Mental illness is a leading cause of disability, with approximately 970 million people being affected worldwide(1). Compared with the general population, individuals affected by tuberculosis (TB) are significantly more likely to have poor mental health due to biological and social complexities(Reference Sweetland, Kritski and Oquendo2). Common mental illness, including depression and anxiety disorders, imposes immense costs on healthcare systems and poor quality of life on patients and can be important barriers for TB diagnosis and treatment. Moreover, depression may lead to medication nonadherence resulting in unfavourable TB treatment outcomes and ongoing community transmission(Reference Pachi, Bratis and Moussas3), which has been cited as the fundamental obstacle in effective TB control(Reference Suryanarayana, Rajalakshmi and Jaigopal4). Therefore, mental health is an integral part of achieving WHO’s The End TB Strategy and eliminating the global TB epidemic(5). Unfortunately, the majority of TB and mental illness occur in low- and middle-income countries(Reference Rathod, Pinninti and Irfan6), where mental health is often overlooked due to a myriad of challenges such as lack of mental health specialists, limited treatment options, perceived stigma and other competing public health priorities(Reference Rathod, Pinninti and Irfan6).
Meanwhile, individuals with TB often experience malnutrition(Reference Sinha, Davis and Saag7), which may be exacerbated by food insecurity. Globally, about 2 billion people suffer from moderate to severe levels of food insecurity(8). According to The United States Department of Agriculture, a household is defined as food insecure if at least one member experiences insufficient or inconsistent access to nutritionally adequate and safe food(9). A survey of patients with TB/HIV co-infection in multiple African cities reported that only 10 % TB patients feel they are food secure(Reference Caesar, Crush, Frayne, Crush and McCordic10). Food insecurity not only negatively affects general physical health but also has important ramifications for adverse mental health. For instance, studies have shown that adults with food insecurity are more likely to have anxiety disorder, depression and suicidal ideation compared with those who are food secure(Reference Leung, Epel and Willett11).
Globally, Botswana has one of the highest TB incidence – 300 cases per 100 000 population in 2017(12). About 60 % of all TB patients also have co-morbid HIV infection(12), which has been suggested as a risk factor in psychiatric populations(Reference Peltzer, Naidoo and Matseke13,14) . People living with HIV/AIDS may have an increased risk of developing mood disorders and clinical depressive symptoms triggered by distress associated with initial HIV diagnosis, disease progression, difficult life circumstances, hospitalisation, HIV-induced brain injury and adverse effects of antiretroviral medications(14). The most recent data indicate that psychiatric morbidity in the country contributes an estimated 4·6 % of the global burden of disease(15). There are 0·5 mental health outpatient facilities, 2·5 psychiatrists and 15·2 psychologists available per million population in spite of the heavy burden of psychological distress(15).
For the past few decades, Botswana has been experiencing rapid urbanisation and positive economic growth with a significant decline in poverty incidence(Reference Acquah, Kapunda and Legwegoh16). At the same time, many challenges remain, including food insecurity. The Food and Agricultural Organisation ( FAO) indicated that the average prevalence of severe food insecurity was 41·3 % between 2016 and 2018(17). It is crucial to understand the relationship between food insecurity and mental health among the socially vulnerable TB patients because, while the expansion and integration of mental health care can be a challenging and gradual task in resource-limited settings, social support programmes, particularly food supplementation, may be relatively low intensity and straightforward for policy delivery and implementation. The objectives of our study were to examine the prevalence of depression and anxiety among newly diagnosed TB patients in Botswana and to explore the association between those conditions with food insecurity and HIV co-infection.
Methods
Study design, population and eligibility
We conducted a cross-sectional study and recruited participants with newly diagnosed pulmonary or extrapulmonary TB from primary healthcare clinics in Gaborone, Botswana. Participants were deemed eligible if they were at least 18 years old with a verified TB diagnosis and were recruited by our research staff at the time of receiving TB diagnosis. According to national guidelines during the study period, the first-line diagnostic test for TB was sputum smear microscopy or GeneXpert MTB/RIF for patients who experienced prolonged general pulmonary TB symptoms such as cough, fever, shortness of breath, night sweats, weight loss and haemoptysis(18). Patients with indications for culture testing, such as symptomatic individuals at high risk of multidrug-resistant TB, also underwent examination by mycobacterial culture and drug susceptibility testing with the Mycobacteria Growth Indicator Tube method. A chest radiography was performed in symptomatic individuals who had negative sputum smears or negative Xpert MTB/RIF. For patients who had negative results in all testing, TB diagnosis was made by a physician based on clinical presentations and/or lymph node examinations. A rapid HIV test was conducted among participants who have not had an HIV test done before, or for those who have had a negative result in previous testing.
Data collection
Socio-demographic data were collected via face-to-face interview and included age, gender, education, monthly income, marital status, smoking and alcohol history. We also collected clinical information that included TB symptoms, duration of the symptoms, HIV test result and HIV diagnosis date if any, antiretroviral treatment (ART) history, and results of smear microscopy, Xpert MTB/RIF, mycobacterial culture and chest X-ray. CD4 T-cell data closest to the time of enrolment were extracted and integrated from the national HIV database for HIV-infected participants.
We administered standardised questionnaires to assess the presence and severity of depressive symptoms, anxiety symptoms and household food insecurity of enrolled participants. For simplicity, we will use depression and anxiety to describe depressive symptoms and anxiety symptoms, respectively, hereinafter.
Depression was assessed by the Patient Health Questionnaire (PHQ-9), a nine-item self-report tool that screened depression as participants identified how much each item statement applied to them within the past 2 weeks(Reference Kroenke, Spitzer and Williams19). Examples of item statements include ‘feeling down, depressed or hopeless’ and ‘little interest or pleasure in doing things’. Answer choices were scored on a Likert-type scale that ranged from ‘not at all’, ‘several days’, ‘more than half the days’, to ‘nearly every day’. PHQ-9 has been validated in multiple sub-Saharan African countries to assess depression, including Botswana(Reference Motlhatlhedi, Setlhare and Ganiyu20).
Anxiety was assessed by Zung Self-Rating Anxiety Scale (ZUNG), a twenty-item self-report scale that measured anxiety levels(Reference Zung21). Similar to PHQ-9, participants indicated how much each of the twenty statements applied to them within the past several days and replies included ‘none or a little of the time’, ‘some of the time’, ‘good part of the time’ and ‘most or all of the time’. Examples of item statements include ‘I feel afraid for no reason at all’ and ‘I get upset easily or feel panicky’.
Finally, food insecurity was evaluated by the Household Food Insecurity Access Scale, a nine-item questionnaire widely used across different cultures, including Botswana(Reference Acquah, Kapunda and Legwegoh16), to distinguish food-insecure households and to determine the degree of food insecurity(Reference Coates, Swindale and Bilinsky22). Examples on this questionnaire include ‘In the past four weeks, did you worry that your home would not have enough food?’ and ‘In the past four weeks, did you or anyone in the house go to sleep at night hungry because there was not enough food?’ Answer choices were scored on a Likert-type scale that ranged from ‘never’, ‘rarely (1 or 2 times)’, ‘sometimes (3–10 times)’, to ‘often (more than 10 times)’.
All questionnaires were prepared in English and Setswana, both official languages of Botswana. Study interview was conducted in the preferred language of the participant. We restate that data collection tools (i.e. PHQ-9 and ZUNG) used in this study were suggestive of symptoms of depression and anxiety, and not used for clinical diagnoses. All participants with PHQ-9 scores indicative of depression and suicidal ideation were provided with appropriate psychiatric referrals.
Statistical analysis
The outcomes of interest for this study were the prevalence of depression and anxiety among newly diagnosed TB patients, and the roles of food insecurity and HIV status on (a) depression, and (b) anxiety. Patients were categorised as having depression if they scored 10 points or more on PHQ-9(Reference Kroenke, Spitzer and Williams19), and having anxiety if they scored 36 points (raw score) or more on ZUNG(Reference Zung21). Prevalence estimates of depression and anxiety were calculated as the number of observed participants with depression and anxiety, respectively, divided by the total number of enrolled participants, and the 95 % confidence limits were obtained by the Wilson score method. Based on Household Food Insecurity Access Scale scores, participants were categorised as ‘food secure’, ‘mildly insecure’, ‘moderately insecure’ or ‘severely insecure’ households(Reference Coates, Swindale and Bilinsky22). For the purpose of analysis, we dichotomised participants as experiencing ‘food security or mild insecurity’ or ‘moderate to severe insecurity’(Reference Maxwell, Coates and Vaitla23). Monthly income was categorised as having ‘no income’ or ‘some income’. As recommended for estimating prevalence ratios in cross-sectional studies where the outcome of interest was common, Poisson regression models with robust variance were used to examine the bivariate associations between selected covariates and each of the mental disorder outcomes(Reference Zou24). Independent covariates were selected based on a priori knowledge of predictors of adverse mental health, which include age, gender, HIV status, monthly income, education, marital status and food insecurity. We excluded education and marital status in the multivariable model due to the relatively small overall sample size of our study. Subsequently, we analysed the bivariate associations between age, gender, monthly income, ART status, CD4 T-cell count, food insecurity and each outcome among participants infected with HIV. Similarly, multivariable model was constructed to determine the independent effect of each covariate, regardless of their bivariate associations. However, because this is a smaller subset of participants, we present the model including only food insecurity, ART status and CD4 T-cell count in this paper. Estimates were similar between the smaller model and the full model.
Data analysis was carried out in SAS version 9.4 (SAS Institute). In accordance with recent guidelines, no alpha cut-off was specified for statistical significance(Reference Greenland, Senn and Rothman25).
Results
Between January and December 2019, 180 participants were recruited into the study. Overall, 99 (55·0 %) were HIV positive, 64 (35·6 %) were female, 155 (86·1 %) were single, 137 (76·1 %) were diagnosed with pulmonary TB, 17 (9·4 %) had no formal education and 65 (36·1 %) had no income (Table 1). Among those who were HIV co-infected, the median duration since HIV diagnosis was 43 months, median CD4 T-cell count was 335 and 31 (31·3 %) had never taken ART, of whom 90 % were newly diagnosed with HIV at or within one month of study enrolment. Over half of all participants reported being food secure, and 15 (8·4 %), 17 (9·5 %) and 52 (29·0 %) reported experiencing mild insecurity, moderate insecurity and severe insecurity, respectively (Table 1).
Table 1 Characteristics of study participants in Botswana, 2019 (n 180)
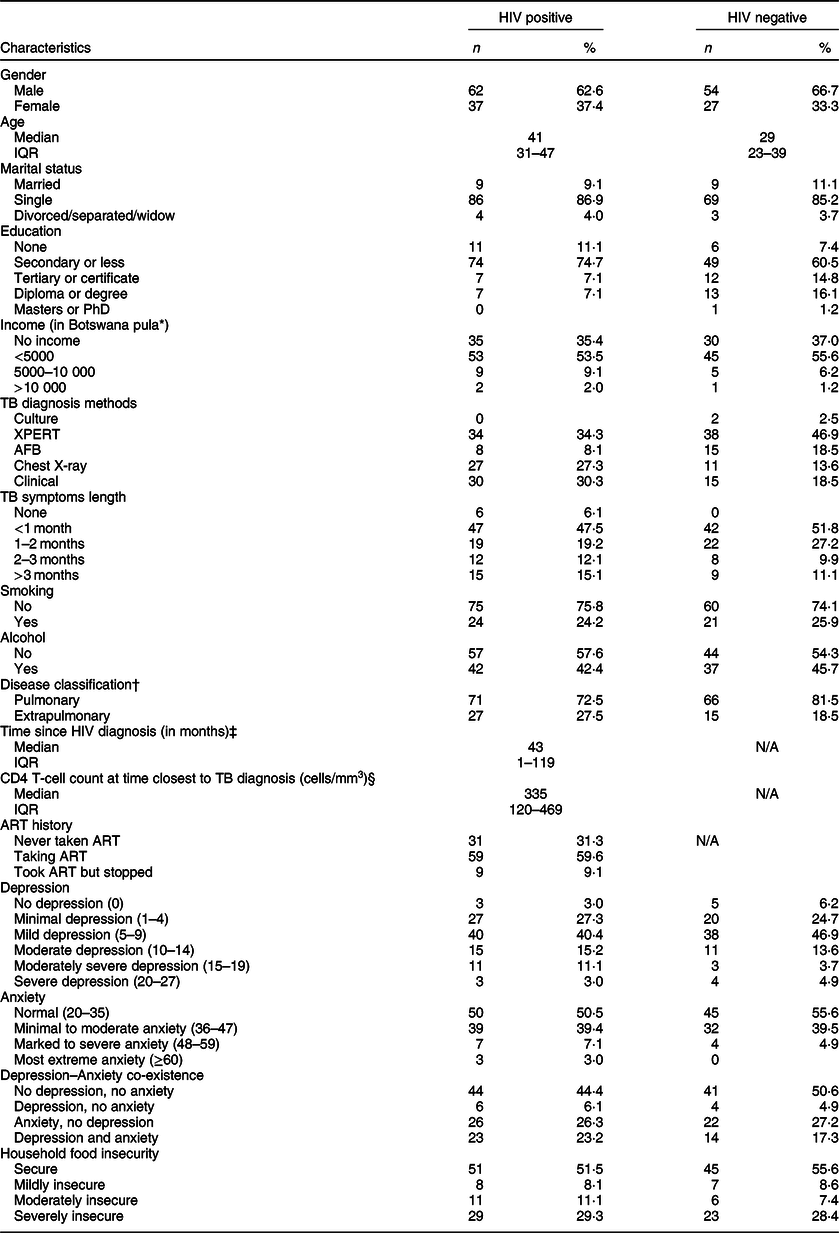
IQR, interquartile range; TB, tuberculosis; XPERT, GeneXpert MTB/RIF; AFB, acid-fast Bacilli smear; ART, antiretroviral therapy.
* 5000 Botswana pula is approximately 465 US dollars.
† n = 1 missing data on disease classification.
‡ n = 2 missing data on HIV diagnosis date.
§ n = 14 missing data on CD4 T-cell count.
The overall prevalence of depression, anxiety and co-existence of both conditions was 26·1 %, 47·2 % and 20·6 %, respectively. Among those who were depressed, 26 (14·4 %), 14 (7·8 %) and 7 (3·9 %) had moderate, moderately severe and severe depression, respectively (Table 1). Twenty-seven (15·1 %) participants had PHQ-9 scores indicative of suicidal ideation. Additionally, 71 (39·4 %), 11 (6·1 %) and 3 (1·7 %) had minimal to moderate, marked to severe and most extreme anxiety, respectively.
Table 2 shows the results of Poisson regression analysis of covariates associated with depression. In bivariate analysis, moderate to severe food insecurity was associated with a higher prevalence of depression (crude prevalence ratio (PR) = 2·37; 95 % CI 1·44, 3·91). After adjusting for age, gender, monthly income and HIV co-infection status, the association between moderate to severe food insecurity and depression remained similar (adjusted PR = 2·30; 95 % CI 1·40, 3·78).
Table 2 Correlates of depression* among study participants in Botswana, 2019 (n 180)
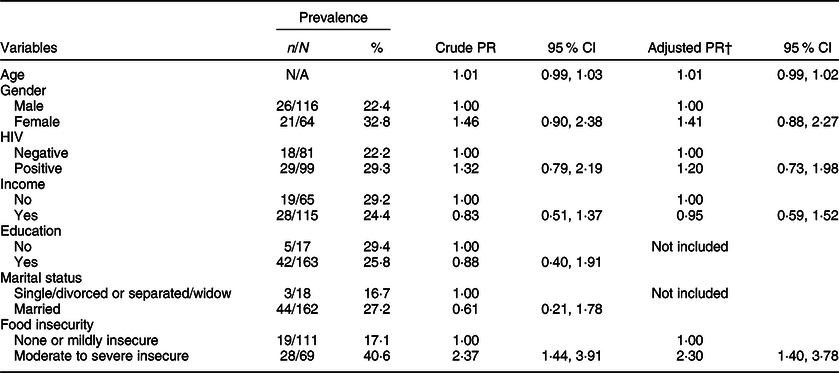
PR, prevalence ratio.
* The presence of depression is defined as scoring equal to or more than 10 points on the Patient Health Questionnaire-9.
† The multivariable Poisson regression analysis adjusted for age, gender, HIV status, income and food insecurity.
Similar findings were observed for the outcome of anxiety; moderate to severe food insecurity was associated with a higher prevalence of anxiety (crude PR = 1·43; 95 % CI 1·06, 1·93; Table 3). The association remained robust in multivariable Poisson analysis after adjusting for age, gender, monthly income and HIV co-infection status (adjusted PR = 1·41; 95 % CI 1·05, 1·91; Table 3).
Table 3 Correlates of anxiety* among study participants in Botswana, 2019 (n 180)
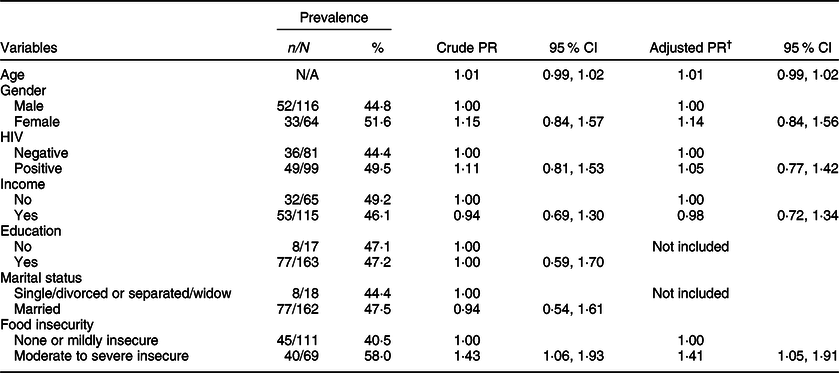
PR, prevalence ratio.
* The presence of anxiety is defined as scoring equal to or more than 36 points (raw score) on the Zung Anxiety Self-Assessment Scale.
† The multivariable Poisson regression analysis adjusted for age, gender, HIV status, income and food insecurity.
Among HIV-co-infected participants, the estimates were similar in the bivariate analysis for depression (crude PR = 2·41; 95 % CI 1·28, 4·55; Table 4) and anxiety (crude PR = 1·54; 95 % CI 1·04, 2·27). Accounting for ART status and CD4 count closest to the time of TB diagnosis, food insecurity continued to be associated with increased symptoms of depression (adjusted PR = 2·33; 95 % CI 1·24, 4·38) and anxiety (adjusted PR = 1·53; 95 % CI 1·03, 2·26) in the multivariable model.
Table 4 Correlates of depression* and anxiety† among HIV-infected participants in Botswana, 2019 (n 99)
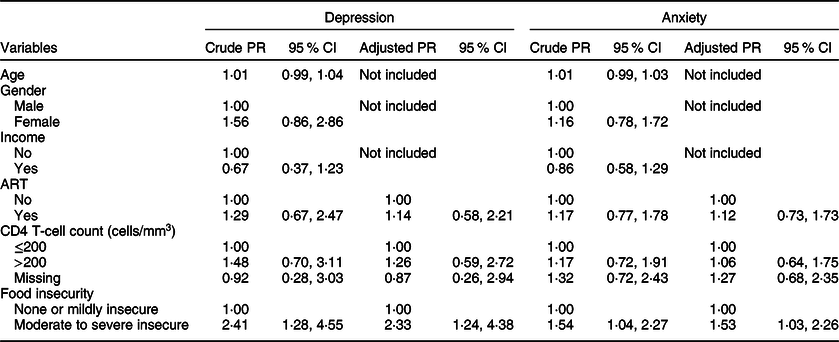
PR, prevalence ratio; ART, antiretroviral therapy.
* The presence of depression is defined as scoring equal to or more than 10 points on the Patient Health Questionnaire-9.
† The presence of anxiety is defined as scoring equal to or more than 36 points (raw score) on the Zung Anxiety Self-Assessment Scale.
Discussion
In our population of TB patients, we showed that household food insecurity was the most important factor associated with symptoms of depression and anxiety, independent of HIV co-infection status, age, monthly income and gender. Our findings confirmed previous research that showed a linkage between food insecurity and poor mental health(Reference Weaver and Hadley26,Reference Jones27) and extended this finding in TB patients. Several reasons may explain this association. First, as a consequence of food insecurity, deficiency in critical micronutrient could lead to depression and anxiety via biological pathways(Reference Rao, Asha and Ramesh28). Additionally, food insecurity encompasses not only hunger and under-nutrition but also uncertainty and distress over the access of food. Thus, food insecurity may compromise mental health by generating instability and unpredictability into lives of the already socio-economically vulnerable TB population(Reference Weaver and Hadley26). This pathway could be independent of the degree of social and economic deprivation, as a global analysis of 149 countries concluded an association between food insecurity and mental illness in a dose-response fashion, after controlling for socio-economic status(Reference Jones27).
To our knowledge, this is the first study that examined the association between food insecurity and mental illness among new TB patients in a high burden setting. In compliance with The End TB Strategy, Botswana has social (e.g. food support) programmes to help TB-affected individuals to improve nutritional status and treatment adherence and alleviate the steep costs associated with the disease and treatment. Specifically, TB patients with confirmed diagnosis may receive food allocations that include cooking oil, beans and maize flour with additive vitamins. However, the food rations are usually not enough to support every member of the household. In addition, TB renders many patients unable to work and contribute to household expenditures, and caregivers may still experience household food insecurity as they face challenges of providing for their family due to income loss(Reference Tanimura, Jaramillo and Weil29). The total financial costs of TB can be catastrophic for patients – up to 58 % of annual individual income and 39 % of household income in low- and middle-income countries, and income loss constitutes the largest portion of all TB-related cost at 60 %(Reference Tanimura, Jaramillo and Weil29). The cyclical nature of the relationship between food insecurity and TB fosters sustained impoverishment and makes it difficult for household to break the cycle without outside intervention. Malnutrition, a consequence of food insecurity, may predispose patients for risks of TB infection and TB disease reactivation(Reference Chandrasekaran, Saravanan and Bethunaickan30).
Comparable with national data released by FAO(17), our survey indicated 38·5 % of the participants had experienced moderate to severe food insecurity prior to receiving their TB diagnosis. Further, a separate survey estimated that only 12 % of households report to be ‘food secure’ in urban parts of Gaborone(Reference Acquah, Kapunda and Legwegoh16). These findings highlight the pervasive food-insecure households among TB patients and those at risk for TB in Botswana, which lend support for food assistance programmes and social protection interventions to target populations who are at high risks for TB. Other research suggests members of households who participate in food supplementation programmes have lower rates of psychological distress; by reducing food insecurity, these programmes can be effective in improving mental health and overall security among vulnerable populations(Reference Oddo and Mabli31).
In this study, we revealed that anxiety and depression were highly prevalent among patients newly diagnosed with TB in Botswana. Approximately 26 % of enrolled TB patients had PHQ-9 scores indicative of moderate to severe depression and 15 % indicative of suicidal ideation. Our estimate was comparable to recent studies in several countries that examined depression (moderate to severe) among TB patients, including Ethiopia (17·6–54·0 %)(Reference Ambaw, Mayston and Hanlon32–Reference Duko, Gebeyehu and Ayano34), South Africa (32·9 %)(Reference Peltzer, Naidoo and Matseke13), Angola (49·4 %)(Reference Paulo and Peixoto35) and China (18·1 %)(Reference Wang, Li and Zhang36). They were, however, considerably lower than those found in Ethiopia using PHQ-9 by Ambaw et al. (Reference Ambaw, Mayston and Hanlon32); these differences may have arisen from variations in study location and population. For example, roughly 45 % of the participants in the Ethiopian study had no formal education and over half were married.
Approximately 46 % of our participants reported anxiety symptoms, comparable with published estimates of other settings: Ethiopia (41·5 %)(Reference Duko, Gebeyehu and Ayano34), Angola (38·3 %)(Reference Paulo and Peixoto35) and China (18·4 %)(Reference Wang, Li and Zhang36). None of those studies utilised the ZUNG scale; instead, Generalized Anxiety Disorder Questionnaire and Hospital Anxiety and Depression Scale were used to assess the presence of anxiety symptoms, which might be an explanation for the discrepant results. Our estimate was much higher than those published by Wang et al. of Chinese TB patients(Reference Wang, Li and Zhang36). One explanation for this discrepancy could be patient characteristics: in the Chinese study, over half of the participants had tertiary or higher education levels; majority were married and patients with extrapulmonary TB were excluded.
We did not find an association between HIV co-morbidity and mental disorders in our sample. This finding is in agreement with some studies(Reference Ambaw, Mayston and Hanlon32,Reference Molla, Mekuriaw and Kerebih33) , but not others(Reference Peltzer, Naidoo and Matseke13,Reference Duko, Gebeyehu and Ayano34) . Studies that reported an association between co-morbidity and mental disorders have posited HIV-associated stigma as the plausible driving force(Reference Peltzer, Naidoo and Matseke13,Reference Duko, Gebeyehu and Ayano34) . Among people with TB, factors such as malnutrition, poor physical health, socio-economic adversities and interaction between these factors may have a stronger influence on depression and anxiety than HIV-related stigma. Furthermore, following WHO’s guidelines in 2015, nearly all sub-Saharan African countries adopted universal HIV care policies irrespective of CD4 T-cell count (‘treat all’), including Botswana. The well-developed, universal access to HIV care may have also influenced mental health in our population of TB patients. Individuals who were co-infected but not on ART predominantly discovered their HIV status at enrolment or within 1 month of study enrolment, and further research is needed among this group as HIV-related stigma may be perceived differently in these individuals.
We note several limitations associated with our research. First, depression and anxiety assessment relied on self-reporting using screening questionnaires. This may lead to self-report bias, particularly under-reporting owing to stigmatisation of psychological disorders. However, we tried to minimise this by training the research staff on administering the questionnaires with consistency and without judgement, and by asking the participants to record their answer on the tablet directly. Second, the PHQ-9 and ZUNG screening tools have not been validated in Botswana, nor in TB patients in Botswana. The English version of these questionnaires was translated by a native speaker of Setswana. Some items on the questionnaires overlap with TB symptoms, including fatigue, loss of appetite and insomnia, which may lead to misclassification of depressive and anxiety cases. As a consequence, our estimate of associations between depression, anxiety and independent variables may be biased towards the null. Third, due to the cross-sectional study design, statistical associations may not be representative of casual relationships between exposure and outcome variables. Finally, confounding by unmeasured variables is a possibility in our study.
Conclusion
In conclusion, we have shown that common mental disorders are highly prevalent and food insecurity is associated with poor mental health among new TB patients. This study highlights the critical need for integration of TB, mental health and social services (i.e. food assistance) to address the TB-mental health co-morbidity in Botswana. Mental illness is increasingly being recognised as an important driver of the TB epidemic and negatively impacts effective TB control(Reference Sweetland, Kritski and Oquendo2). Individually, poor mental health is associated with elevated morbidity and mortality, and greater risk of drug resistance(Reference Pachi, Bratis and Moussas3). Despite the need, mental health counselling and psychiatric services are severely underfunded in low-resource settings where numerous public health priorities compete(Reference Rathod, Pinninti and Irfan6). Social assistance programmes that mitigate costs of TB care and improve food access may ameliorate symptoms of common mental disorders. Current programmes intended to support individuals struggling with food security may require additional measures to identify patients who experience moderate to severe food insecurity and ensure their access to and adequacy of nutritious and sufficient food for the household. Given the global goal of ending TB and rising interest in mental health, longitudinal research is needed to determine the temporal relationship between food insecurity and mental disorders and to identify potential pathways through which food insecurity may elevate the risk of common mental disorders among TB patients.
Acknowledgements
Acknowledgements: We are thankful to the study participants who made this study possible. The authors also acknowledge the following sources of support. Financial Support: This work was supported by the National Institute of Allergy and Infectious Diseases (grant number K01AI118559). The funding source had no role in the design, analysis or writing of this article. Conflicts of interest: There are no conflicts of interest. Authorship: Q.W. contributed to study design, data analysis and led the manuscript writing. M.D. contributed to data collection. A.H.F. contributed to study training, assisted in data collection and supervision. K.M. contributed to study training, assisted in data collection and supervision. C.M. contributed to study supervision. N.M.Z. assisted in the study and manuscript writing. S.S.S. contributed to study design, study supervision and assisted in study analysis and manuscript writing. Ethics of human subject participation: This study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving study participants were approved by the institutional review board (IRB) at University of California, Irvine, and the Human Research Development Committee of the Botswana Ministry of Health. Written informed consent was obtained from all subjects.







