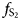Introduction
Tetrahedrite-group minerals are characterised by the general structural formula M (2)A6M (1)(B4C2)X (3)D4 S(1)Y S(2)12Z, where the capital letters indicate several chemical constituents. Among the different species, the most commons belong to the tetrahedrite and tennantite series and are characterised by A and B = Cu+, D = Sb3+ or As3+, and Y and Z = S2–. Different C constituents, usually represented by divalent transition elements, identify the species belonging to these series (Biagioni et al., Reference Biagioni, George, Cook, Makovicky, Moëlo, Pasero, Sejkora, Stanley, Welch and Bosi2020).
‘Tennantite’ was the first name of a mineral belonging to the tetrahedrite group to be introduced by the brothers Richard and William Phillips (R. Phillips, Reference Phillips1819; W. Phillips, Reference Phillips1819) and it honours the English chemist Smithson Tennant (1761–1815). The material studied, from Cornwall, England, was Fe-rich. Later, ‘zincian tennantite’ was reported from some localities (e.g. Freiberg, Saxony, Germany – Plattner, Reference Plattner1846; Lengenbach, Binn Valley, Switzerland – Des Cloizeaux, Reference Des Cloizeaux1855; Miedzianka, Świętokrzyskie Voivodeship, Poland – Morozewicz, Reference Morozewicz1926). Biagioni et al. (Reference Biagioni, George, Cook, Makovicky, Moëlo, Pasero, Sejkora, Stanley, Welch and Bosi2020) renamed these species as tennantite-(Fe) and tennantite-(Zn). Iron and Zn are the most common divalent elements occurring in tennantite-series minerals; moreover, since the publication of the nomenclature of tetrahedrite-group minerals (Biagioni et al., Reference Biagioni, George, Cook, Makovicky, Moëlo, Pasero, Sejkora, Stanley, Welch and Bosi2020), three other species belonging to the tennantite series have been approved by the International Mineralogical Association – Commission on New Minerals, Nomenclature and Classification (IMA-CNMNC), i.e. tennantite-(Hg) (Biagioni et al., Reference Biagioni, Sejkora, Raber, Roth, Moëlo, Dolníček and Pasero2021), tennantite-(Ni) (Wang et al., Reference Wang, Chen, Gu, Hou, Yang, Dong, Guo and Qu2021), and tennantite-(Cd) (Biagioni et al., Reference Biagioni, Kasatkin, Sejkora, Nestola and Škoda2022). Other potential end-member compositions are known in the literature. Among them, compositions corresponding to ideal Cu12As4S13 have been reported, for instance, from Canada, France and Peru (Johan and Le Bel, Reference Johan and Le Bel1980; Thouvenin, Reference Thouvenin1983; Cesbron et al., Reference Cesbron, Giraud, Picot and Pillard1985; Marcoux et al., Reference Marcoux, Moëlo and Milési1994).
The re-examination of a specimen from the Peruvian epithermal deposit of Layo (Marcoux et al., Reference Marcoux, Moëlo and Milési1994) allowed the description of the new mineral species tennantite-(Cu). The new mineral and its name (symbol Tnt-Cu) have been approved by the Commission on New Minerals, Nomenclature and Classification of the International Mineralogical Association (IMA-CNMNC), under the voting number 2020-096. Part of holotype material of tennantite-(Cu) is deposited in the collections of the Department of Mineralogy and Petrology, National Museum in Prague, Cirkusová 1740, 193 00 Praha 9, Czech Republic under the catalogue number P1P 74/2020, in the collections of the Museo di Storia Naturale of the Università di Pisa, Via Roma 79, Calci (PI), under catalogue number 19925, and in the collections of the Mineralogical Museum of Ecole des Mines de Paris (MINES ParisTech) under catalogue number ENSMP 83990.
In this paper the description of this new species belonging to the tetrahedrite group is reported and some crystal-chemical and nomenclature issues are discussed.
Occurrence and physical properties
Tennantite-(Cu), described initially as ‘Cu-excess tennantite’ by Marcoux et al. (Reference Marcoux, Moëlo and Milési1994), was found in the Layo epithermal deposit (15°11’16”S, 72°14’30”W), Castilla Province, Arequipa Department, Peru (Fig. 1). The Layo vein system is formed by anastomosing veins hosted in NNE–SSW-striking fractures in Miocene–Pliocene volcanic rocks belonging to the Tazaca Group. This rock sequence is formed by two distinct successions: the first one is a 300 m thick sequence of ignimbritic lava flows and pyroclastic tuffs, dacitic to rhyolitic in compositions (Pisaca Formation), whereas the second one, known as the Jullujia Formation, is composed by discordant andesitic lava flows and domes, as well as by pyroclastic rocks. In the Layo area, the volcanic rocks are affected by a pervasive propylitic alteration. Around ore vein systems, propylitised rocks occur as relicts within hydrothermally altered silicic-argillic zones. Marcoux et al. (Reference Marcoux, Moëlo and Milési1994) gave further details about the geological background of the Layo epithermal deposit.

Fig. 1. The Layo ore district: location and geology (simplified after Marcoux et al., Reference Marcoux, Moëlo and Milési1994).
Tennantite-(Cu) was collected in the eastern zone of the ore field, in the so-called Vetas 7 and 8. In these veins, a breccia, formed by silicified and alunitised angular rock clasts, is cemented by an ore assemblage composed of complex Cu–As–Fe–Sn sulfides, showing banded and cockade textures related to the rhythmic alternation of pyrite and Cu–As–Sn sulfides, i.e. enargite, tennantite-(Cu), chalcopyrite and vinciennite (Marcoux et al., Reference Marcoux, Moëlo and Milési1994). The mineralogy is dominated by early pyrite succeeded by a late complex Cu–As–Sn association where tennantite-(Cu) is the prominent sulfide (Fig. 2). Tennantite-(Cu) is associated with enargite, chalcopyrite and vinciennite, that often form ameboid oriented patches up to 80 μm in size, sometimes developed from relicts of enargite. Secondary minerals also formed, such as bornite, covellite and digenite, at the expense of chalcopyrite, and scarce luzonite at the expense of enargite.

Fig. 2. (a) Association of tennantite-(Cu) (Tnt, bluish grey), with pyrite (Py, yellowish white), chalcopyrite (Ccp, yellow), enargite (Eng, purplish pale grey), vinciennite (Vcn, orange-brown) and secondary Cu sulfides: bornite (Bn, brown) and covellite (Cv, blue). Veta 8, Layo epithermal deposit. Reflected light microscope (plane polarised light). Mineral symbols after Warr (Reference Warr2021). (b) Holotype material of tennantite-(Cu), associated with the same phases shown in (a), as seen in reflected light microscopy (partly crossed polars). Catalogue number P1P 74/2020.
Tennantite-(Cu) is black in colour, with a black streak and metallic lustre. Mohs hardness was not measured, owing to the small size of the grain studied and the intimate association of other sulfides, but it should be close to 3½–4, in agreement with other members of the tetrahedrite group. Tennantite-(Cu) is brittle, with a conchoidal fracture and an indistinct cleavage. Due to the small size of the grains studied and their admixture with other sulfides, density was not measured; on the basis of the empirical formula and the single-crystal X-ray diffraction data, the calculated density is 4.656 g⋅cm–3.
In reflected light, tennantite-(Cu) is isotropic. It is grey, with a bluish shade (Fig. 2). Internal reflections were not observed. Reflectance values measured in air on the holotype sample using a spectrophotometer MSP400 Tidas at Leica microscope, with a 50× objective, are given in Table 1 and shown in Fig. 3, where the reflectance curve for tennantite-(Cu) is compared with published data for other tetrahedrite-group minerals.

Fig. 3. Reflectance curves for tennantite-(Cu) from the Layo deposit, compared with published data for other tennantite-series minerals. (1) Layo, Arequipa Department, Peru; (2) tennantite-(Fe) from Wheal Jewel, Cornwall, England (Criddle and Stanley, Reference Criddle and Stanley1993; p. 557); (3) tennantite-(Zn) from Tsumeb, Otavi, Namibia (Criddle and Stanley, Reference Criddle and Stanley1993; p. 561); (4) Bi-bearing tennantite-(Zn) from the Bicknoller Quarry, Cornwall, England (Criddle and Stanley, Reference Criddle and Stanley1993; p. 558); (5) Hg-bearing tennantite-(Fe) from the Gortdrum mine, Ireland (Criddle and Stanley, Reference Criddle and Stanley1993; p. 559); (6) Pb-bearing tennantite-(Zn) from the Sark's Hope mine, Sark, Channel Islands (Criddle and Stanley, Reference Criddle and Stanley1993; p. 560); and (7) tennantite-(Hg) from Lengenbach, Switzerland (Biagioni et al., Reference Biagioni, Sejkora, Raber, Roth, Moëlo, Dolníček and Pasero2021).
Table 1. Reflectance data for tennantite-(Cu).*

*The reference wavelengths required by the Commission on Ore Mineralogy (COM) are given in bold.
Chemical data
Quantitative chemical analyses were carried out using a Cameca SX 100 electron microprobe (National Museum of Prague, Czech Republic) and the following experimental conditions: wavelength dispersive spectroscopy mode, accelerating voltage = 25 kV and beam current = 20 nA, beam diameter = 1 μm. Standards (element, emission line) were: chalcopyrite (CuKα and SKα), pyrite (FeKα), ZnS (ZnKα), NiAs (AsLβ), Sn (SnLα), Sb2S3 (SbLα) and PbTe (TeMα). The contents of other sought elements with Z > 8 (Ag, Au, Bi, Cd, Co, Ga, Ge, Hg, In, Mn, Cl, Ni, Pb, Se and Tl) were below detection limits. Matrix correction by the PAP procedure (Pouchou and Pichoir, Reference Pouchou, Pichoir and Armstrong1985) was applied to the data. Electron back-scattered images showed that tennantite-(Cu) is slightly zoned, with a domain richer in Sb, 30–40 μm in size, located on the margin of the grain. Results are given in Table 2.
Table 2. Chemical data for tennantite-(Cu) and Sb-rich tennantite-(Cu).

n = number of spot analyses.
X-ray crystallography
Single-crystal X-ray diffraction intensity data were collected on an anhedral grain of tennantite-(Cu), 60 μm × 40 μm × 30 μm in size, using a Bruker Apex II diffractometer (50 kV and 30 mA) equipped with a Photon II CCD detector and graphite-monochromatised MoKα radiation (Dipartimento di Scienze della Terra, Università di Pisa, Italy). The detector-to-crystal distance was set at 50 mm. Data were collected using φ scan mode in 0.5° slices, with an exposure time of 30 s per frame, and they were corrected for Lorentz, polarisation, absorption and background effects using the software package Apex3 (Bruker AXS Inc., Reference Bruker AXS Inc.2016). The refined unit-cell parameters are a = 10.1710(10) Å, V = 1052.2(2) Å3; and space group I $\overline 4$![]() 3m. The crystal structure of tennantite-(Cu) was refined using Shelxl-2018 (Sheldrick, Reference Sheldrick2015) starting from the structural model of Johnson and Burnham (Reference Johnson and Burnham1985). The following neutral scattering curves, taken from the International Tables for Crystallography (Wilson, Reference Wilson1992) were used initially: Cu vs □ at M(2), Cu vs Fe at M(1), As vs Sb at X(3), S at S(1) and S(2) sites.
3m. The crystal structure of tennantite-(Cu) was refined using Shelxl-2018 (Sheldrick, Reference Sheldrick2015) starting from the structural model of Johnson and Burnham (Reference Johnson and Burnham1985). The following neutral scattering curves, taken from the International Tables for Crystallography (Wilson, Reference Wilson1992) were used initially: Cu vs □ at M(2), Cu vs Fe at M(1), As vs Sb at X(3), S at S(1) and S(2) sites.
Several cycles of isotropic refinement converged to R 1 = 0.115, confirming the correctness of the structural model. The modelling of the racemic twin suggested that the structure had to be inverted. An anisotropic refinement for cations only converged to R 1 = 0.0280. The relatively large U eq value of the M(2) site suggested its split nature, in agreement with previous authors (Andreasen et al., Reference Andreasen, Makovicky, Lebech and Karup-Møller2008; Welch et al., Reference Welch, Stanley, Spratt and Mills2018). After the addition of the split position, found in the difference-Fourier map, the R 1 value was lowered to 0.0210. The site occupancy factors (s.o.f.) at the two split positions M(2a) and M(2b) were constrained to be 1, as no Cu excess at the M(2) site was detected. After several cycles of anisotropic refinement for all the atoms, the R 1 converged to 0.0178 for 263 unique reflections with F o > 4σ(F o) and 24 refined parameters. Details of the data collection and crystal structure refinement are reported in Table 3. Fractional atomic coordinates and equivalent isotropic displacement parameters are reported in Table 4, whereas Table 5 reports selected bond distances and Table 6 the weighted bond-valence sums (BVS) calculated according to the bond parameters of Brese and O'Keeffe (Reference Brese and O'Keeffe1991). The crystallographic information file has been deposited with the Principal Editor of Mineralogical Magazine and is available as Supplementary material (see below).
Table 3. Summary of crystal data and parameters describing data collection and refinement for tennantite-(Cu).

1 w = 1/[σ2(F o2)+1.3875P].
2 Flack (Reference Flack1983)
Table 4. Sites, Wyckoff positions, site occupancy factors (s.o.f.), fractional atom coordinates and equivalent isotropic displacement parameters (Å2) for tennantite-(Cu).

Table 5. Selected bond distances (Å) for tennantite-(Cu).

Table 6. Weighted bond-valence sums (in valence unit) for tennantite-(Cu).*

*Note: left and right superscripts indicate the number of equivalent bonds involving cations and anions, respectively. The following site populations were used: M(2a) = Cu0.669; M(2b) = Cu0.166; M(1) = Cu0.900Fe0.097Zn0.003; X(3) = As0.95Sb0.05.
Powder X-ray diffraction data were not collected, owing to the small size of the available grains and admixture with other phases (in particular, vinciennite). Table 7 reports the calculated X-ray powder diffraction pattern.
Table 7. X-ray powder diffraction data for tennantite-(Cu).*

*Intensity and d hkl were calculated using the software PowderCell2.4 (Kraus and Nolze, Reference Kraus and Nolze1996) on the basis of the structural model given in Table 4. Only reflections with I calc > 1 are listed. The five strongest reflections are given in bold.
Results and discussions
Chemical formula
As discussed in previous papers (e.g. Sejkora et al., Reference Sejkora, Biagioni, Vrtiška and Moëlo2021), there are different approaches to recalculate the chemical formulae of tetrahedrite-group minerals. The two better ones normalise the number of atoms on the basis of ΣMe = 16 atoms per formula unit (apfu) or on the basis of (As + Sb + Te + Bi) = 4 apfu. The former approach assumes that no vacancies occur at the M(2), M(1), and X(3) sites, whereas the latter is based mainly on the results discussed by Johnson et al. (Reference Johnson, Craig and Rimstidt1986) who revealed that negligible variations in the ideal number of X(3) atoms usually occur.
The first approach gives the chemical formula Cu11.36(10)Fe0.58(3)Zn0.02(1)(As3.80(7)Sb0.23(1))Σ4.03S12.67(24), whereas the other normalisation strategy corresponds to the formula Cu11.27(31)Fe0.57(3)Zn0.02(1)(As3.77(1)Sb0.23(1))Σ4.00S12.57(47). These formulae are in agreement with previous results of Marcoux et al. (Reference Marcoux, Moëlo and Milési1994). Their average formula, recalculated on the basis of ΣMe = 16 apfu, taking into account the average formula given in their Table 3, is Cu11.37Fe0.66Zn0.02(As3.74Sb0.21)Σ3.95S13.51. Copper, Fe and Zn contents agree with those found in this work, as well as the As/(As+Sb) atomic ratios.
The simplified formula of tennantite-(Cu) is Cu6Cu4(Cu,Fe,Zn)2(As,Sb)4S13, corresponding to the end-member formula Cu+6(Cu+4Cu2+2)As4S13. It corresponds to (in wt.%) Cu 51.56, As 20.26, S 28.18, total 100.00.
The Sb-enriched domain corresponds to the chemical formula (based on ΣMe = 16 apfu) Cu11.35(2)Fe0.65(1)Zn0.03(1)(As2.78(4)Sb1.10(2)Te0.09(1))Σ3.97S12.85(8). This domain is characterised by a distinctly higher Sb content [Sb/(As+Sb+Te) atomic ratio passing from 0.06 to 0.28] and detectable Te content.
Crystal structure description
Tennantite-(Cu) is isotypic with the other members of the tetrahedrite group. Its crystal structure (Fig. 4) can be described as a framework of corner-sharing M(1)S(1)4 tetrahedra with cages hosting X(3)S(1)3 trigonal pyramids around S(2)M(2)6 octahedra (e.g. Johnson et al., Reference Johnson, Craig and Rimstidt1988).

Fig. 4. Crystal structure of tennantite-(Cu). Several atoms in front of the unit cell have been suppressed, to enhance atom coordination. S(1): yellow; S(2): orange; X(3): green; M(1): blue (Cu) and dark green (Fe), with representation of some tetrahedra (blue); M(2a) and M(2b): blue (Cu) and white (vacancy).
The tetrahedrally coordinated M(1) site is a mixed (Cu,Fe) site, with only minor amounts of Zn. The refined site scattering is 171.48 electrons per formula unit (epfu), to be compared with the value calculated from the site population proposed on the basis of electron microprobe data, M (1)(Cu5.40Fe0.58Zn0.02), i.e. 172.26 epfu. Average bond distance is 2.3075 Å. The BVS at the M(1) site, 1.36 valence units (vu), agrees with a site with Cu+ mixed with higher valency cations.
The M(2) site is split into two sub-positions, M(2a) and M(2b), separated by 0.60 Å, whereas the distance between two neighbouring M(2b) positions is 1.20 Å. These distances are shorter than those observed in Cu-rich unsubstituted tennantite described by Makovicky et al. (Reference Makovicky, Karanović, Poleti, Balić-Žunić and Paar2005), having M(2a)–M(2b) and M(2b)–M(2b) distances of 1.08 and 2.00 Å, respectively. The M(2a) position has a triangular planar coordination, whereas M(2b) has a flat trigonal pyramidal one. This feature agrees with previous studies (e.g. Andreasen et al., Reference Andreasen, Makovicky, Lebech and Karup-Møller2008; Welch et al., Reference Welch, Stanley, Spratt and Mills2018). Average bond distances are 2.230 and 2.307 Å for M(2a) and M(2b) positions, respectively. Such values can be compared with those reported in Cu-pure and unsplit M(2) sites occurring in tetrahedrite and tennantite studied by Wuensch (Reference Wuensch1964) and Wuensch et al. (Reference Wuensch, Takéuchi and Nowacki1966), respectively, i.e. 2.259 and 2.240 Å, respectively, as well as with those reported by Makovicky et al. (Reference Makovicky, Karanović, Poleti, Balić-Žunić and Paar2005), i.e. 2.217 Å for Cu2A and 2.467 Å for Cu2B. The Cu2B of Makovicky et al. (Reference Makovicky, Karanović, Poleti, Balić-Žunić and Paar2005) is also close to the As site, 2.41 Å, whereas the M(2b)–X(3) distance is 2.835 Å in the sample from Layo. In this latter sample, copper was the only cation at the M(2) site, in agreement with electron microprobe data. The larger size of the M(2b) position is probably due to its average nature. During the refinement of tennantite-(Cu) from Layo, an unconstrained refinement of the s.o.f. at the M(2a) and M(2b) sites was performed, resulting in M(2a) = Cu0.650(19) and M(2b) = Cu0.162(9), with a site population at M(2) corresponding to M (2)Cu5.84, not suggesting any Cu excess. For this reason, as described above, the sum of the s.o.f. at M(2a) and M(2b) was constrained to be 1. On the contrary, Makovicky et al. (Reference Makovicky, Karanović, Poleti, Balić-Žunić and Paar2005) found a slight Cu excess, with a surplus of ca. 10%. The BVS at the M(2) site [i.e. M(2a) + 2×M(2b)] (Table 6) is 1.04 valence units (vu), in agreement with the presence of monovalent cations.
The splitting of the M(2) position is variable in the tetrahedrite–tennantite sub-group. In Fe-bearing compounds synthesised at 450°C (Fe between ~ 0.3 and 2 apfu), Andreasen et al. (Reference Andreasen, Makovicky, Lebech and Karup-Møller2008) only found a split (24g) site. A single-crystal synchrotron X-ray diffraction study of synthetic Cu12Sb4S13 and Cu12As4S13 was performed recently by Hathwar et al. (Reference Hathwar, Nakamura, Kasai, Suekuni, Tanaka, Takabatake, Iversen and Nishibori2019) from room temperature down to 70 K. Whereas in synthetic Cu12Sb4S13 there is only a single (12e) site (but with a high atomic displacement perpendicular to the triangle) the use of high resolution data allowed the resolution of the M(2) site of synthetic Cu12As4S13 into six (24g) sub-positions.
The X(3) site shows an average bond distance of 2.266 Å. Taking into account the electron microprobe data, the site occupancy (As0.95Sb0.05) can be proposed. It corresponds to a mean atomic number of 33.90 electrons, to be compared with the refined mean atomic number of 34.03 electrons. Assuming idealised X–S distances of 2.26 and 2.45 Å for As3+ and Sb3+, respectively (calculated according to the bond parameters of Brese and O'Keeffe, Reference Brese and O'Keeffe1991), an average X(3)–S(1) distance of 2.270 Å can be expected. The BVS is 3.06 vu.
The S(1) site is four-fold coordinated and is bonded to two M(1), one M(2) [i.e. M(2a) or one of the two mutually-exclusive M(2b)] and one X(3). Its BVS is 2.04 vu. S(2) is octahedrally coordinated by atoms hosted at M(2) sites, with a BVS of 2.16 vu. Both S sites were found fully occupied.
Coupling the results of the crystal structure refinement and the electron microprobe analysis, the structural formula of holotype tennantite-(Cu) can be written as M (2)Cu6.00M (1)(Cu5.40Fe0.58Zn0.02)X (3)(As0.95Sb0.05)4S13.
Relation between unit-cell parameter and chemical composition
Unit-cell parameter of tennantite-(Cu) from Layo (i.e. a = 10.171 Å) agrees with that of synthetic sample No. 2052 of Makovicky et al. (Reference Makovicky, Tippelt, Forcher, Lottermoser, Karup-Møller and Amthauer2003), having composition Cu11.24Fe0.57As3.93S13.00 and unit-cell parameter a = 10.174(8) Å. The ‘Cu-rich unsubstituted tennantite’ of Makovicky et al. (Reference Makovicky, Karanović, Poleti, Balić-Žunić and Paar2005) has a = 10.1756(9) Å. Applying the relationships between chemistry and unit-cell parameter proposed by Johnson et al. (Reference Johnson, Craig and Rimstidt1987), to the sample from Layo one obtains a value of 10.22 Å; a better fit is obtained using the relations proposed by Charlat and Lévy (Reference Charlat and Lévy1975), i.e. 10.19 Å.
Several recent studies on synthetic Fe-free Cu12As4S13 gave the following unit-cell parameter a = 10.163(7) Å (Levinsky et al., Reference Levinsky, Candolfi, Dauscher, Tobola, Hejtmánek and Lenoir2019), with composition actually Cu11.81As4.19S12.67, based on ΣMe = 16 apfu; a = 10.1572(2) Å at 293 K (Yaroslavzev et al., Reference Yaroslavzev, Kuznetsov, Dudka, Mironov, Buga and Denisov2021); and a = 10.1554(6) Å at 300 K (Hathwar et al., Reference Hathwar, Nakamura, Kasai, Suekuni, Tanaka, Takabatake, Iversen and Nishibori2019).
Comparison between tennantite-(Cu) and previous findings of Cu-rich tennantite
Natural members of the tennantite series are usually characterised by the formula Cu6(Cu4Me 2)As4S13, where Me is commonly Fe and Zn. However, synthetic Cu12As4S13 has been synthesised, in some cases showing a Cu excess with respect to the ideal 12 apfu. For instance, Maske and Skinner (Reference Maske and Skinner1971) studied the system Cu–As–S and found a compositional field Cu12+xAs4+yS13, with 0 < x < 1.72 and 0 < y < 0.08. Unit-cell variation from 10.168 to 10.222 Å was reported for compositions ~Cu12As4S13 and ~Cu14As4S13, respectively. Lind and Makovicky (Reference Lind and Makovicky1982) highlighted an analytical problem during electron microprobe analysis of synthetic tetrahedrite-group phases; indeed, those compositions having Cu > 12 apfu gave the same analytical results as those having 12 Cu apfu. This effect was noted for both Sb- and As-members of this sulfosalt group.
In literature, the term ‘Cu-excess’ was first used by Marcoux et al. (Reference Marcoux, Moëlo and Milési1994) to indicate a Cu content higher than 10 apfu. According to the work of Lind and Makovicky (Reference Lind and Makovicky1982) and Makovicky et al. (Reference Makovicky, Karanović, Poleti, Balić-Žunić and Paar2005), this term ought to be reserved to compounds with Cu over 12 apfu. Some of these Cu-rich tennantite-series minerals (Cu > 10 apfu) correspond to what is now classified as tennantite-(Cu), whereas others are simply Cu-rich varieties of tennantite-(Fe). For instance, sample M14 of Charlat and Lévy (Reference Charlat and Lévy1974), from Trevisane, Cornwall, England, having a chemical formula Cu10.70Fe1.27Zn0.03As3.97S12.90, may be labelled as tennantite-(Fe), as its formula can be recast as Cu6[Cu4(Fe1.27Cu0.70Zn0.03)]As3.97S12.90. On the contrary, samples from Huaron, Peru (Thouvenin, Reference Thouvenin1983), Chizeuil, France (Cesbron et al., Reference Cesbron, Giraud, Picot and Pillard1985) and Lornex, Canada (Johan and Le Bel, Reference Johan and Le Bel1980) correspond to tennantite-(Cu); at the French locality, a potential tetrahedrite-(Cu) was also reported.
Other findings of tennantite-(Cu) were reported by Kouzmanov et al. (Reference Kouzmanov, Ramboz, Bailly and Bogdanov2004) from the Radka deposit, Bulgaria. The samples studied by these authors show Cu contents ranging from 10.88 to 11.26 apfu, Fe between 0.79 and 1.14 apfu, and Zn below the detection limit. The observed As/(As+Sb+Bi) atomic ratio is in the range 0.90–0.98. Ideally, samples from this locality correspond to the formula Cu11Fe(As3.8Sb0.2)S13.
Catchpole et al. (Reference Catchpole, Kouzmanov and Fontboté2012) reported chemical data of tetrahedrite-group minerals from the Morococha base metal district, Peru. Chemical compositions vary between tetrahedrite-(Zn) in the Ag–Pb zone to tennantite-(Zn) in the Zn–Pb–Ag and Zn–Cu zones, and to tennantite-(Cu) in the Cu zone. Actually, in this last zone Cu varies between 11.10 and 11.60 apfu, and Fe is the second most abundant C constituent (ranging from 0.44 to 0.70 apfu), with low contents of Zn (from below the detection limit to 0.23 apfu). The As/(As+Sb+Te) atomic ratio ranges between 0.59 and 0.88.
Repstock et al. (Reference Repstock, Voudouris and Kolitsch2015) described Cu-rich tennantite from the Pefka mine, Greece; at this locality, tennantite-(Cu) shows As/(As+Sb+Te) atomic ratios ranging between 0.73 and 0.94 (also samples with Sb > As were observed) and Fe/(Fe+Zn+Hg) varying between 0.01 and 0.75, i.e. from nearly Fe-free samples, with Zn as the second most abundant C constituent, to Fe-rich phases.
Velebil et al. (Reference Velebil, Hyršl, Sejkora and Dolníček2021) described Zn-free tennantite samples from Julcani ore district, Peru, with 0.61–0.94 Fe apfu and Sb only up to 0.09 apfu which also correspond to tennantite-(Cu).
Finally, Voudouris et al. (Reference Voudouris, Repstock, Spry, Frenzel, Mavrogonatos, Keith, Tarantola, Melfos, Tombros, Zhai, Cook, Ciobanu, Schaarschmidt, Rieck, Kolitsch and Falkenberg2022) reported an interesting In- and Cu-rich tennantite from the Pefka mine, Greece. This occurrence deserves further discussion below.
Notwithstanding these previous occurrences of tennantite-(Cu), the first structural characterisation of a pure Cu-tennantite-series mineral was reported by Makovicky et al. (Reference Makovicky, Karanović, Poleti, Balić-Žunić and Paar2005) using a sample from Cerro Atajo Cu–Au deposit, in the Province of Catamarca, Argentina. Its chemical formula, based on (As + Sb) = 4 apfu, is Cu12.5(As3.92Sb0.08)S12.4. Makovicky et al. (Reference Makovicky, Karanović, Poleti, Balić-Žunić and Paar2005) found ~10% excess of Cu, with s.o.f. at the split M(2a) and M(2b) sites of Cu0.75(2) and Cu0.17(1), resulting in a site population at M(2) corresponding to M (2)Cu6.54. As discussed above, such a Cu excess was not found in the sample from Layo.
Nomenclature issues in Cu-rich tennantite
Type material of tennantite-(Cu) has a chemical composition close to Cu11.4Fe0.6(As3.75Sb0.25)S13 = ACu6[BCu4.0C(Cu1.4Fe0.6)]D(As3.75Sb0.25)S13. Following Biagioni et al. (Reference Biagioni, George, Cook, Makovicky, Moëlo, Pasero, Sejkora, Stanley, Welch and Bosi2020), this chemistry can be idealised to the end-member formula Cu+10Cu2+2As4S13, assuming that formally divalent Cu2+ is the most abundant C constituent. However, in agreement with the results of Mössbauer studies performed by Makovicky et al. (Reference Makovicky, Tippelt, Forcher, Lottermoser, Karup-Møller and Amthauer2003) on synthetic Fe-bearing tennantite, the most probable composition of the sample studied from Layo could be ACu6[BCu4.0C(Cu2+0.8Cu+0.6Fe3+0.6)]D(As3.75Sb0.25)S13. Indeed, sample 2052 of Makovicky et al. (Reference Makovicky, Tippelt, Forcher, Lottermoser, Karup-Møller and Amthauer2003), with chemical formula Cu11.24Fe0.57As3.93S13 (similar to that of the natural Peruvian specimen), showed Fe3+ as the dominant iron species. If so, applying the site-total-charge approach (Bosi et al., Reference Bosi, Hatert, Hålenius, Pasero, Miyawaki and Mills2019), the end-member formula Cu6(Cu+5Fe3+)As4S13 = Cu11Fe3+As4S13 is achieved. This result is in agreement with the hypothesis of Marcoux et al. (Reference Marcoux, Moëlo and Milési1994) about the presence of Fe3+ in the specimen from Layo, due to the high $f_{{\rm S}_ 2}$![]() .
.
It is worth noting that a virtually Fe-free or Fe-poor tennantite-(Cu) is reported by Repstock et al. (Reference Repstock, Voudouris and Kolitsch2015). Analyses 4 and 10 of these authors (see their table 3) correspond (on the basis of ΣMe = 16 apfu) to Cu10.99Ag0.01(Zn0.89Fe0.01)(As3.00Sb1.03Te0.07)(S12.97Se0.01) and Cu11.14(Zn0.79Fe0.09)(As2.33Sb1.66)S13.54, whereas analyses 7 and 8 have compositions Cu11.68Ag0.01(Fe0.18Zn0.08)(As3.80Sb0.25)S13.22 and Cu11.62(Fe0.29Zn0.09)(As3.76Sb0.24)S13.42, respectively. The two former analyses have a possible site population at M(1) corresponding to M (1)[Cu4.00(Cu1.10Zn0.89Fe0.01)] and M (1)[Cu4.00(Cu1.12Zn0.79Fe0.09)], with the C constituent represented by formally divalent Cu (Zn is the second most abundant C constituent). The remaining two analyses show Cu2+ as the dominant C constituent, even considering Fe as Fe3+: M (1)[Cu4.00(Cu2+1.56Cu+0.18Fe3+0.18Zn0.08)] and M (1)[Cu4.00(Cu2+1.33Cu+0.29Fe3+0.29Zn0.09)]. The end-member formula Cu6(Cu+4Cu2+2)As4S13 can be applied to all these samples.
Thus, the solid solution from the Fe pole to the Cu pole would correspond ideally to the following sequence (‘ionic’ model): (1) Cu+10Fe2+2 → (2) Cu+10.5Fe2+Fe3+0.5 → (3) Cu+11Fe3+ → (4) Cu+10.5Cu2+Fe3+0.5 → (5) Cu+10Cu2+2. Compositions (1) to (3) correspond to the substitution rule 2Fe2+ → Cu+ + Fe3+, and compositions (3) to (5) to Cu+ + Fe3+ → 2Cu2+. This sequence, controlled by an increase of $f_{{\rm S}_ 2}$![]() , indicates that iron oxidation precludes the appearance of formally divalent copper. According to nomenclature rules, one ought to distinguish three species: (i) ‘tennantite-(Fe2+)’, from formula (1) up to formula (2), (ii) ‘tennantite-(Fe3+)’, from formula (2) up to formula (4), and (iii) ‘tennantite-(Cu2+)’, from formula (4) up to formula (5).
, indicates that iron oxidation precludes the appearance of formally divalent copper. According to nomenclature rules, one ought to distinguish three species: (i) ‘tennantite-(Fe2+)’, from formula (1) up to formula (2), (ii) ‘tennantite-(Fe3+)’, from formula (2) up to formula (4), and (iii) ‘tennantite-(Cu2+)’, from formula (4) up to formula (5).
On this basis, composition of tennantite-(Cu) from the Layo epithermal deposit falls in the field of ‘tennantite-(Fe3+)’. Nevertheless, studies of natural and synthetic samples of tetrahedrite-(Cu) and tennantite-(Cu) using various physical methods revealed a very complex crystal chemistry, not completely understood up to now. After initial examinations in the 1970s, the first detailed 57Fe-Mössbauer studies were performed on Fe-bearing tetrahedrite in the 1990s (Charnock et al., Reference Charnock, Garner, Pattrick and Vaughan1989; Makovicky et al., Reference Makovicky, Forcher, Lottermoser and Amthauer1990 and references herein) and completed by Nasonova et al. (Reference Nasonova, Presniakov, Sobolev, Verchenko, Tsirlin, Wei, Dikarev and Shevelkov2016) and Sobolev et al. (Reference Sobolev, Presniakov, Nasonova, Verchenko and Shevelkov2017). Iron-bearing synthetic tennantite was studied by Makovicky et al. (Reference Makovicky, Tippelt, Forcher, Lottermoser, Karup-Møller and Amthauer2003). Though the first studies confirm major Fe2+ towards the Fe pole, and major Fe3+ towards the Cu pole, examination of tennantite indicates the presence of Fe2+ down to 0.5 Fe apfu, as well as mixed valence Fe. Mixed valence iron seems to represent a substantial fraction of total iron at room T, owing to charge-transfer phenomena between Cu and Fe. For instance, at a content of 0.5 Fe apfu, Makovicky et al. (Reference Makovicky, Tippelt, Forcher, Lottermoser, Karup-Møller and Amthauer2003) estimated a formal valence ranging between +2.68 and +2.69 (+2.68 for sample 2052). The oxidation state of Cu was determined by Pattrick et al. (Reference Pattrick, van der Laan, Vaughan and Henderson1993) and Gainov et al. (Reference Gainov, Dooglav, Pn'kov, Mukamedshin, Savinkov and Mozgova2008) on natural tetrahedrite and tennantite, and by Di Benedetto et al. (Reference Di Benedetto, Bernardini, Cipriani, Emiliani, Gatteschi and Romanelli2005) on synthetic Cu12Sb4S13. These three studies revealed the presence of divalent Cu in all Cu-rich samples. Nevertheless, though Di Benedetto et al. (Reference Di Benedetto, Bernardini, Cipriani, Emiliani, Gatteschi and Romanelli2005) proposed two Cu2+ apfu, located on the Cu1 site, Pattrick et al. (Reference Pattrick, van der Laan, Vaughan and Henderson1993), confirmed by Gainov et al. (Reference Gainov, Dooglav, Pn'kov, Mukamedshin, Savinkov and Mozgova2008), indicates that Cu2+ located on the Cu2 triangular site, is sometimes present for compositions excluding it according to the ionic model. Moreover, in normal conditions, pure Cu12Sb4S13 and Cu12Sb4S13 are metallic (Lu and Morelli, Reference Lu and Morelli2013), which would correspond to partial replacement of Cu2+ by Cu+ and one ligand hole (i.e. mobile S electron).
It thus appears that in Cu-rich tetrahedrite/tennantite one may have coexistence of Fe3+, Fe2+, Cu2+ and Cu+ (with ligand hole). The distinction between three species envisaged above on the basis of a simple ionic model is not pertinent, and it is more convenient, for nomenclature purposes, to consider only two species, tennantite-(Fe) and tennantite-(Cu). The sample from Layo can thus be classified as tennantite-(Cu), as Cu is more abundant than Fe as the C constituent.
A special case is represented by In-bearing tennantite-series minerals reported by Voudouris et al. (Reference Voudouris, Repstock, Spry, Frenzel, Mavrogonatos, Keith, Tarantola, Melfos, Tombros, Zhai, Cook, Ciobanu, Schaarschmidt, Rieck, Kolitsch and Falkenberg2022) from Pefka, Greece. These samples show up to 0.893 In apfu, with 11.049 Cu apfu, thus corresponding to the end-member Cu6(Cu5In3+)As4S13. As no ambiguity in the oxidation state of In can be assumed, this phase should be regarded as different from tennantite-(Cu), and it may represent the new species ‘tennantite-(In)’.
Genesis of tennantite-(Cu)
The ore assemblages where tennantite-(Cu) occurs are usually composed of enargite, Cu3As5+S4, vinciennite, Cu+7Cu2+3Fe2+2Fe3+2SnAs5+S16, mawsonite, Cu+6Fe3+2SnS8, and chalcopyrite, Cu+Fe3+S2 (e.g. Chizeuil, France – Cesbron et al., Reference Cesbron, Giraud, Picot and Pillard1985; Layo, Peru – Marcoux et al., Reference Marcoux, Moëlo and Milési1994; Radka, Bulgaria – Kouzmanov et al., Reference Kouzmanov, Ramboz, Bailly and Bogdanov2004); these assemblages are typical of high-sulfidation conditions. Tennantite-(Cu) usually replaces enargite, probably as a consequence of decreasing $f_{{\rm S}_ 2}$![]() in the crystallisation environment, usually during the early stages of the evolution of epithermal systems. On the contrary, Makovicky et al. (Reference Makovicky, Karanović, Poleti, Balić-Žunić and Paar2005) proposed that ‘Cu-excess tennantite-(Cu)’ from Catamarca, Argentina, was related to (Fe/Zn)-poor late-stage hydrothermal solutions. However, the samples show inclusions of luzonite, the low T-dimorph of enargite (Maske and Skinner, Reference Maske and Skinner1971), and watanabeite, ideally Cu4(As,Sb)5+2S5; thus, a genesis similar to those proposed for other occurrences cannot be discarded.
in the crystallisation environment, usually during the early stages of the evolution of epithermal systems. On the contrary, Makovicky et al. (Reference Makovicky, Karanović, Poleti, Balić-Žunić and Paar2005) proposed that ‘Cu-excess tennantite-(Cu)’ from Catamarca, Argentina, was related to (Fe/Zn)-poor late-stage hydrothermal solutions. However, the samples show inclusions of luzonite, the low T-dimorph of enargite (Maske and Skinner, Reference Maske and Skinner1971), and watanabeite, ideally Cu4(As,Sb)5+2S5; thus, a genesis similar to those proposed for other occurrences cannot be discarded.
Conclusion
The description of tennantite-(Cu) adds further complexity to the tetrahedrite group, confirming on one side the structural plasticity of these chalcogenides, hosting several metals typical of hydrothermal settings, and on the other side their role in recording the crystallisation conditions of ore assemblages.
In addition to improving the knowledge of ore mineralogy, the description of this new phase gives further information about the crystal chemistry of tetrahedrite-group minerals, with possible technological implications, as revealed by several recent studies focusing on the thermoelectric properties of these compounds (e.g. Chetty et al., Reference Chetty, Bali and Mallik2015; Levinsky et al., Reference Levinsky, Candolfi, Dauscher, Tobola, Hejtmánek and Lenoir2019).
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1180/mgm.2022.26
Acknowledgements
CB acknowledges financial support from the Ministero dell'Istruzione, dell'Università e della Ricerca through the project PRIN 2017 “TEOREM – deciphering geological processes using Terrestrial and Extraterrestrial ORE Minerals”, prot. 2017AK8C32. The study was also financially supported by the Ministry of Culture of the Czech Republic (long-term project DKRVO 2019-2023/1.II.d; National Museum, 00023272) for JS and ZD. The comments of Peter Leverett and two anonymous reviewers improved the original manuscript.