α-Tocopherol (α-Tocopherol refers to total α-Tocopherol stereoisomers) is the most abundant form of vitamin E in human plasma, which plays an important role in the health of mothers and infants, especially in the early life of offspring(Reference Azzi1). Studies shows that α-Tocopherol, a powerful chain-breaking antioxidant, prevents propagation of free-radical reaction and resists oxidative stress associated with pre-term delivery, low birth weight and pre-eclampsia during pregnancy. Antioxidant barrier supported by α-Tocopherol is of vital importance in the development of the central nervous system and maturation of the placenta(Reference Hanson, Lyden and Furtado2).
α-Tocopherol has three chiral carbons, and as a result there are potentially eight stereoisomers. However, only the RRR-α-Tocopherol is known as the physiologically active vitamer. In contrast, all-rac-α-Tocopherol, a mixture of the eight possible stereoisomers, has 50 % difference in relative activity compared with RRR-α-Tocopherol according to IOM(3). RRR-α-Tocopherol is the primary stereoisomer in all brain regions (> 66 %) and cumulative with age, especially the frontal cortex, hippocampus and visual cortex of infants, the key areas responsible for memory and learning, suggesting it is vital for the development of memory and intelligence in infants(Reference Kuchan, Jensen and Johnson4). RRR-α-Tocopherol can also stabilise the membrane and protect DHA from oxidative damage by antioxidant or other mechanisms as well as promote the differentiation and synapse formation of brain nerve cells(Reference Stimming, Mesch and Kersting5,Reference Raederstorff, Wyss and Calder6) .
According to the WHO, breast milk is the ideal food for infants under 6 months of age(7), and its nutrient content and composition dynamically change with the lactation stage to adapt to the growth needs of the offspring. As a result, breast milk is the predominately source of vitamin E for infants, and its content and isomer composition have a significant influence on the growth and development of infants.
The umbilical cord is an important organ that connects the placenta and the fetus as well as the main way for the fetus to obtain nutrients from the mother. The level of nutrients in umbilical cord plasma reflects the composition and content requirements of nutrients provided by the mother to the fetus, which directly affects the growth and development of the fetus(Reference Hauta-Alus, Kajantie and Holmlund-Suila8).
Thus, studying the content of α-Tocopherol and the distribution of chiral isomers in breast milk at different stages can better reflect the changes in content and composition requirements of infants during their growth. Similarly, through the study of maternal plasma and umbilical cord plasma, we can have a knowledge of the content and priority of α-Tocopherols provided by the mother to the fetus.
This study aimed to investigate natural RRR-α-Tocopherol and synthetic stereoisomers in Chinese maternal plasma, cord plasma and breast milk from different regions of China, which had not been studied at present and provide a reference for further guidance on maternal diet and the addition of vitamin E content and configuration in infant formula. The abbreviations and definitions of some nouns in the article are shown in Table 5.
Materials and methods
Background of participants
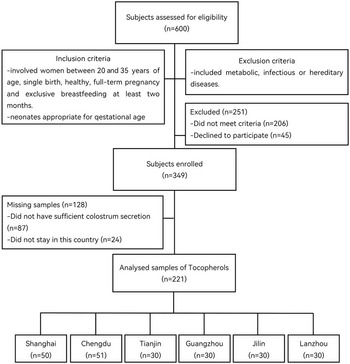
The study was a joint research project carried out by Shanghai Jiao Tong University, Sun Yat-sen University, Nankai University, Sichuan University, Lanzhou University and Jilin University at 2018 (Fig. 1). We chose six representative regions (Shanghai is located in Eastern China; Guangzhou: Southern China; Tianjin: Northern China; Chengdu: Western China; Lanzhou: Northwest China; Changchun: Northeast China) for the characterisation of breast milk, maternal plasma and cord plasma in accordance with the geographical location. Based on published literature and practical feasibility, at least thirty longitudinal samples were enrolled in each city. Inclusion criteria involved women between 20 and 35 years of age, single birth, healthy, full-term pregnancy, and exclusive breast-feeding at least 2 months, and neonates appropriate for 37–42 weeks of pregnancy with Apgar score > 8. Exclusion criteria included metabolic, infectious or hereditary diseases. The study was approved by medical ethics research boards in six regions as well as registered in http://www.chictr.org.cn/ (ChiCTR1800015387). All subjects have written informed consents.

Fig. 1. Flow chart of sample collection.
Data collection
Maternal medical history and anthropometric information were collected through the hospital medical record system and questionnaires, including height, pre-gestation weight and BMI, pre-delivery weight and BMI, delivery mode, education level, number of deliveries, and pregnancy. Neonatal gestational age, sex, length, and weight were also collected.
Sample collection
During delivery, 5 ml each of the maternal peripheral venous plasma and the neonatal umbilical venous plasma was collected by obstetricians or nurses, stored in a vacuum heparin lithium plasma collection tube, mixed and transported to the laboratory within 30 min by cold chain without light. The venous blood was centrifuged at 1500 × g for 15 min at 4°C to obtain the upper layer of clarified plasma. The upper layer of plasma was absorbed and placed into a 500-µl cryo-storage tube (SIGMA V2881) covering with tin foil for protection from light and stored in a refrigerator at −80°C for later analysis. During the period of 1–5 d, 10–15 d and 40–45 d after the delivery, breast milk samples were collected in any day of each period from 09.00 to 11.00, about 2 h after the first feeding in the morning to avoid circadian influence on the results. All the milk from a single full breast was emptied through a breast pump, mixed thoroughly and took out 15 ml of milk in a clean test tube, which was transported to the laboratory within 5 h through a low-temperature cold chain and immediately frozen in a –80°C refrigerator and divided into colostrum, transition milk or mature milk.
Chemical analysis
The methodology was modified from a previous method by Romeu-Nadal et al.(Reference Gaur, Kuchan and Lai9). After saponification and extraction into heptane, α-Tocopherol concentration was determined by HPLC with a fluorescence detector (Agilent 1260 Infinity). After methylation in corresponding methyl ethers, the eight stereoisomers of α-Tocotrienols, RRR-, RSS-, RRS-, RSR-, SSS-, SSR-, SRS- and SRR-α-Tocotrienols(Reference Szewczyk, Chojnacka and Górnicka10), which were subsequent separation by UPLC (Waters, Acquity UPLC H-Class) and the concentrations were determined by fluorescence detector (Shimadzu, RF-20AXS). The linear correlation coefficient is greater than or equal to 0·999.
Statistical analysis
Although the contents of α-Tocopherol and its eight stereoisomers in human milk were skewed distribution, we tested the changes in α-Tocopherol of breast milk over different lactation stages with a repeated-measures ANOVA because of our large enough sample size. Contents of α-Tocopherol and its eight stereoisomers in maternal plasma and cord plasma among six regions were compared by Mann–Whitney U test. Contents of α-Tocopherol and chiral isomers in breast milk, maternal plasma and cord plasma between six regions were compared by the Kruskal–Wallis test due to the non-normal distribution of the data. Mann–Whitney U test was used for pairwise comparisons after significant differences confirmed to exist (P < 0·05). For determining significant correlations among different nutrient concentrations of α-TOH and its eight stereoisomers and characteristics of the parturient women and their newborns, data were analysed via Spearman’s correlation. All data were carried out by Statistical Package for the Social Sciences version 23.0 with two-tailed tests, and statistical significance set at P < 0·05.
Results
In this longitudinal study, 663 breast milk samples were collected at three stages from early to late lactation in 221 healthy Chinese women from six different regions (Shanghai: n 50; Chengdu: n 51; Tianjin: n 30; Guangzhou: n 30; Lanzhou: n 30; and Changchun: n 30). The characteristics of the postpartum women and their newborns are summarised in Table 1. The average age of the women is 29·7 ± 3·5 years, and the weight gain was well.
Table 1. Characteristics of the parturient women and their newborns
(Numbers and percentages; mean values and standard deviations, n 221)

The concentrations of α-Tocopherol and stereoisomers studied at different stages of lactation in addition to maternal plasma and cord plasma of which concentrations considered to be in normal ranges are shown in Table 2. As we can see, significant differences according to the different stages of lactation as well as the different plasma samples were observed for α-Tocopherol and its stereoisomers (P < 0·001). The concentrations of α-Tocopherol and its stereoisomers in maternal plasma were significantly higher than those in cord plasma (P < 0·001). As for the three stages of milk, those in colostrum were significantly higher compared with those during other periods (P < 0·001). With the advancement of lactation, a decrease was observed in the transitional and mature milk. Meanwhile, the concentrations in mature milk of α-Tocopherol and RRR were the lowest in different stages of milk (P < 0·05), while the others found to have no significant differences between transitional and mature milk (P > 0·05).
Table 2. α-Tocopherol and stereoisomers concentrations in maternal plasma, cord plasma and human milk at different lactation stages of 221 sample sets (mg/l)
(Mean values and standard deviations; median values and percentiles)
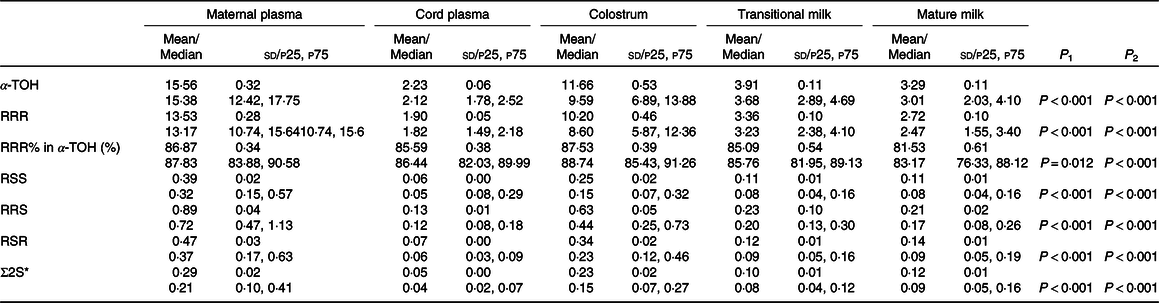
P1, α-Tocopherol and stereoisomers concentrations in maternal plasma and cord plasma are compared by Mann–Whitney U test with α value (α = 0·05); P2, α-Tocopherol and stereoisomers concentrations in human milk at different lactation stages are compared by repeated-measures ANOVA.
* Σ2S = SSS + SSR + SRS + SRR.
Data are reported as mean ± sd and median (p25, p75).
α-Tocopherol and RRR concentrations in maternal plasma, cord plasma and breast milk from different stages of lactation in the six regions are shown in Table 3. There was no significant difference in most regions, and only a few areas had differences in specific nutrient (P < 0·05). α-Tocopherol and RRR were higher in maternal plasma samples from Shanghai, Chengdu, Guangzhou, Jilin and Lanzhou, but the lowest from Tianjin. Similarly, in cord plasma samples, the contents of α-Tocopherol and RRR were higher from Shanghai, Chengdu and Lanzhou, followed by Tianjin and Guangzhou with the lowest from Jilin (P < 0·05). The α-Tocopherol and RRR contents in colostrum and transitional milk from Chengdu, Lanzhou, Tianjin, Shanghai and Guangzhou were higher; in contrast, those from Jilin were slightly lower. Similarly, α-Tocopherol and RRR concentrations in mature milk from Shanghai were significantly higher than those from other regions (P < 0·05). Meanwhile, Jilin had the lowest concentrations among the six regions (P < 0·05).
Table 3. α-Tocopherol and stereoisomers concentrations in maternal plasma, cord plasma and human milk from six regions
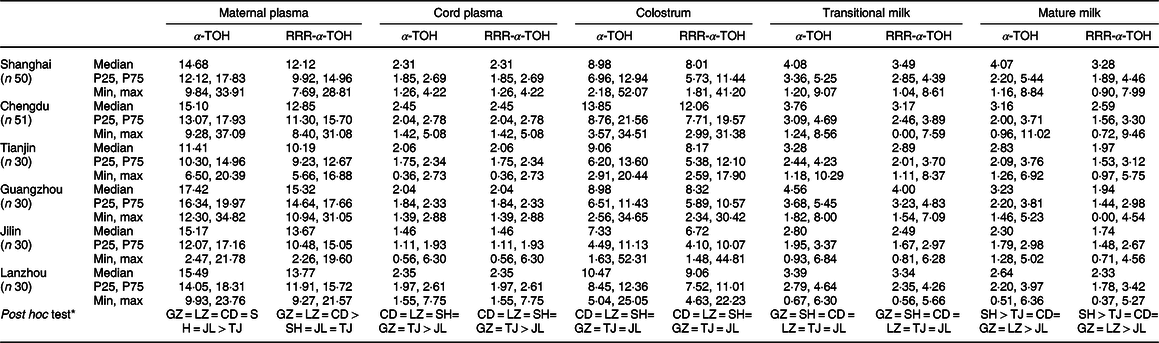
SH, Shanghai; CD, Chengdu; TJ, Tianjin; GZ, Guangzhou; JL, Changchun; LZ, Lanzhou.
* Compared by Mann–Whitney U test with α value (α = 0.05).
Associations among concentrations of α-Tocopherol and RRR in maternal plasma, cord plasma, human milk and characteristics of parturient women as well as their newborns are provided in Table 4. RRR concentrations in colostrum and the number of pregnancies and deliveries were also positively related to the concentrations in cord plasma and transitional milk (P < 0·05). Weight and BMI before gestation were negatively correlated with the concentrations in cord plasma (P < 0·05). Concentrations in cord plasma were negatively associated with weight and BMI before delivery, while the former was also negatively related to concentrations in maternal plasma and transitional milk (P < 0·05). Similarly, the pregnancy weight gain was negatively related to concentrations in maternal plasma, transitional milk and mature milk (P < 0·05). As for the characteristics of newborns, only the gestational age was found to have positive relationships with concentrations in cord plasma and transitional milk (P < 0·05).
Table 4. The associations among concentrations of α-Tocopherol and RRR in maternal plasma, cord plasma and human milk and characteristics of parturient women
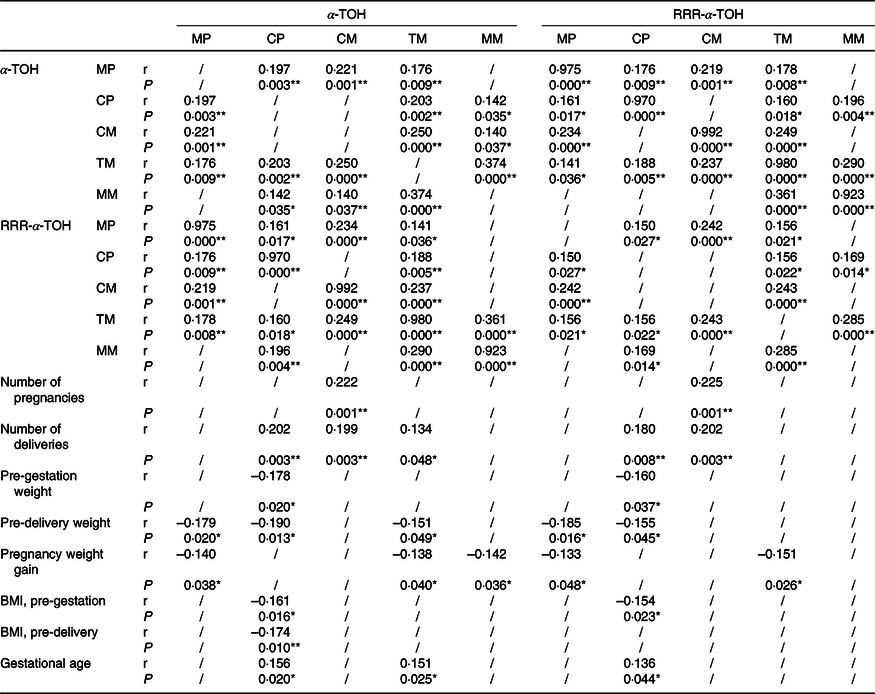
MP, maternal plasma; CP, cord plasma; CM, colostrum; TM, transitional milk; MM, mature milk.
*P < 0·05; **P < 0·01.
/, No statistically significant correlation is found between the two.
Table 5. Abbreviations and definitions

Discussion
This multi-centre, large-sample study was jointly conducted by six schools, aimed to investigate the natural occurring RRR-α-Tocopherol and synthetic α-Tocopherol stereoisomers in maternal plasma, cord plasma and different stages of lactation from six regions of China. It is the first case to report that α-Tocopherol and RRR contents in different kinds of samples from multiple regions of China are of great significance to comprehensively and accurately reflect the α-Tocopherol content and configuration requirements during maternal to fetal and breast milk changes, which provides a reference for further guidance on maternal diet and the addition of vitamin E content and configuration in infant formula.
There were significant differences among the five samples for α-Tocopherol and its stereoisomers, reflecting differences between mothers and fetus in addition to nutritional needs of three lactation stages. The results of α-Tocopherol content in this study are basically consistent with the conclusions in other reports. Clemente H.A et al. (Reference Clemente, Ramalho and Lima11) found that maternal supplementation with natural or synthetic vitamin E can increase vitamin E concentrations in colostrum, and the natural form was more efficient in increasing the levels. Hanson et al. (Reference Hanson, Lyden and Furtado2) assessed the levels of α-Tocopherol in Midwestern USA and found that the average level was 12·51 ± 4·67 mg/l in maternal plasma and 1·95 ± 0·80 mg/l in the cord plasma from 189 pairwise samples. Tijerina-Saenz A et al. (Reference Tijerina-Saenz, Innis and Kitts12) reported that α-Tocopherol in breast milk from sixty breast-feeding women at 1 month postpartum was 2·32 ± 0·11 g/l in Canada.
As we can see, the concentrations of α-Tocopherol and RRR in maternal plasma were higher than breast milk, which was significantly higher than umbilical cord plasma. This phenomenon also existed in the finding of Kuchan M. J. et al. (Reference Kuchan, DeMichele and Schimpf13), who measured the concentrations of RRR-αT of cord plasma and maternal plasma in both uncomplicated and complicated pregnancies, and the latter was significantly higher than the former. Pregnancy is a state of increased oxidative stress. Antioxidants including α-Tocopherol can offer defence against tissue-damaging effects of reactive oxygen species. Studies have shown that before and after childbirth, as newborns transition from a hypoxic environment to a hyperoxic environment, fetal tissues are exposed to high concentrations of free radicals(Reference Woods, Cavanaugh and Norkus14). Deficiency of α-Tocopherol may cause a variety of diseases, such as congenital malformations, respiratory diseases, and retinopathy, and affect the central nervous system, increasing neonatal mortality, especially in pre-term infants(Reference Fares, Feki and Khouaja-Mokrani15,Reference Brion, Bell and Raghuveer16) . In this study, the level of α-Tocopherol in fetus was only 14·26 % of that in mothers, lower than that shown in another report(Reference Fares, Sethom and Khouaja-Mokrani17). However, 4 to 6 d after breast-feeding, the level of newborns is almost consistent to adults(Reference Lima, Dimenstein and Ribeiro18). It suggests that newborns complement their α-Tocopherol deficiency by breast milk intake(Reference Clemente, Ramalho and Lima11), and high level of α-Tocopherol in colostrum contributes to flip adverse conditions in newborns. As the colostrum turns to mature milk, the α-Tocopherol concentration gradually decreases(Reference Ostrea, Balun and Winkler19), which is consistent with our findings.
Although there were significant differences among five kinds of samples in this longitudinal study, they were both highly enriched in the natural occurring RRR-α-Tocopherol. Meanwhile, we find that the RRR contents in maternal plasma, cord plasma and breast milk from different stages were significantly higher than other configurations, all accounting for more than 80 % of the total α-Tocopherol content. Liu Z et al. (Reference Liu, Neuringer and Erdman20) found out that RRR-α-Tocopherol, which is the naturally occurring stereoisomer of α-Tocopherol, preferentially presented in breast milk and deposited in brain. This pointed out that RRR-α-Tocopherol with higher proportion, higher biological activity and easier to deposit in the baby’s brain among the eight stereoisomers should be added to milk powder instead of adding synthetic all-rac-α-Tocopherol with lower transmission efficiency and lower biological activity, which most formulas on the market contains.
The low α-Tocopherol concentration and high RRR proportion in umbilical cord plasma may be due to the low transfer efficiency of placenta aimed plasma lipids and the function of α-Tocopherol transporter (α-TTP) on the placenta, which can specifically transport RRR(Reference Kuchan, Jensen and Johnson4). Studies have shown that RRR is the most active chiral isomer of α-Tocopherol. Maternal supplementation with RRR-α-Tocopherol can significantly increase the ratio of RRR in breast milk and plasma, while supplemental all-rac-α-Tocopherol will reduce the ratio(Reference Gaur, Neuringer and Erdman21), suggesting that the supplemental form of α-Tocopherol is of great significance to mothers and fetus. Furthermore, in the study of Ranard K. M. et al. (Reference Ranard, Kuchan and Bruno22), compared with natural α-T, there was a danger of altering myelin gene expression in the cerebellum when the adolescent mice were exposed to high-dose synthetic α-T, which may result in morphological and functional abnormalities later in life. This increases the necessity of studying the form of vitamin E in maternal supplement and infant formula milk powder, and it is also the guiding significance of this paper.
Besides, this study shows regional differences both in α-Tocopherol and RRR among maternal plasma, cord plasma and different stages of milk. Yong Xue et al.(Reference Xue, Campos-Giménez and Redeuil23) have reported the concentration of α-Tocopherol from three cities in China, including Beijing, Suzhou, and Guangzhou that similarly showed regional differences. According to the theory of de Sousa Rebouças A et al. (Reference de Sousa Rebouças, Costa Lemos da Silva and Freitas de Oliveira24), who demonstrated that the pre-existing vitamin E levels in milk and diet are determinants for the greater effect of supplementation, α-Tocopherol is so greatly affected by diet that further analysis about the effect of regional dietary intake is required. Silva et al. (Reference da Silva, de Sousa Rebouças and Mendonça25) found that there was a high degree of inadequacy in vitamin intake and a reduction in serum α-Tocopherol. This suggests that the adequacy of vitamin E content in lactating mothers is very important to ensure mother’s health. Our project has collected questionnaires for maternal diets; therefore, regional dietary data will continue to be analysed in the future. At present, daily dietary supplements, oils and formula powders are mainly added with the synthetic α-Tocopherol as vitamin E(Reference Stone, LeClair and Ponder26), which leads to the existence of non-natural configuration of α-Tocopherol in body of mothers and fetus. The presence of 10 to 20 % of the synthetic α-Tocopherol in maternal plasma, cord plasma and breast milk in this study suggests that the intake of synthetic configuration will affect α-Tocopherol in plasma and milk in addition to the distribution of isomers and reduced ratio of RRR.
There were correlations between α-Tocopherol and RRR content in maternal plasma, cord plasma and milk, suggesting that the maternal α-Tocopherol content and configuration ratio will have effect on the fetal nutrition reserve. Da Silva Ribeiro et al. reported that in order to ensure adequate fetal α-Tocopherol, supplementation with RRR or higher doses of synthetic α-Tocopherol may be required (Reference da Silva Ribeiro, Lima and Medeiros27). In almost all kinds of samples, there were negative correlations among α-Tocopherol, RRR and weight-related indicators, such as pre-gestation weight, pre-delivery weight, pregnancy weight gain, pre-gestation BMI and pre-delivery BMI. It is suggested that we can further explore the impact of weight gain during pregnancy on the fetal α-Tocopherol reserve.
In general, this study has three obvious advantages. First, this study is the first case in China to report the distribution of natural occurring RRR-α-Tocopherol and synthetic α-Tocopherol stereoisomers in the three stages of maternal plasma, umbilical cord plasma and breast milk in different regions. Second, through the collection of population samples and a large number of experiments, this study comes to the conclusion that although different regions had significant differences in α-Tocopherol content, the content law was the same: maternal plasma > colostrum > transition milk > mature milk > cord plasma, and the content of RRR-α-Tocopherol was significantly higher in maternal plasma, cord plasma and human milk than that of the other seven configurations, which is consistent with the research conclusions at home and abroad. Third, this study evaluated the needs of pregnant women and early life for α-Tocopherol and its different configurations, so as to provide reference for guiding the rational supplement of pregnant women and the addition of α-Tocopherol in infant formula milk powder. However, this study has not reported the relationship between diet and the content and configuration of α-Tocopherol in maternal plasma, cord plasma and human milk, as well as needed to be supplemented by more powerful clinical evidence. Meanwhile, the survey scope is limited to pregnant women, and the conclusion cannot be applied to other populations.
The results of this study indicate that the RRR-α-Tocopherol is the absolute predominant configuration of α-Tocopherol, suggesting that the RRR-α-Tocopherol may be a more ideal form of vitamin E supplementation for pregnant women and infants.
Acknowledgements
This research was supported by Abbott Nutrition Research & Development Center, Shanghai, China.
M. C. conceived and designed the study protocols. K. W. and Z. L. contributed to the subject recruitment and the sample collection and conducted the sample determinations. Z. L. wrote the manuscript. G. D. and S. W. were mainly responsible for data analysis and the final content. All the authors read and approved the final manuscript.
None of the authors has any conflicts of interest to declare.