wechsler2004, Reference Wechsler2011, Reference Wechsler2016) were used. For inhibition, indicators included commission errors (CPT-II and CPT-III), the Stroop task (Albaret & Migliore, Reference Albaret and Migliore1999), and the stop-signal reaction time form the Stop-Signal Task from the Cambridge Neuropsychological Test Automated Battery (CANTAB; Cambridge Cognition Ltd., 2013). Flexibility was assessed with the Color Trails Test and the extra-dimensional errors from the Intra-/Extra-Dimensional Shift task (CANTAB). For updating, the digit span (backward span) and Letter–Number Sequencing Task (Letter–Number Span) from the age-appropriate Wechsler Intelligence Scale were used, as well as the between error score from the Spatial Working Memory task (CANTAB). Finally, initiation was assessed with a verbal and nonverbal fluency task (Sevino, Reference Sevino1998), considering number of items produced. Tasks were chosen to evaluate different aspects of attention and EF in different modalities (verbal and nonverbal) and with different types of tools (paper/pencil and computerized tasks). EF subdomains were regrouped based on the a priori construct stated by the author who developed the test. Correlations between indicators are available in Supplementary Table 1. Tests from the CANTAB were administered using the CANTABeclipse version 6, on a portable touch-screen tablet running on a Windows-based PC system. Detailed descriptions of the tasks can be found on the CANTAB website (https://www.cambridgecognition.com). As shown in Table 1, all the tasks were completed for 196 (83.40%) time points.
Table 2. Details of the measures of executive functions and attention and information about missing data
Intellectual functioning
Intellectual functioning was assessed using the Wechsler Intelligence Scale for children (aged 6–16 years) or adults (aged 17 years and above) (Wechsler, Reference Wechsler1997, Reference Wechsler2004, Reference Wechsler2011, Reference Wechsler2016). Due to the longitudinal design of this study, different versions of the test battery were used. Therefore, only the Full-Scale Intellectual Quotient (FSIQ) is reported. FSIQ at first time point was missing for two participants (both with 22q11DS) for whom only data on EF and attention were collected.
Statistical Analyses
As previously mentioned in the participant description, the dataset included both cross-sectional and longitudinal data. For descriptive statistics, groups were compared at baseline (first assessment) on age, gender, and FSIQ using SPSS 25 (IBM). Trajectories of performance with age were examined using mixed model regression analyses in MATLAB R2018b (Mathworks) (for studies using similar approaches, see Maeder et al., Reference Maeder, Schneider, Bostelmann, Debbané, Glaser, Menghetti and Eliez2016; Mancini et al., Reference Mancini, Sandini, Padula, Zöller, Schneider, Schaer and Eliez2019; Mutlu et al., Reference Mutlu, Schneider, Debbané, Badoud, Eliez and Schaer2013). This method is optimally suited for studies combining participants with variable number of time points and with different time intervals between assessments (Shaw et al., Reference Shaw, Greenstein, Lerch, Clasen, Lenroot, Gogtay and Giedd2006; Thompson, Hallmayer, & O’Hara, Reference Thompson, Hallmayer and O’Hara2011). Within-subject factor was modeled as a nested variable, whereas population parameters (age and diagnosis) were modeled as fixed effects (Dedrick et al., Reference Dedrick, Ferron, Hess, Hogarty, Kromrey, Lang and Lee2009). For each variable, a constant, linear, quadratic, or cubic model was fitted using the nlmefit function in MATLAB. The best model was selected based on the Bayesian information criterion (BIC) method. Statistical significance for differences in trajectories between groups was assessed using a likelihood ratio test. Developmental trajectories resulting from this type of analysis can reveal group differences (i.e., trajectories that follow a parallel path but not on the same intercept) and/or interactions with age (i.e., trajectories that do not follow the same path). To fully grasp the pattern of development with age, raw scores were used in the analysis. As different versions of tests were pooled together at times, the test version was included as a covariate where appropriate (Conners’ Continuous Performance Test, Color Trails Test, and Wechsler batteries).
RESULTS
Comparison of Developmental Trajectories Between 22q11DS and Healthy Controls
Developmental trajectories followed a linear or a quadratic model for most included variables, suggesting an effect of age in a majority of EF and attentional domains (see Table 3 for details). Only two measures of flexibility, one measure of inhibition, and additional updating measures showed constant trajectories, indicating no relationship with age.
Table 3. Results from the mixed model regression analyses. Group comparison (22q11DS vs. controls)
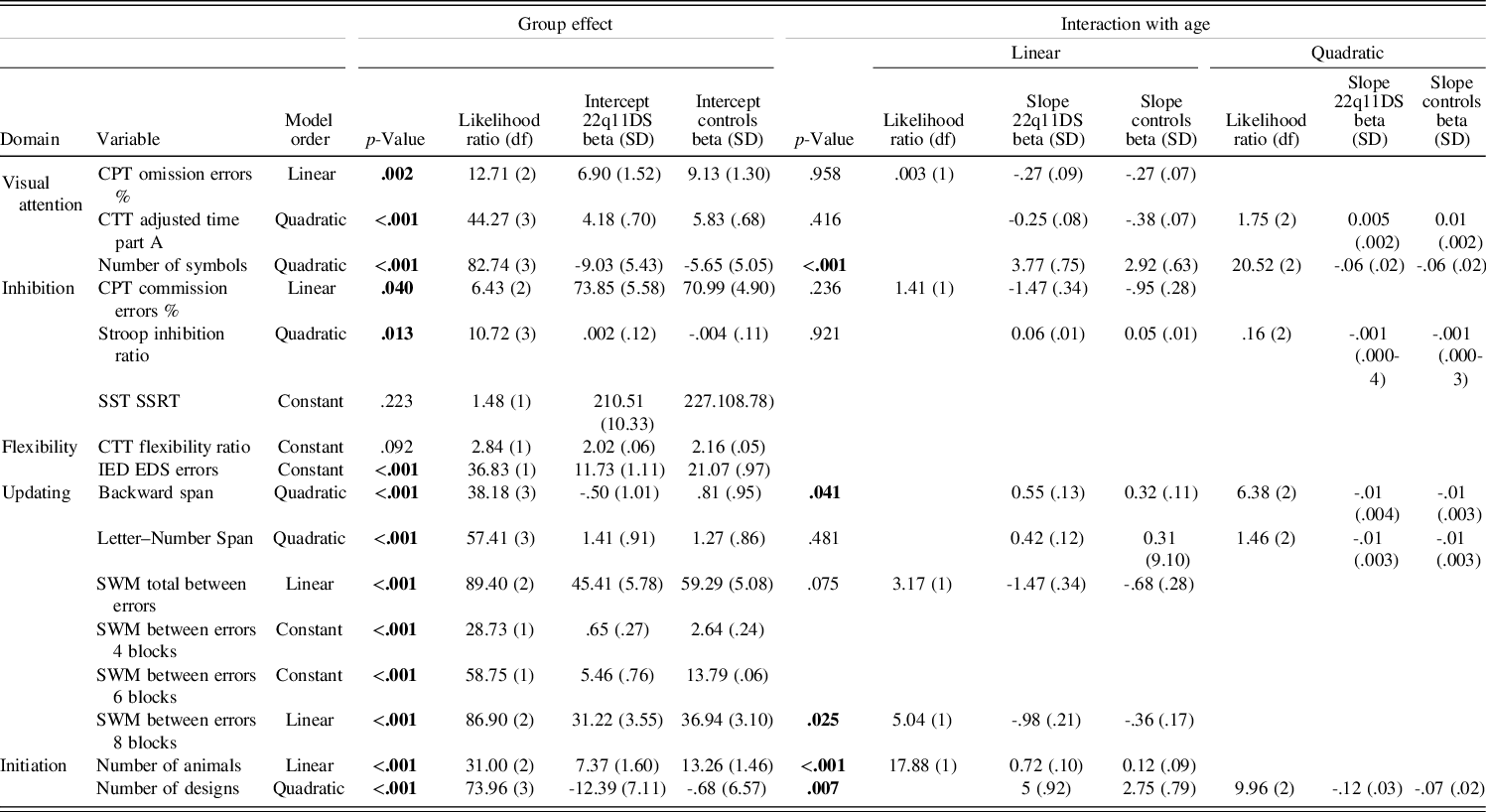
Significant values at the .05 level are displayed in bold.
Significant group differences were observed in all measures of visual focal attention, with lower performance in the 22q11DS group for Conners’ Continuous Performance Test omission error % (p = .002), Color Trails Test Adjusted time part A (p < .001), and number of symbols (p < .001). Only the latter displayed a significant interaction with age (p < .001), with 22q11DS participants improving less with age and reaching a plateau earlier than the control group (for a visual representation of the different trajectories, see Figure 2).
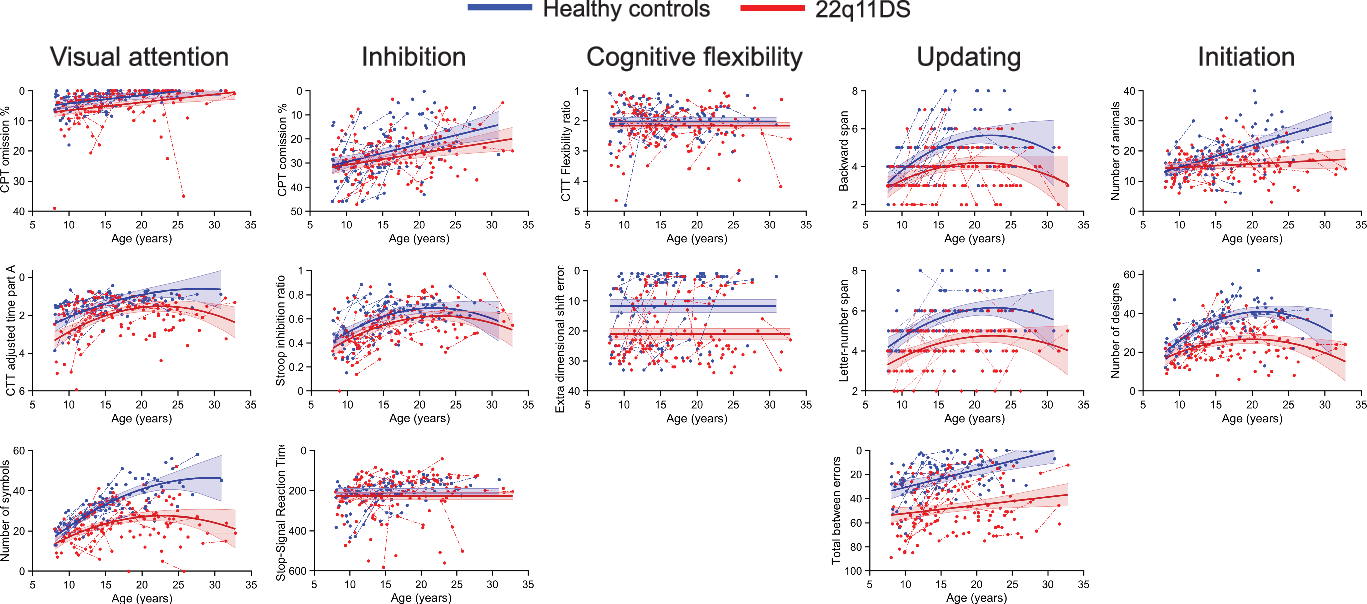
Fig. 2. Developmental trajectories of executive functions and attention domains. The solid lines show the developmental model best fitting the data. Data points from a single subject are connected by a dotted line. Scores from the 22q11DS group are displayed in red and healthy controls in blue. Note that in all sub-figure, scores depicted toward the top of the figure represent better performance whereas score depicted toward the bottom represent worse performance. To improve clarity of interpretation, scales of the y axis have therefore been reversed when lower scores indicated better performance (e.g., less errors), with low scores at the top of the figure and high scores at the bottom.
Inhibition measures showed mixed results depending on the task. Both cognitive inhibition (measured by the Stroop inhibition ratio) and motor inhibition (measured by Conners’ Continuous Performance Test commission error %) yielded significant group differences with better performance in the control group (respectively: p = .013 and p = .040) and similar developmental trajectories across groups. No group difference was, however, observed for stop signal reaction time (p = .223).
Out of the flexibility measures, only the extra-dimensional shift errors from the Intra-/Extra-Dimensional Shift task showed a significant difference, with higher rates of errors for the 22q11DS group (p < .001). The Color Trails Test Flexibility ratio showed comparable performance between groups (p = .092).
Updating measures were significantly poorer in the 22q11DS group in both verbal and nonverbal performance (p < .001). Only the backward span displayed a significant interaction with age (p = .041), with a smaller performance increase with age in the 22q11 group. Interaction with age was not significant for Letter–Number Span (p = .481) and Spatial Working Memory Total between errors only reached trend level (p = .075). Post hoc analyses on the Spatial Working Memory task dividing results according to the working memory load showed a significant interaction with age (p = .025) at the highest load (between errors 8 boxes). Specifically, error rate was diminishing drastically with age in the control group, but changes with age in the 22q11DS group were minimal. Significant group effects were found in all loads (between errors 4 boxes p < .001; between errors 6 boxes p < .001; between errors 8 boxes p < .001) characterized systematically by higher error rates in the 22q11DS group.
Finally, initiation showed significant group effects with better performance in the control group in both number of animals (p < .001) and number of designs (p < .001). In both variables, significant interactions with age showed that performance of the 22q11DS group prematurely reached a plateau compared to healthy controls (number of animals: p < .001 and number of designs: p = .007).
DISCUSSION
The goal of this study was to delineate EF and focal attention development in 22q11DS. To address this, we comprehensively characterized childhood-to-adulthood developmental trajectories, in several domains using multiple tasks per domain, a broader age range (8–35 years), and a partially longitudinal design. Overall, age-related improvement was observed in a majority of cognitive variables. Results moreover point to a variety of developmental trajectories across domains or tasks, with 22q11DS participants showing both developmental deficits and developmental lags compared to healthy controls.
No Evidence for Cognitive Decline
When considering raw score changes in 22q11DS, participants demonstrated an age-related performance increase (i.e., raw score increase) for almost all examined domains of EF and focal attention. This is in line with the literature on healthy controls demonstrating continuous development during childhood, extending to early adulthood (e.g., Romine & Reynolds, Reference Romine and Reynolds2005). When comparing 22q11DS with controls, however, examination of raw scores yielded patterns of both developmental deficit (i.e., lower levels of performance but regular improvement) and developmental lag (i.e., improvement at a slower pace with age) in this sample during this age window. In contrast, neither developmental maturation (i.e., initial cognitive impairment but development catches up with the control group) nor deterioration (i.e., decline in absolute ability) were observed. Previous studies examining changes in neurocognitive measures in samples with smaller age ranges have often suggested patterns of developmental deficit in 22q11DS (Antshel, Fremont, Ramanathan, & Kates, Reference Antshel, Fremont, Ramanathan and Kates2017; Chawner et al., Reference Chawner, Doherty, Moss, Niarchou, Walters, Owen and van den Bree2017; Hooper et al., Reference Hooper, Curtiss, Schoch, Keshavan, Allen and Shashi2013). Some exceptions are observed, with evidence for a developmental maturation of processing speed in Chawner et al. (Reference Chawner, Doherty, Moss, Niarchou, Walters, Owen and van den Bree2017), as well as evidence for a developmental lag of sustained attention in Hooper et al. (Reference Hooper, Curtiss, Schoch, Keshavan, Allen and Shashi2013) and working memory (verbal and visual) in Antshel et al. (Reference Antshel, Fremont, Ramanathan and Kates2017). Discrepancies with our findings could come either from the limited age window examined in these studies (as they did not considered adults but only focused on development across childhood) or from the study design (i.e., mix of cross-sectional and longitudinal measures) in our study. Nevertheless, in line with our results, no previous study reported deterioration for measures of EF and focal attention. Indeed, as previously demonstrated, prevalence of individual decline from one visit to another was observed but did not differ from the control group, reflecting developmental fluctuation rather than a 22q11DS-specific pattern of deterioration (Chawner et al., Reference Chawner, Doherty, Moss, Niarchou, Walters, Owen and van den Bree2017). Regarding cognitive decline, previous studies using overall intellectual abilities as an indicator of cognitive functioning have shown that some individuals with 22q11DS do present a more severe deterioration over time (Duijff et al., Reference Duijff, Klaassen, Swanenburg de Veye, Beemer, Sinnema and Vorstman2013). Particularly, in a large sample from a collaborative study including over 800 22q11DS carriers, early cognitive decline of verbal intellectual abilities (verbal IQ) was suggested as a robust indicator for the emergence of a psychotic illness (Vorstman et al., Reference Vorstman, Breetvelt, Duijff, Eliez, Schneider, Jalbrzikowski and Bassett2015). However, results should be interpreted carefully as the analyses were based on standardized composite scores. Indeed, while a drop in standardized scores reads as a decline, it may result from two different processes: either from a loss of ability (deterioration) or a slower pace of improvement leading to a gap compared to controls (lag).
Diversity of Developmental Patterns Across Domains
Exploration of multiple cognitive domains in the same sample highlighted different patterns of developmental trajectories across domains. This in line with previous results reporting different developmental models depending on the domains examined (Antshel et al., Reference Antshel, Fremont, Ramanathan and Kates2017; Maeder et al., Reference Maeder, Schneider, Bostelmann, Debbané, Glaser, Menghetti and Eliez2016; Shapiro et al., Reference Shapiro, Tassone, Choudhary and Simon2014). Different patterns across cognitive domains were also reported in Chawner et al. (Reference Chawner, Doherty, Moss, Niarchou, Walters, Owen and van den Bree2017); however, measures of EF and attention (spatial working memory, spatial planning, set-shifting, and visual attention) only yielded a single type of pattern (developmental deficit). This contrasts with results of the current study and evidence from previous literature, suggesting that EF and focal attention show that different developmental patterns across domains. Indeed, some domains show a steady increase in raw performance with age (developmental deficit), whereas others display a gap with the control group that widens with age (developmental lag). Differences in findings likely originate form methodological differences between studies (chosen tasks, age range, and cross-sectional of longitudinal design).
Only two domains (inhibition and initiation) yielded a consistent developmental pattern on all tasks, while the other three (flexibility, updating, and focal attention) yielded different developmental models, depending on the task. Furthermore, using different outcome measures (speed vs. accuracy), we showed that accuracy mostly distinguished 22q11DS from controls, whereas speed sometimes did not show any group differences (e.g., for stop-signal reaction time). These results highlight that even when measuring the same domain, changes in tasks, testing modality (verbal vs. nonverbal) or outcome measure can yield different developmental pattern. In this context, future studies may use latent variable approaches to extract communalities across variables sharing variance, which may provide a better way to identify similar patterns of development in 22q11DS. However, such undertaking would need larger samples with comparable age groups, which might be particularly challenging in the context of a rare genetic condition such as 22q11DS.
Clinical Implications
Results from this study have several implications for clinicians and caregivers. First, different patterns of development were observed across domains and sometimes across tasks from one domain or outcome measure. This result should be considered in relation to neuropsychological assessments. Not only does it suggest that different types of indicators can give very different results, but it also indicates that depending on the chosen task or indicator, performance could be only partially represented. With regard to intervention, specific patterns of development for a certain ability should help guiding professionals towards different strategies. Indeed, particular attention should be given to domains exhibiting developmental lag and action should be taken to prevent the gap from widening during adolescence, for example, by introducing early cognitive training targeting the affected domain. Similarly, in domains showing developmental deficits, compensatory strategies could be implemented depending on identified strength in the cognitive profile.
Second, across all domains of EF and focal attention, impairments and/or divergence of developmental trajectories were observed in childhood or early adolescence. This implies that cognitive and educational interventions should be implemented as early as possible during childhood to prevent or lessen future impairments (e.g., Cioni, Inguaggiato, & Sgandurra, Reference Cioni, Inguaggiato and Sgandurra2016; Cutler-Landsman, Reference Cutler-Landsman2020; Wass, Reference Wass2015).
Limitations
Firstly, the developmental trajectories modeled in this paper originated from both cross-sectional and longitudinal data, with a relatively small proportion of participants with two assessments (28.42% of participants). We argue that adding this longitudinal data and combining between- and within-subject variability in one study provides a better estimation of developmental patterns compared to only using cross-sectional data such as in previous studies (e.g., Morrison et al., Reference Morrison, Chawner, van Amelsvoort, Swillen, Vingerhoets, Vergaelen and van den Bree2020; Shapiro et al., Reference Shapiro, Tassone, Choudhary and Simon2014). However, the present findings will need replication in future studies including predominantly longitudinal data.
Secondly, although the examined age range was much larger in our sample compared to most of the previous studies, it remained limited from school age to young adulthood due to the following reasons. On one hand, we had to limit the age range to ensure that the same task could be used across the entire sample. On the other hand, as the Swiss longitudinal cohort focuses on childhood and adolescence, participants older than 35 years of age are only rarely included. Literature on adults 22q11DS carriers older than 30 years of age is still very scarce; however, there is evidence for early onset of neurodegenerative disorders (such as Parkinson’s disease), increasing the risk for cognitive decline in this population (Butcher et al., Reference Butcher, Kiehl, Hazrati, Chow, Rogaeva, Lang and Bassett2013; Fung et al., Reference Fung, Butcher, Costain, Andrade, Boot, Chow and Bassett2015). Future studies should therefore further extend the age range in order to investigate lifespan developmental trajectories, particularly at later adult stages.
Thirdly, only cognitive tasks were selected for this study. Additional questionnaires with observations from the parents could provide supplementary information to the developmental picture of EF and attention in 22q11DS, by increasing ecological validity. For example, analysis of the predicting value of questionnaires measuring EF suggested that parents reports are more sensitive than cognitive performance when it comes to identify children at risk of negative developmental outcome (Albert et al., Reference Albert, Abu-Ramadan, Kates, Fremont and Antshel2018).
Fourthly, tasks were clustered together in different subdomains of EF based on their theoretical construct (i.e., previous research indicating/establishing that tasks measure the respective domain). However, as shown in the correlation matrix provided in supplementary material, correlations between tasks within a certain a priori domain (e.g., cognitive flexibility) were not always significant. This suggests that although some tasks are thought to measure the same theoretical construct, they may actually measure mechanisms that are not correlated. This is an important limitation to consider in regard to the findings of different developmental patterns within a single domain. A data-driven grouping of task into a similar construct, for example, using correlations or principal component analysis (as applied in Fiksinski et al., Reference Fiksinski, Breetvelt, Lee, Boot, Butcher, Palmer and Bassett2019), should be considered in future work.
Finally, patterns of maturation were solely examined based on accuracy or speed indicators extracted from behavioral tasks. However, previous studies using functional magnetic resonance imagery to study working memory have shown significant differences in brain activation during a task, while behavioral results were comparable between groups (Harrell et al., Reference Harrell, Zou, Englander, Hooper, Keshavan, Song and Shashi2017; Montojo et al., Reference Montojo, Ibrahim, Karlsgodt, Chow, Hilton, Jonas and Bearden2014). Combined with evidence of atypical maturation of brain regions who support these abilities in 22q11DS (Ramanathan et al., Reference Ramanathan, Mattiaccio, Coman, Botti, Fremont, Faraone and Kates2017; Schaer et al., Reference Schaer, Debbané, Bach Cuadra, Ottet, Glaser, Thiran and Eliez2009), future work should focus on linking neuroimaging and behavioral results in order to get a more fine-grained understanding of the developmental mechanisms and their underlying neural pathways.
Conclusion
In sum, the current findings confirm and extend knowledge on the developmental patterns of EF and focal attention in 22q11DS. Results indicated age-related improvements on most of the domains examined, although some tasks did not. Contrasting with previous research, the inclusion of a larger age range in this study uncovered not only developmental deficits of individuals with 22q11DS (i.e., lower levels of performance), but also developmental lags for certain cognitive domains (i.e., delayed onset or slower pace of developmental improvement). Specifically, individuals with 22q11DS had worse inhibition as well as a delayed development of initiation skills compared to healthy controls. In contrast, developmental differences between the two groups seemed less clear regarding cognitive flexibility, updating, and visual focal attention, for which performance appeared to strongly depend on the tasks selected to assess a given domain. Overall, findings of the current study demonstrated that EF and focal attention are not affected as a unitary construct, but instead different patterns of development are found across domains and tasks in 22q11DS requiring specific and adapted intervention strategies.
SUPPLEMENTARY MATERIAL
To view supplementary material for this article, please visit https://doi.org/10.1017/S135561772100059X
ACKNOWLEDGMENTS
The authors are grateful to all the families for their participation and their commitment to the study. We also would like to thank our colleagues who participated in data collection and processing: Laura Abdili, Joëlle Bagautdinova, Charlène Bernard, Giulia Binarelli, Mathilde Bostelmann, Aude Burckel, Léa Chambaz, Lucie Chambeyron, Lydia Dubourg, Clémence Feller, Marina Goncalves, Marc Jeanneret, Fiona Journal, Eva Micol, Léa Moreau, Virginie Pouillard, Justine Quiblier, and Alexandra Zaharia. Finally, we would like to thank Karin Bortolin, Valentina Mancini, and Emilie Joly-Burra for their guidance regarding statistical analyses. We are also grateful to Clémence Feller and Joëlle Bagautdinova for proofreading the manuscript.
FINANCIAL SUPPORT
This study was supported by grants from the Swiss National Science Foundation (SNSF) to Prof. Stephan Eliez (#324730_144260 and #320030_179404) and The National Centre of Competence in Research (NCCR) “Synapsy – The Synaptic Bases of Mental Diseases” (#51NF40-158776 and #51NF40-185897). Maude Schneider is supported by an Ambizione grant from the Swiss National Science Foundation (#PZ00P1_174206).
CONFLICT OF INTEREST
The authors have nothing to disclose.







