Introduction
The Artedidraconidae includes small- to medium-sized benthic species endemic to the Southern Ocean commonly known as plunderfishes. The genus Pogonophryne is the most species-rich group within the family, consisting of more than 25 recognized species, most of which have been described in the last decade (e.g. Eakin et al. Reference Eakin, Eastman and Matallanas2008, Reference Eakin, Eastman and Near2009, Balushkin et al. Reference Balushkin, Petrov and Prutko2010, Balushkin & Korolkova Reference Balushkin and Korolkova2013, Balushkin & Spodareva Reference Balushkin and Spodareva2013, Shandikov & Eakin, Reference Shandikov and Eakin2013, Shandikov et al. Reference Shandikov, Eakin and Usachev2013, Spodareva & Balushkin Reference Spodareva and Balushkin2014). The taxonomic composition of the genus was first described on the basis of body coloration, number of vertebrae and second dorsal fin rays, distinguishing three main groups of fish: the unspotted group (Pogonophryne albipinna), the dorsally unspotted group (Pogonophryne scotti) and the dorsally spotted group (Eakin Reference Eakin1977, Reference Eakin, Gon and Heemstra1990). More recently, the last group was further subdivided in three different groups (Pogonophryne marmorata, Pogonophryne barsukovi, Pogonophryne mentella), each including one or more species (Balushkin & Eakin Reference Balushkin and Eakin1998).
Compared to other genera of plunderfishes, Pogonophryne is characterized by a wide and depressed head, poorly to well-developed post-temporal ridges, a wide interorbital space and a snout as long as the orbit diameter (Eakin Reference Eakin, Gon and Heemstra1990). Most species are only known from the holotype or type series, with occurrence limited to single spots or restricted areas around the Antarctic continent. A few other more abundant species have generally a circum-Antarctic distribution, including areas as far north as the South Orkney Islands (Eakin Reference Eakin, Gon and Heemstra1990). The species of Pogonophryne are distributed over a wide depth range, being found on the continental shelf and slope from inshore waters down to more than 2500 m depth (Eastman Reference Eastman2017).
Because of their low abundance and generally deep distribution, the biological characteristics of many species of Pogonophryne are virtually unknown (Eakin Reference Eakin, Gon and Heemstra1990). The few data available from the literature come primarily from the Weddell Sea, where a total of 12 species have been collected during several past cruises of the Research Vessel (RV) Polarstern carried out in the summer from 1983 to 2000 (Ekau Reference Ekau1988, Schwarzbach Reference Schwarzbach1988, Arntz & Gutt Reference Arntz and Gutt1999, Arntz & Brey Reference Arntz and Brey2001). Based on stomach contents analysis, Pogonophryne includes primarily benthic or suprabenthic feeders, which rely on small prey found on the sea floor or above it (Olaso et al. Reference Olaso, Rauschert and de Broyer2000). From an ecomorphological point of view, Pogonophryne species have smaller eyes, medium or larger mental barbels and a more specialized diet than other artedidraconids (Lombarte et al. Reference Lombarte, Olaso and Bozzano2003). Among the most abundant species, P. marmorata was found to feed on amphipods, isopods, mysids and occasionally on polychaetes, whereas P. barsukovi and P. permitini preferentially fed on amphipods, isopods and molluscs (Schwarzbach Reference Schwarzbach1988, Olaso et al. Reference Olaso, Rauschert and de Broyer2000, Lombarte et al. Reference Lombarte, Olaso and Bozzano2003). Pogonophryne scotti relied on larger prey, such as fishes, decapods, euphausiids and cumaceans, having the largest mouth among the four species studied (Lombarte et al. Reference Lombarte, Olaso and Bozzano2003).
Macroscopic analyses of gonads gathered some preliminary information on the reproductive biology of P. barsukovi, P. mentella and P. scotti, although this was based only on one or two specimens each (Duhamel et al. Reference Duhamel, Kock, Balguerias and Hureau1993). Absolute fecundity was positively related to fish size and ranged from ~1200 eggs/female for P. barsukovi (20–24 cm total length (TL)) to ~10 000 eggs/female for P. scotti (28 cm TL). Egg size in mature females was similar among the three species (2.2–2.6 mm). According to the stage of gonad development at sampling time, P. barsukovi and P. mentella were considered late autumn spawners, whereas P. scotti probably spawned in winter (Duhamel et al. Reference Duhamel, Kock, Balguerias and Hureau1993).
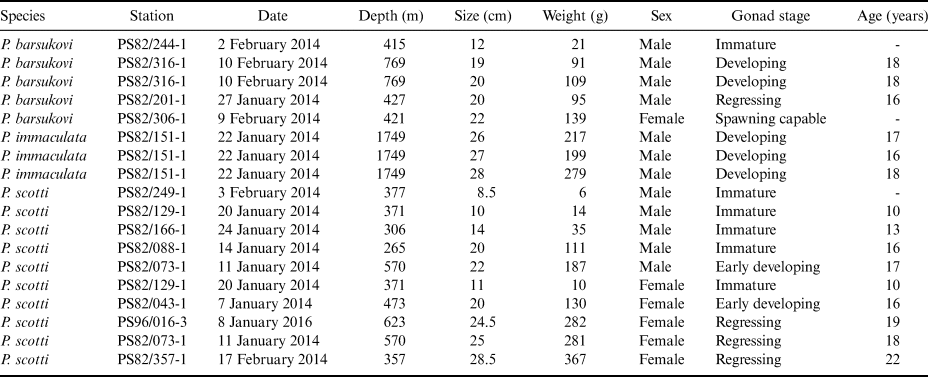
The aim of this study is to fill in the gap of knowledge on some life history traits of Pogonophryne species. Starting from an abundant sampling for the genera obtained during two recent cruises of the RV Polarstern in the southern Weddell Sea (PS82 and PS96, Table I), we analysed 18 specimens of plunderfishes belonging to three different species (Pogonophryne barsukovi Andriashev, 1967; Pogonophryne immaculata Eakin, 1981; Pogonophryne scotti Regan, 1914). Our results provide further insight into their reproductive traits based on both macroscopic and histological analyses of gonads, as well as the first data on individual ages estimated by means of otolith readings.
Table I. Sampling data of barbeled plunderfishes (genus Pogonophryne) during the Polarstern cruises (PS82 and PS96) conducted in the Weddell Sea in the 2013–14 and 2015–16 summer seasons.
Materials and methods
Fish sampling
Fish samples were collected during two bottom trawl (BT) surveys carried out aboard the RV Polarstern in the south-eastern Weddell Sea (cruise ANT-XXIX/9, PS82, 19 December 2013–5 March 2014, and cruise ANT-XXXI/2, PS96, 6 December 2015–14 February 2016) (Table I) (Knust & Schröder Reference Knust and Schröder2014, Schröder Reference Schröder2016). Sampling was conducted using two different gears: an otter BT with a mouth opening of 2.5–3.2 m × 16–18 m and a mesh size of 40 mm in the cod end towed at 3 knots for 30 min, and an Agassiz trawl (AGT) with a rigid frame of 3.5 m and a mesh size of 10 mm in the cod end towed at 1 knot for 10 min. The surveyed area encompassed the continental shelf located in the eastern and western sides of the Filchner Trough, with one sample collected at the mouth of the trough (Fig. 1).

Fig. 1. Map of the study area, showing the geographical positions of sampling stations in the south-eastern Weddell Sea for Pogonophryne barsukovi (black crosses), Pogonophryne immaculata (yellow crosses) and Pogonophryne scotti (red crosses).
At the end of each tow, fish samples were sorted to species and individually measured as total length (TL, cm) and weighed as total mass (TM, g). Sex and gonad stage of development were macroscopically assessed according to a five-point scale (Kock & Kellermann Reference Kock and Kellermann1991). The species identification within the genus Pogonophryne was carried out according to body morphology (Balushkin & Eakin Reference Balushkin and Eakin1998, Eakin et al. Reference Eakin, Eastman and Matallanas2008, Reference Eakin, Eastman and Near2009) and genetic analyses. Each fish was dissected and the gonads weighed as total mass (GM, g) and stored in Dietrich solution for further analysis. Sagittal otoliths were removed from the otic capsule, cleaned of adhering tissue and stored dry. A small portion of fin or muscle was preserved in 95% ethanol at 4°C for genetic analyses.
Laboratory activities
For histological analysis, gonad subsamples were dehydrated and embedded in paraplast. Following a standard protocol, serial transverse sections of 5–7 μm were obtained using a rotary microtome and put on slides, dried at room temperature and stained with haematoxylin and eosin (Pearse Reference Pearse1985). Gonad sections were then viewed at 10–100× magnification under a light microscope (Leica DM400B) linked to a digitized computer video system (Leica Application Suite 4.3.0) through a charge-coupled device camera (Leica DFC 420). Histological stage of gonad maturity was assigned for both sexes according to a five-phase scale (Brown-Peterson et al. Reference Brown-Peterson, Wyanski, Saborido-Rey, Macewicz and Lowerre-Barbieri2011). In spawning females, a subsample of gonads was weighed and washed out in a Petri dish to manually separate oocytes of various sizes. All oocytes were then counted and measured as maximum diameter using a stereomicroscope. In order to estimate total fecundity (Ftot, eggs/female), the number of late vitellogenic oocytes in the whole gonads was inferred from the weighed subsample applying the gravimetric method (Murua et al. Reference Murua, Kraus, Saborido-Rey, Witthames, Thorsen and Junquera2003). The relative fecundity (Frel, eggs g-1) was derived from the Ftot and TM of fish. The extent of reproductive investment or gonadal development was assessed in both sexes by calculating the gonadosomatic index (GSI), expressed as gonad mass as a percentage of total body mass.
Sagittal otoliths were measured along the two major axes (length, OL; width, OW) with an accuracy of 0.01 mm and weighed (OM, mg). For ageing purposes, one otolith for each individual was randomly selected and burned in an oven at 350°C to enhance the contrast of the inner structure. Otoliths were then embedded in epoxy resin (Crystalbond 509 Amber) to obtain transverse sections through a grinding and polishing process. Otolith sections were viewed under reflected light using a stereomicroscope at 25–40× magnifications. According to the growth pattern commonly described in notothenioids (e.g. North Reference North1988, White Reference White, Di Prisco, Maresca and Tota1991), individual age was estimated by counting the translucent/opaque zones from the primordium to the otolith margin, assuming that they were laid down annually. Two blind readings were carried out by a single reader and the mean value taken as individual age.
Results
Pogonophryne barsukovi
Four males ranging between 12 and 20 cm TL and 21 and 109 g and a single female of 22 cm TL and 139 g were collected by BT at four stations located between 416 and 769 m depth (Fig. 1). The smaller male (12 cm TL) was immature (GSI 0.01%), with thread-like testes consisting exclusively of spermatogonia (Fig. 2a). Two males (GSI 3.7–4.4%) were in the developing phase, with lobules containing all stages of spermatogenesis, such as spermatogonia, spermatocytes, spermatids and spermatozoa (Fig. 2b). The other male (GSI 3.2%) was regressing, with large and empty lumina and lobules with a few residual spermatozoa and scattered spermatogonia at the periphery (Fig. 2c). The single female (GSI 7.9%) was in early spawning condition, with large ovaries composed of primary growth (pre-vitellogenic), cortical alveoli and vitellogenic oocytes with coalescent yolk granules (Fig. 2d). Gonad maturation in this female followed a group-synchronous development, showing two well-separated groups of pre-vitellogenic and vitellogenic oocytes of 0.1–1.6 mm and 2.3–3.0 mm, respectively (Fig. 3). Ftot and Frel were estimated to be 1097 eggs/female and 7.9 eggs g-1, respectively.

Fig. 2. Micrographs of gonad histological sections of Pogonophryne spp. at various stages of development. Pogonophryne barsukovi: a. immature male, b. developing male, c. regressing male, d. spawning-capable female. Pogonophryne immaculata: e. developing male. Pogonophryne scotti: f. immature male, g. developing male, h. immature female, i. developing female, l. regressing female. A = atretic oocytes, CA = cortical alveoli, L = lumen, Oo = oogonia, PG = primary growth oocytes, POF = postovulatory follicles, Sc = spermatocytes, Sg = spermatogonia, St = spermatids, Sz = spermatozoa, Vtg = vitellogenic, Yg = yolk granules.

Fig. 3. Size frequency of pre-vitellogenic (grey bars) and vitellogenic oocytes (black bars) in the ovary of a spawning female of Pogonophryne barsukovi.
Sagittal otoliths had an ovate shape (Fig. 4a), measuring 4.4–5.4 mm (OL) and 3.2–3.5 mm (OW) and weighing 27.6–32.5 mg. Based on transverse sections obtained only from the three largest males (19–20 cm TL), age estimates ranged between 16 and 18 years (Fig. 4b).

Fig. 4. Micrographs of whole otoliths (proximal side up, on the left) and sectioned otoliths (on the right) of Pogonophryne spp. a. & b. Pogonophryne barsukovi (male, 18 years old). c. & d. Pogonophryne immaculata (male, 16 years old). e. & f. Pogonophryne scotti (female, 10 years old).
Pogonophryne immaculata
Three adult males ranging between 26 and 28 cm TL and 199 and 279 g were caught by the AGT net deployed at 1750 m on the steep slope north of Halley Bay (Fig. 1). All specimens were in the developing phase (GSI 0.5–0.8%), being characterized by large testes with lobules containing cysts of spermatogonia, spermatocytes and spermatids, as well as evident lumina free of spermatozoa (Fig. 2e).
Sagittal otoliths had an ovate shape (Fig. 4c), with a size range of 4.1–5.4 mm (OL) and 3.9–4.2 mm (OW) and a TM of 31.3–48.2 mg. As in the previous species, individual age estimates for all specimens caught ranged between 16 and 18 years (Fig. 4d).
Pogonophryne scotti
Pogonophryne scotti was the most common and abundant species within the genus in both cruises. The species was collected in three AGT and five BT stations at 473–623 m and 265–377 m depths, respectively, mainly located on the shelf east of the Filchner Trough (Fig. 1). Fish size ranged between 8.5 and 22 cm TL and 6 and 187 g for males (n = 5) and between 11.0 and 28.5 cm TL and 10 and 367 g for females (n = 5). Four males were immature (GSI 0.01–0.02%), with very small gonads characterized by lobules filled solely by spermatogonia and with no visible lumina (Fig. 2f). The largest male (22 cm TL) was in the early developing sub-phase (GSI 0.04%), with testes of increased size and spermatocysts dominated by spermatogonia and spermatocytes (Fig. 2g). The smallest female (11 cm TL) was immature (GSI 0.4%), with ovaries filled by oogonia and primary growth oocytes (Fig. 2h). A single female was in the early developing sub-phase (GSI 1.5%), with the germinal epithelium consisting of primary growth and cortical alveoli oocytes (Fig. 2i). The remaining females were all regressing (GSI 0.4–1.2%), with flaccid ovaries containing postovulatory follicles and atretic oocytes, as well as a few primary growth and cortical alveoli oocytes (Fig. 2l).
Sagittal otoliths had a triangular shape (Fig. 4e), with a size range of 3.2–6.6 mm (OL) and 2.3–4.7 mm (OW) and a TM of 9.0–63.7 mg. Based on individual otolith readings, performed on all specimens except for the smallest male, age estimates were 10–16 and 10–22 years in males and females, respectively (Fig. 4f).
Discussion
Spatial distribution
The two recent cruises in the southern Weddell Sea (PS82 and PS96) confirmed the presence of P. barsukovi and P. scotti in this area, strengthening the hypothesis they had a circum-Antarctic distribution (Eakin Reference Eakin, Gon and Heemstra1990). Their depth distributions were quite similar to each other and fell within the range reported elsewhere (Eastman Reference Eastman2017). The three specimens of P. immaculata analysed in this study represent the first occurrences of this species in the Weddell Sea. Indeed, this rare species was only known from the holotype collected at the South Orkney Islands (Eakin Reference Eakin, Gon and Heemstra1990) and seven other specimens caught on the continental slope of the Ross Sea (Eakin et al. Reference Eakin, Eastman and Near2009). With a depth range of ~1200–2500 m (Eastman Reference Eastman2017), P. immaculata is by far the deepest-living species of Pogonophryne and the second deepest-living species among notothenioids (Eakin et al. Reference Eakin, Eastman and Near2009).
Reproductive traits
Except for a juvenile, all of the other specimens of P. barsukovi were adults close to the maximum size reported so far for the species (25 cm TL) (Eastman Reference Eastman2019). The evidence of males in the developing phase of gonad maturity and a single female in early spawning condition indicates that this species probably spawns in early autumn in the Weddell Sea. The reproductive effort of females in terms of GSI, Ftot and Frel falls within the ranges previously reported (Duhamel et al. Reference Duhamel, Kock, Balguerias and Hureau1993), closely resembling another species of comparable maximum size (Pogonophryne ventrimaculata, 26 cm TL) reported in the same area (Ekau Reference Ekau1991).
Males of P. immaculata collected in the Weddell Sea were larger than reported in literature for samples collected in the Ross Sea (22–25 cm TL) (Eakin et al. Reference Eakin, Eastman and Near2009). This observation enables us to update the maximum size of the species from 25 to 28 cm TL. Based on the developmental stage of their gonads, P. immaculata should probably spawn in autumn.
Pogonophryne scotti was the most common species within the genus, consistent with the results obtained during a recent seabed imaging survey conducted in the Weddell Sea (La Mesa et al. Reference La Mesa, Piepenburg, Pineda-Metz, Riginella and Eastman2019). Notably, the lack of large adult males in the samples could be related to their spatial segregation during parental egg guarding, as described in the northern Weddell Sea in the same period (February–March) (Jones & Near Reference Jones and Near2012). The presence of regressing or post-spawning females with ovaries containing postovulatory follicles indicates a recent spawning, possibly occurring in spring or early summer. Compared to the other two species, P. scotti showed greater fecundity, consistent with their maximum size reported in the literature (32 cm TL) (Eastman Reference Eastman2019).
Interestingly, nesting males of P. scotti exhibited an evident sexual dimorphism in the size and colour of the anterior lobe of the second dorsal fin (Jones & Near Reference Jones and Near2012), a characteristic shared with P. barsukovi (Eakin Reference Eakin, Gon and Heemstra1990, present data) and P. immaculata (Eakin et al. Reference Eakin, Eastman and Near2009). As sexual dichromatism is generally correlated with male parental care and nesting behaviour (Mank et al. Reference Mank, Promislow and Avise2005) and considering their similar low fecundities, we hypothesize that P. barsukovi and P. immaculata also evolved similar reproductive strategies, including parental care and nest guarding.
Age and growth
As has been reported for many other notothenioids (reviewed in North Reference North1988, White Reference White, Di Prisco, Maresca and Tota1991, Kock & Everson Reference Kock, Everson, Di Prisco, Pisano and Clarke1998), the use of otolith sectioning appears to provide a method for estimating the age of barbeled plunderfishes. Although based on presumed annual deposition of neighbouring opaque and translucent zones, this study is the first attempt to age specimens of Pogonophryne spp. Pogonophryne barsukovi and P. immaculata attained the same maximum age. These estimates were significantly higher than those reported for other (smaller) artedidraconids, such as Artedidraco skottsbergi and Dolloidraco longedorsalis (Meneghesso et al. Reference Meneghesso, Riginella, La Mesa, Donato and Mazzoldi2017). Pogonophryne scotti was characterized by an evident sexual dimorphism in body size and longevity, with females being larger and older than males. In general, all species of Pogonophryne investigated in this study share common traits, such as small maximum size and longevity of nearly 20 years, which suggest a relatively low body growth rate, consistent with their low scope for activity and sluggish mode of life (Zimmermann & Hubold Reference Zimmermann, Hubold, Di Prisco, Pisano and Clarke1998).
Acknowledgements
We sincerely thank the captain and all the crew members and personnel working aboard the RV Polarstern during the cruises ANT-XXIX/9 (PS82) and ANT-XXXI/2 (PS96) for their support during field sampling activities. The authors are also grateful to Nils Koschnick, Magnus Lucassen, Maj Wetjen, Kai Wätjen and Rainer Knust (Alfred Wegener Institute Helmholtz Centre for Polar and Marine Research) for their support in sample collection. We wish to thank two anonymous reviewers, whose comments greatly improved the early draft of the manuscript.
Author contributions
MLM conceived and wrote the paper, FD performed the histological analysis of gonads, ER and CP conducted the field sampling and TJN and EP performed the genetic analyses. Each author contributed significantly to data analyses and manuscript editing before submission.
Financial support
CP acknowledges financial support from the University of Padua (BIRD164793/16, SEED2019) and from the European Marie Curie project ‘Polarexpress’ Grant No. 622320. This study was conducted within the project 2013/C1.07, and MLM acknowledges financial support of the Italian National Antarctic Research Program (PNRA).








