Background
The coronavirus disease 2019 (COVID-19) pandemic has challenged the ability of Emergency Medical Services (EMS) providers to maintain personal safety while providing a range of standard respiratory interventions for patient care at the scene and in transport. Many patients transported by EMS require respiratory therapies, including bag valve mask ventilation, intubation, nebulized medications, continuous positive airway pressure (CPAP) non-invasive ventilation, heated high-flow nasal cannula oxygen therapy (HHFNC), and even surgical airway management. These treatments and procedures are considered aerosol-generating procedures (AGPs) and have the potential to increase spread of infectious viral agents, such as COVID-19, posing an elevated risk of infection to providers utilizing them. Reference Pasnick, Carlos, Dela Cruz, Gross, Garrison and Jamil1,Reference Cournoyer, Grand’Maison and Lonergan2 Particularly, EMS providers are at risk while performing AGPs given the small, confined space of an ambulance or helicopter and a lack of effective ventilation to mitigate aerosolization of infectious agents. Consequently, many EMS systems have limited the use of AGPs despite the potential patient benefit.
Several studies have found EMS providers can be actively infected during transport of COVID-19 patients. Early in the pandemic, New York City (New York USA) saw a significant increase in 9-1-1 calls for complaints likely to necessitate AGP use, as well as an increase in high-acuity calls, including cardiopulmonary arrest. There was a resultant increased exposure of EMS staff to aerosolizing procedures and risk of contracting COVID-19. Reference Prezant, Lancet and Zeig-Owens3 Another study demonstrated that 16.3% of encounters for COVID-19 had one or more EMS-performed AGPs with a subsequent incidence of COVID-19 infection in EMS personnel of 0.57 infections/10,000 person-days. Reference Brown, Schwarcz and Counts4 One study looking specifically at nebulized medication therapy in COVID-19 patients found that 67% of medical personnel developed infection themselves, further noting that the chance of virus transmission would be significantly higher in the limited space of an ambulance compared to in-hospital. Reference Heinzerling, Stuckey and Scheuer5 Quarantine after exposure has also been a notable issue with huge implications for the EMS system workforce. A study of 274 EMS encounters with COVID-19 confirmed patients found 151 person-exposures resulting in 981 quarantine days. Reference Murphy, Barnard and Drucker6 The increased necessity for AGPs related to increased volumes of respiratory complaints in the field necessitates updated personal protective equipment (PPE) to minimize prehospital exposures. The risk of COVID-19 infection and/or mandatory quarantine following exposure could potentially be mitigated by novel PPE technologies.
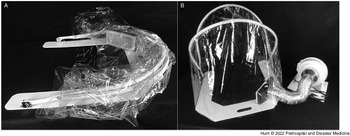
During the COVID-19 pandemic, the University of Michigan Department of Emergency Medicine (Ann Arbor, Michigan USA), Michigan Center for Integrative Research in Critical Care (M-CIRCC; Ann Arbor, Michigan USA), and the University of Michigan College of Engineering (Ann Arbor, Michigan USA) collaborated with a local manufacturing company to develop a device capable of mitigating these risks. The device (AerosolVE BioDome; Inspire Rx LLC; Ann Arbor, Michigan USA) consists of a clear plastic drape on a collapsible frame that can be secured to a back board or stretcher under the patient. The tent is fitted with a high-efficiency particulate air (HEPA) filter connected to a fan motor. This design allows ambient air to be pulled into the tent and passed through the HEPA filter before being released back into the ambient environment (Figure 1). This creates a negative pressure environment within the tent, allowing infectious particles produced and aerosolized by the patient and/or any AGP to be contained within the tent and filtered before release into ambient air. The device is designed to accommodate use of HHFNC, CPAP/bi-level positive airway pressure (BiPAP), nebulized aerosol therapies, endotracheal intubation/invasive ventilation, and cardiopulmonary resuscitation (CPR). The tent is designed to allow for up to eight entry ports to be cut into the plastic to allow multiple health care workers to access various parts of the patient (Figure 2). The fan motor pulls room air through the tent at 5,600 liters/minute. When compared to current Centers for Disease Control and Prevention (CDC; Atlanta, Georgia USA) recommendations for hospital negative pressure rooms of at least 12 air exchanges per hour (ACH), 7 the tent produces 900 ACH, or 75-times more air exchanges than current hospital room recommendations. The plastic drape, hose, rigid frame, and backboard are designed to be disposable. The fan motor and filter are designed to be reusable with a filter life of 12 months with continuous use and much longer with intermittent use.

Figure 1. AerosolVE BioDome.
Note: A) AerosolVE BioDome in collapsed position; B) AerosolVE BioDome in operating position.

Figure 2. AerosolVE BioDome Access Opening Positions.
Note: AerosolVE BioDome with locations of operational openings used during testing. Three identical openings were present on the opposite side.
The AerosolVE BioDome and a similar negative pressure helmet were initially developed for use in hospitals. Previous publications have demonstrated their efficacy in reducing air particle counts and in safely allowing advanced AGPs to be performed on COVID-19 patients. Reference Haas, Bassin and Kota8–Reference Haas, Bassin, Puls and Ward11 The prehospital transport environment varies significantly from a hospital room and transport vehicle platforms (jet versus helicopter versus ground ambulance) are also not equivalent. Thus, testing the device in each different transport platform is integral to ensure efficacy and safety.
The objective of this study was to test the effects of the negative pressure tent device on air particle counts in healthy volunteers undergoing a variety of AGPs in simulated prehospital settings. The hypothesis was the AerosolVE BioDome would prevent increases in air particle counts in the ambient cabin air under a variety of AGPs.
Methods
This was an open-label study of the efficacy of the AerosolVE BioDome system. The study enrolled 15 healthy volunteers, twelve men and three women, and distributed them amongst three transport platforms: a LearJet 75 configured for medical transport, a Eurocopter EC155 medical helicopter, and a Ford E450 modular ambulance. While not a requirement, all volunteers had been fully vaccinated for COVID-19 prior to participation. Each participant was screened for signs and symptoms of COVID-19 or other respiratory illness prior to enrollment. Sodium chloride particles, generated by a TSI 8026 particle generator (TSI Inc; Shoreview, Minnesota USA), were emitted near the subjects’ mouths to simulate bioaerosol generation associated with viral respiratory infections and to maximally test the system. A TSI 3007 condensation particle counter (TSI Inc; Shoreview, Minnesota USA) was used to detect and quantify air particle counts at different locations, including particle leakage from the tent and particle concentration inside the tent. The device is capable of detecting and quantifying particles in the range of 0.01 to >1.0µm which would include the size of the COVID-19 virus (0.1µm). Reference Bar-On, Flamholz, Phillips and Milo12 This study was approved by the University of Michigan Institutional Review Board and required written consent (protocol # HUM00192223).
Baseline ambient air particle counts were obtained in the closed cabin of the selected transport platforms without oxygen (O2) devices on and without active particle generation. This determined the ambient particle counts related to dust and other environmental particles. The participant was then placed supine on the tent backboard and the rigid frame and plastic drape placed over them, covering the entire EMS transport stretcher. Six 10cm port holes (two next to the participant’s head, two on each side of the participant) were cut into the plastic to simulate a working configuration necessary for airway management and IV access and management (Figure 2). The particle generator was inserted into the tent through one of the access ports and turned on. This was meant to simulate uniform active expiration and aerosolization of infectious particles from a patient. Particle counts were obtained in numerous locations around the tent and in various locations about the transport platform’s cabin (Figure 3A-C). After baseline counts were obtained without use of an O2 device, the procedure was repeated with the participant wearing a non-rebreather mask at 15L/minute O2 flow, a CPAP mask with pressure of 5cm of water, and a high-flow nasal cannula (HFNC) with 30L/minute of flow. The tests and measurements were performed in succession without any break in the cabin to ensure no inadvertent disruption of ambient particle levels (eg, dust in the outside air entering the cabin that would affect interpretation of subsequent particle counts). The participant was able to remain in the tent for each change of the O2 delivery device. At each testing location, ten particle count measurements were obtained and the mean recorded. This accounted for respiratory variation and other environmental factors that may cause small shifts in particle counts at that location (eg, participants breathing in particles resulting in a momentary reduction in counts inside the tent). Primary outcome was the difference in ambient particle counts and counts close to the tent compared to counts inside the tent with the filter motor off and on. Particle counts in the tent with the motor off were considered a worst-case scenario for AGP production since the boundaries of the tent are in almost identical proximity to where providers will be when caring for a patient undergoing an AGP.

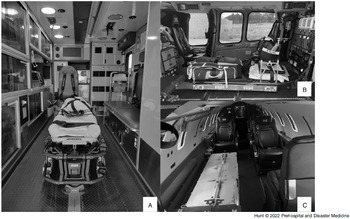
Figure 3. Emergency Medical Services Transport Platforms.
Note: Patient compartment view of each transport platform. Driver/pilot compartment not visible. A) Ground Ambulance; B) Medical Helicopter; and C) Medical Jet.
Statistical Analysis
The primary outcome of particle count inside tent with filter on compared to particle count in the ambient air and the environment close to the tent was analyzed using a one-way ANOVA as this was a comparison of means. Statistical significance was considered as α=0.05. All data were analyzed using PRISM 9 (GraphPad Software; San Diego, California USA).
Results
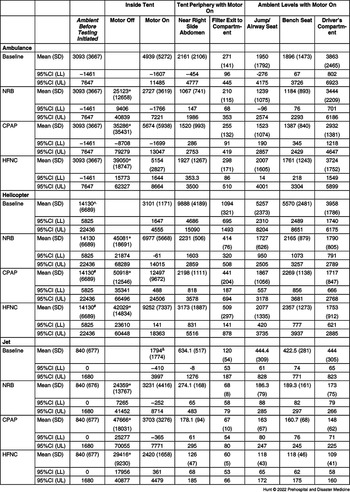
Table 1 presents the mean particle counts with and without the use of each O2 delivery device. For all transport platforms, with the tent fan off, the particle generator alone and with all AGPs produced particle counts inside the tent significantly higher than ambient particle counts (P <.0001). With the tent fan powered on, particle counts near the tent, where EMS providers are expected to be located, showed no significant elevation compared to baseline ambient particle counts during the use of the particle generator alone or with use of any of the AGPs. This also held true for other compartments within transport platforms. During the helicopter test series, the ambient particle counts were higher than the other transport platforms, likely due to the environment within the hanger housing the helicopter. Despite this, the fan and filter system were capable of reducing particle counts adjacent to the tent to levels below ambient levels through its entraining of air throughout the helicopter.
Table 1. Mean (SD) and 95% CI for Particle Counts for Each Transport Platform and AGP

Abbreviations: AGP, aerosol-generating procedure; NRB, non-rebreather mask; CPAP, continuous positive airway pressure; HFNC, high-flow nasal cannula.
* Denotes significant difference between “Inside Tent Motor Off” and all other locations (P<.0001).
^ Denotes significant difference between “Ambient Air Level” and “Close to Tent” (P<.0001).
# Denotes significant difference between “Ambient Air Level” and “Filter Exit to Compartment” (P<.0001).
& Denotes significant difference between “Inside Tent” and “Filter Exit to Compartment” (P<.0001).
Discussion
The results of this analysis demonstrated that with the AerosolVE BioDome activated (fan on), no significant increase in the ambient particle counts near the tent nor in the cabin occurred under all AGP conditions across each transport platform. The particle count in the tent during the AGP without the motor on (levels significantly higher than ambient pre-treatment levels) was used as a surrogate for worst-case particle exposure to an EMS provider close to the patient in the absence of the tent.
The AerosolVE BioDome appears to effectively contain the release of aerosolized particles into the patient care compartment and thus may reduce risks to the patient attendant(s). Interestingly, during the serial tests, a gradual decline in the cabin air particle counts of the various transport vehicles was seen. As the AerosolVE BioDome is designed to entrain cabin air into the tent, which is subsequently filtered, this shows a gradual improvement in air quality and improved safety during the duration of the simulated care event.
For simplicity, the testing protocol excluded the use of a nebulizer, a common respiratory care device used in the prehospital setting, as previous testing had shown similar aerosolized particle generation from the particle generator and the nebulizer. Reference Haas, Bassin and Kota8 In seeking to test the device in a “worst-case scenario” (no air exchange), the study did not utilize built-in HVAC (ie, heat, ventilation, air conditioning) in the patient compartment of the ambulance. Any HVAC use would only serve to improve the ambient air quality by creating air exchange.
While a number of negative pressure/isolation “tents” have gained Emergency Use Authorization (EUA) for use within hospitals, none, to the authors’ knowledge, are approved for prehospital use, despite the protracted nature of the COVID-19 pandemic. Furthermore, some of these hospital-use tents rely on regular wall suction to produce a negative pressure within the tent and to prevent AGP-related increases in ambient particles, the efficacy of which has not been reported.
Given the impact on the workforce of infection and/or mandatory quarantine, many EMS systems have reduced their transport of patients with known or highly suspected COVID-19 infections or have modified treatment protocols (eg, using supraglottic airways rather than oral endotracheal intubation, metered-dose inhalers rather than nebulizer treatment) to reduce risks of EMS personnel. The AerosolVE BioDome can likely reduce EMS provider exposure during transport of any patient with unknown COVID-19 status, regardless of symptoms, and allow for maximal patient therapy.
Transport of COVID-19 positive military personnel has seen similar challenges as those described in the civilian EMS sector. Additionally, the necessity for multiple-patient transport is higher in the military sector. Negative pressure transport conexes have been designed to transport multiple infected individuals, but simultaneous use of AGPs within these containment systems has not been reported. Reference Correll13 Use of devices like the AerosolVE BioDome may offer additional options and improved safety for care staff.
Limitations
This study was conducted on healthy volunteers who were breathing normally (not coughing) with simulated infectious droplets dispersed within the tent. The study elected to use a small convenience sample of volunteers rather than a large population as the focus of this study was to determine the device’s efficacy in preventing increases in ambient particle counts in the EMS transport cabin. Given the efficiency of the device in its ability to filter particles, it is unlikely that additional test participants would have made a significant difference in the results obtained. Future studies will be required examining the device and particle counts with real patients being transported and undergoing AGPs. In addition, studies will be required to examine EMS providers’ ability to actively manage and engage patients under the tent for more advanced AGPs such as endotracheal intubation or surgical airway management.
As mentioned above, testing in the helicopter or jet with HVAC cabin air modulation was not possible due to high levels of environmental particles with the aircraft running. While it is believed this creates a “worst-case scenario” in that no cabin air is exchanged, it is difficult to say with certainty how HVAC would have impacted results.
The study design originally intended to use CPAP pressure at 10cm of water and HFNC with 60L/minute of flow. However, these levels of respiratory support were uncomfortable to the healthy volunteers and thus reduced to 5cm of water and 30L/minute, respectively. There were no adverse or safety events reported by participants or observed by the study team during the conduct of this study.
Conclusion
Given the risk posed by communicable respiratory diseases to prehospital medical providers, novel protective devices may improve safety for these caretakers while still enabling use of respiratory therapies known to increase aerosolization and risk of transmission of infectious agents. Development and testing of these devices are of paramount importance. While this is certainly true during the current pandemic, the same could be said about traditional “respiratory virus season,” when the function of the prehospital system depends heavily on a healthy workforce. The AerosolVE BioDome demonstrated efficacy in creating a negative pressure environment around simulated patients and provided significant filtration of simulated respiratory droplets, thus making the confined space of various EMS transport vehicle types potentially safer for EMS personnel.
Conflicts of interest/funding
Kevin Ward has intellectual property regarding the AerosolVE BioDome through the University of Michigan which has been licensed to Inspire Rx LLC. Kevin Ward, Benjamin Bassin, and Nathan Haas have equity in Inspire Rx LLC. Benjamin Bassin is the Chief Medical Officer for Inspire Rx LLC. The remaining authors have no competing interests.