Introduction
Attention-deficit/hyperactivity disorder (ADHD) is a common, childhood-onset neurodevelopmental disorder and an important risk factor for many psychiatric [Reference Fayyad, Sampson, Hwang, Adamowski, Aguilar-Gaxiola and Al-Hamzawi1,Reference Nigg2] and general medical disorders [Reference Nigg2] across the life span. Symptoms of ADHD may persist to an older age in a substantial number of individuals with ADHD [Reference Dobrosavljevic, Solares, Cortese, Andershed and Larsson3]. However, the extent to which individuals with ADHD are at increased risk for dementia and mild cognitive impairment (MCI) is unclear. Dementia is characterized by a significant decline in cognition, behavior and in the ability to perform everyday activities, whereas MCI is defined by the presence of impairment in one or more cognitive domains without affecting functional independence and without fulfilling the diagnostic criteria for dementia [Reference Winblad, Palmer, Kivipelto, Jelic, Fratiglioni, Wahlund and Nordberg4].
Studies in humans [Reference Tzeng, Chung, Lin, Yeh and Huang5–Reference Ivanchak, Abner, Carr, Freeman, Seybert and Ranseen8] and animal models of ADHD [Reference Zhang, Du, John, Kapahi and Bredesen9-Reference Dellu-Hagedorn, Trunet and Simon10] have indicated an association between antecedent symptoms of ADHD and cognitive deficits in later life, but available literature is limited and conflicting. Three studies, including a population-based study from Taiwan [Reference Tzeng, Chung, Lin, Yeh and Huang5], a U.S. hospitalization discharge, cohort study [Reference Fluegge and Fluegge6], and a case-control study from Argentina [Reference Golimstok, Rojas, Romano, Zurru, Doctorovich and Cristiano7], found an increased risk for dementia in people with ADHD. In contrast, a cross-sectional study from the United States [Reference Ivanchak, Abner, Carr, Freeman, Seybert and Ranseen8] did not find a significant association between childhood symptoms of ADHD and dementia/MCI in later life. Limitations of these studies were the use of inpatient-care data that cover more severe cases of ADHD and dementia [Reference Fluegge and Fluegge6], while most patients with ADHD are treated in outpatient care, and over-representation of males and individuals aged 18–54 years old in the study population [Reference Tzeng, Chung, Lin, Yeh and Huang5]. The included individuals [Reference Tzeng, Chung, Lin, Yeh and Huang5] might not represent the population at risk, as it has been reported that dementia is more prevalent in women and the risk of developing dementia before age 50 is low [Reference Ruitenberg, Ott, van Swieten, Hofman and Breteler11]. Other limitations were the retrospective assessment of childhood ADHD symptoms in older adults with and without cognitive impairment [Reference Golimstok, Rojas, Romano, Zurru, Doctorovich and Cristiano7-Reference Ivanchak, Abner, Carr, Freeman, Seybert and Ranseen8], allowing for recall bias and misclassification.
Additionally, it has been proposed [Reference Callahan, Bierstone, Stuss and Black12] that ADHD might lead to development of cumulative health-compromising factors along the lifespan, which in turn present risk factors for developing MCI and dementia in later life. Low educational attainment, common metabolic disorders or metabolic syndrome (i.e., hypertension, type 2 diabetes [T2D] and obesity), sleep disorders, head injuries, and psychiatric disorders (i.e., depression, anxiety, substance use disorder [SUD], and bipolar disorder), are associated with ADHD [Reference Fayyad, Sampson, Hwang, Adamowski, Aguilar-Gaxiola and Al-Hamzawi1,Reference Nigg2], and well-established risk factors for dementia [Reference Norton, Matthews, Barnes, Yaffe and Brayne13–Reference Li, Li, Li, Zhang, Zhao and Zhu16]. Unfortunately, only a few studies have explored the potential impact of these factors on the association between ADHD and dementia, with inconsistent findings. One study reported an increased risk for dementia in people with ADHD even after adjusting for socioeconomic status, general medical and psychiatric comorbidities [Reference Tzeng, Chung, Lin, Yeh and Huang5]. In contrast, another study [Reference Fluegge and Fluegge6] found that the association between ADHD and dementia was no longer significant after controlling for metabolic dysregulation.
The current study aimed to extend previous research [Reference Tzeng, Chung, Lin, Yeh and Huang5–Reference Ivanchak, Abner, Carr, Freeman, Seybert and Ranseen8,Reference Callahan, Bierstone, Stuss and Black12] by utilizing large-scale population data from national registers in Sweden, and a retrospective cohort design to investigate the association of ADHD with dementia and MCI. We additionally aimed to investigate to what extent these associations are affected by educational attainment, comorbid metabolic disorders (i.e., hypertension, T2D, and obesity), sleep disorders, head injuries, and psychiatric disorders (i.e., depression, anxiety, SUD, and bipolar disorder). We also investigated whether a potentially increased risk for dementia is specific to ADHD, or shared with comorbid developmental disorders [Reference Selinus, Molero and Lichtenstein17]. Finally, since prevalence rates of ADHD [Reference Fayyad, Sampson, Hwang, Adamowski, Aguilar-Gaxiola and Al-Hamzawi1] and dementia [Reference Ruitenberg, Ott, van Swieten, Hofman and Breteler11] differ between men and women, we aimed to explore whether their associations differ by sex.
Method
Data sources
We used data from multiple Swedish population-based registers. All individuals registered in Sweden are assigned a unique personal identity number that enables a linkage of these registers [Reference Ludvigsson, Almqvist, Bonamy, Ljung, Michaëlsson and Neovius18]. The Total Population Register (TPR) includes all individuals in Sweden born since 1932, who were alive in 1963 and later. We used the following demographic data from the TPR: date of birth, sex, date of death, and migration [Reference Ludvigsson, Almqvist, Bonamy, Ljung, Michaëlsson and Neovius18]. The National Patient Register (NPR) covers all primary, and up to eight secondary diagnoses from inpatient hospital admissions since 1987, and information from the outpatient register since 2001 [Reference Ludvigsson, Andersson, Ekbom, Feychting, Kim and Reuterwall19]. The Cause of Death Register (CDR) contains information of all deaths since 1952 [Reference Brooke, Talbäck, Hörnblad, Johansson, Ludvigsson and Druid20]. All diagnoses in the NPR and CDR were classified according to the International Classification of Diseases (ICD) versions 7/8/9/10. The Prescribed Drug Register (PDR) covers data on all dispensed medication prescriptions since July 1, 2005 using the Anatomical Therapeutic Classification (ATC) system, with a date of prescription and dosage. Longitudinal integration database for health insurance and labour market studies register (LISA) covers information on educational attainment for individuals aged ≥ 16 since 1990 [Reference Ludvigsson, Svedberg, Olén, Bruze and Neovius21].
Study population
The total cohort consisted of 3,591,689 individuals born between 1932 and 1963, who were alive and resided in Sweden in 2001 (Figure 1). We excluded individuals who emigrated from Sweden and died before 2001, and before age 50, and those who immigrated to Sweden after 2001 and aged 50 and above. A diagnosis of ADHD has been mostly available from the outpatient care medical files recorded in the NPR since 2001. The beginning of the follow-up was set at age 50. The end of the follow-up was set at the date of a diagnosis of dementia/MCI, emigration, death, or December 31, 2013 (the last date with available data), whichever came first.
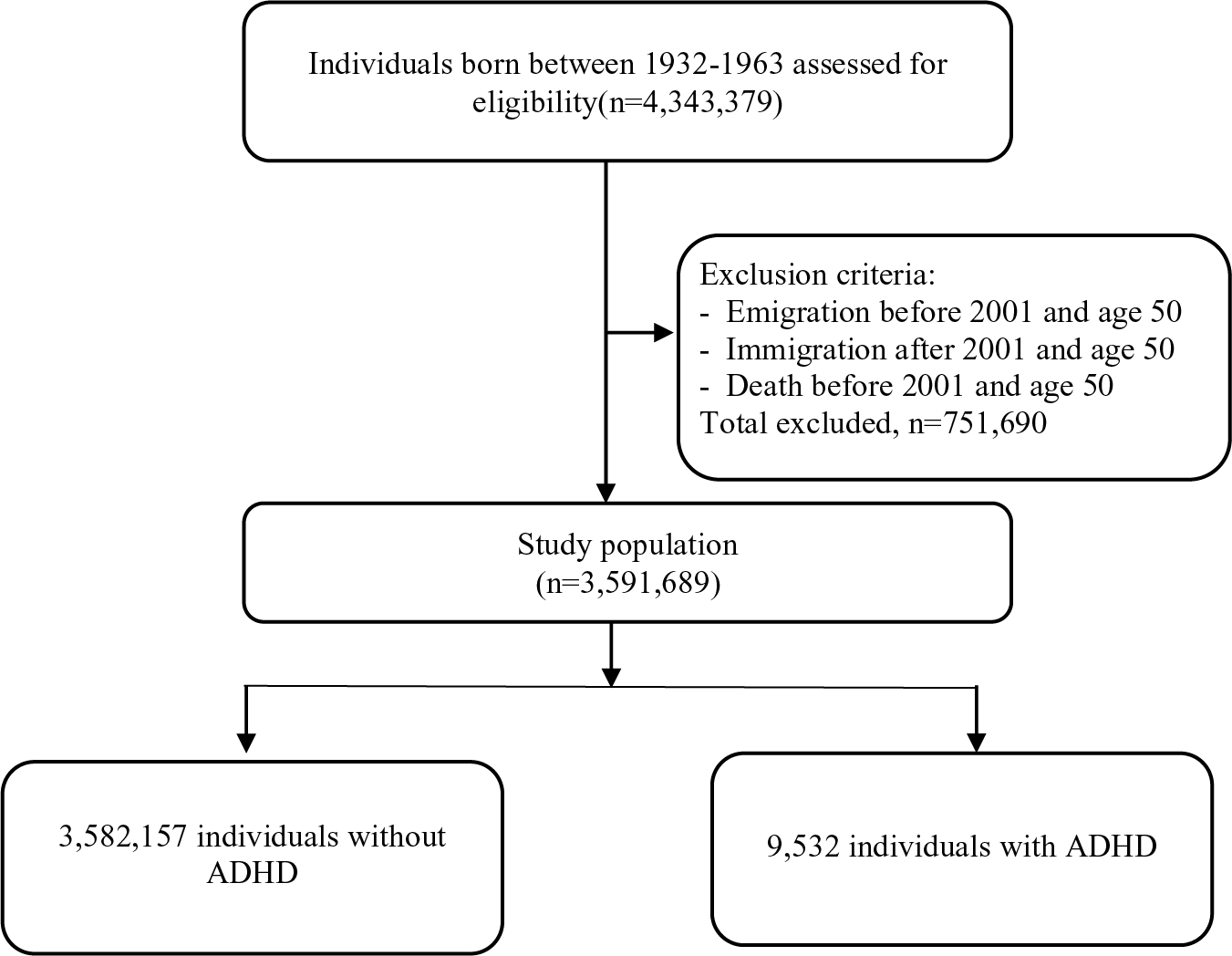
Figure 1. Flowchart of the study population selection process.
Measures of ADHD
ADHD cases were defined as individuals who received an ICD-9/10 diagnosis from the NPR [Reference Larsson, Rydén, Boman, Långström, Lichtenstein and Landén22], or dispensed medication prescription for ADHD treatment according to the ATC codes from the PDR [Reference Zetterqvist, Asherson, Halldner, Långström and Larsson23] (Supplementary Table S1), at any age. Medication prescriptions can be considered as valid indicators of ADHD diagnoses, and both ADHD diagnoses from the NPR and medication prescriptions from the PDR are provided exclusively by specialists in Sweden [Reference Larsson, Rydén, Boman, Långström, Lichtenstein and Landén22]. To additionally ensure that ADHD cases reflect clinically relevant diagnoses, we performed a sensitivity analysis by including only individuals with a confirmed diagnosis of ADHD from the NPR [Reference Ghirardi, Brikell, Kuja-Halkola, Freitag, Franke and Asherson24].
Measures of dementia and MCI
We included a diagnosis of dementia or MCI recorded at age 50 or older to minimize the risk of misdiagnosis with conditions with similar clinical presentations (i.e., ADHD) when dementia/MCI is diagnosed at a younger age [Reference Pollak25]. The following types of dementia were included: Alzheimer’s disease (AD), vascular dementia, and other dementias, with diagnostic codes according to the ICD-8/9/10 from the NPR and the CDR, or medication prescription for AD according to the ATC codes from the PDR (Supplementary Table S1) [Reference Eriksson, Lundholm, Narasimhalu, Sandin, Jin and Gatz26]. MCI was identified from the NPR in accordance with the ICD-10 (Supplementary Table S1) [Reference Winblad, Palmer, Kivipelto, Jelic, Fratiglioni, Wahlund and Nordberg4].
Covariates
The following variables, associated with ADHD and/or dementia in previous research, were addressed as covariate sets: (a) sex [Reference Fayyad, Sampson, Hwang, Adamowski, Aguilar-Gaxiola and Al-Hamzawi1,Reference Ruitenberg, Ott, van Swieten, Hofman and Breteler11] and birth year [Reference Ruitenberg, Ott, van Swieten, Hofman and Breteler11]; (b) educational attainment (highest level of education by age 50 with categories: compulsory education ≤9 years/upper secondary/postsecondary/postgraduate) [Reference Fayyad, Sampson, Hwang, Adamowski, Aguilar-Gaxiola and Al-Hamzawi1,Reference Norton, Matthews, Barnes, Yaffe and Brayne13]; (c) metabolic disorders: hypertension, T2D, and obesity [Reference Nigg2,Reference Norton, Matthews, Barnes, Yaffe and Brayne13]; (d) sleep disorders (organic and nonorganic) [Reference Nigg2,Reference Shi, Chen, Ma, Bao, Han and Wang15]; (e) head injuries [Reference Nigg2,Reference Li, Li, Li, Zhang, Zhao and Zhu16]; (f) psychiatric disorders: depression, anxiety, SUD, and bipolar disorder; [Reference Fayyad, Sampson, Hwang, Adamowski, Aguilar-Gaxiola and Al-Hamzawi1,Reference Nigg2,Reference Norton, Matthews, Barnes, Yaffe and Brayne13,Reference Zilkens, G Bruce, Duke, Spilsbury and B Semmens14] (g) other developmental disorders (i.e., autism spectrum disorder, intellectual disability, motor disorders, and learning disorders) [Reference Selinus, Molero and Lichtenstein17]. We extracted a first diagnosis of included disorders and head injuries, coded according to the ICD-8/9/10 from the NPR (Supplementary Table S1) and acquired at any age.
Statistical analysis
We used a Cox proportional hazards model to test the association of ADHD with dementia/MCI, by comparing the rate of having these disorders between individuals with and without ADHD from the age of 50 years, with attained age as the underlying timescale. Since ADHD is defined according to the ICD-10 criteria by a childhood-onset of the symptoms [27], we considered individuals receiving a diagnosis of ADHD during the study period as exposed from the start of follow-up (i.e., from age 50). Hazard ratio (HR) was estimated with 95% confidence intervals (CI), with adjustment for sex and birth year, followed by separate adjustments for each covariate set.
We used Wilcoxon two-sample test to evaluate differences in median age of dementia/MCI diagnosis, and median age in 2013 between individuals with and without ADHD. When distribution of frequencies for covariates differed between groups with and without ADHD, we used logistic regression models to inspect associations between ADHD and the covariates, presented as odds ratios (OR) with 95 % CIs and adjusted for sex and birth year.
To investigate whether the case definition of ADHD based on a confirmed diagnosis would affect the results, we conducted a sensitivity analysis by excluding cases with ADHD medication prescription only.
Main and sensitivity analyses were presented collapsed across sex and stratified by sex. We used SAS version 9.4 (SAS Institute, Inc.) for data management and R version 3.6.1 for data analyses.
Results
Descriptive characteristics of the study population
The study population covered 3,591,689 individuals born between 1932 and 1963 (Figure 1). We identified 9,532 (0.3%) people diagnosed with ADHD diagnosis/medication prescription, among which 5,168 (54.2%) were male (Table 1). Median age of ADHD diagnosis/medication prescription was 52 years (IQR 48─57). By the end of the follow-up, 55,194 (1.5%) individuals developed dementia, and 23,507 (0.6%) developed MCI, with a median follow-up time of 14.13 years for both dementia and MCI. Median age at diagnosis for dementia and MCI was significantly lower in individuals diagnosed with ADHD compared to those without ADHD (Table 1; Wilcoxon Two-Sample Test: for dementia, Z = −9.02, p < 0.0001; for MCI, Z = −11.55, p < 0.0001). At the end of 2013 individuals with ADHD were younger than those without ADHD (Table 1; Wilcoxon Two-Sample Test: Z = −80.03, p < 0.0001). All covariates were significantly associated with ADHD (adjusted for sex and birth year, Table 2).
Table 1. Descriptive characteristics of the study population.
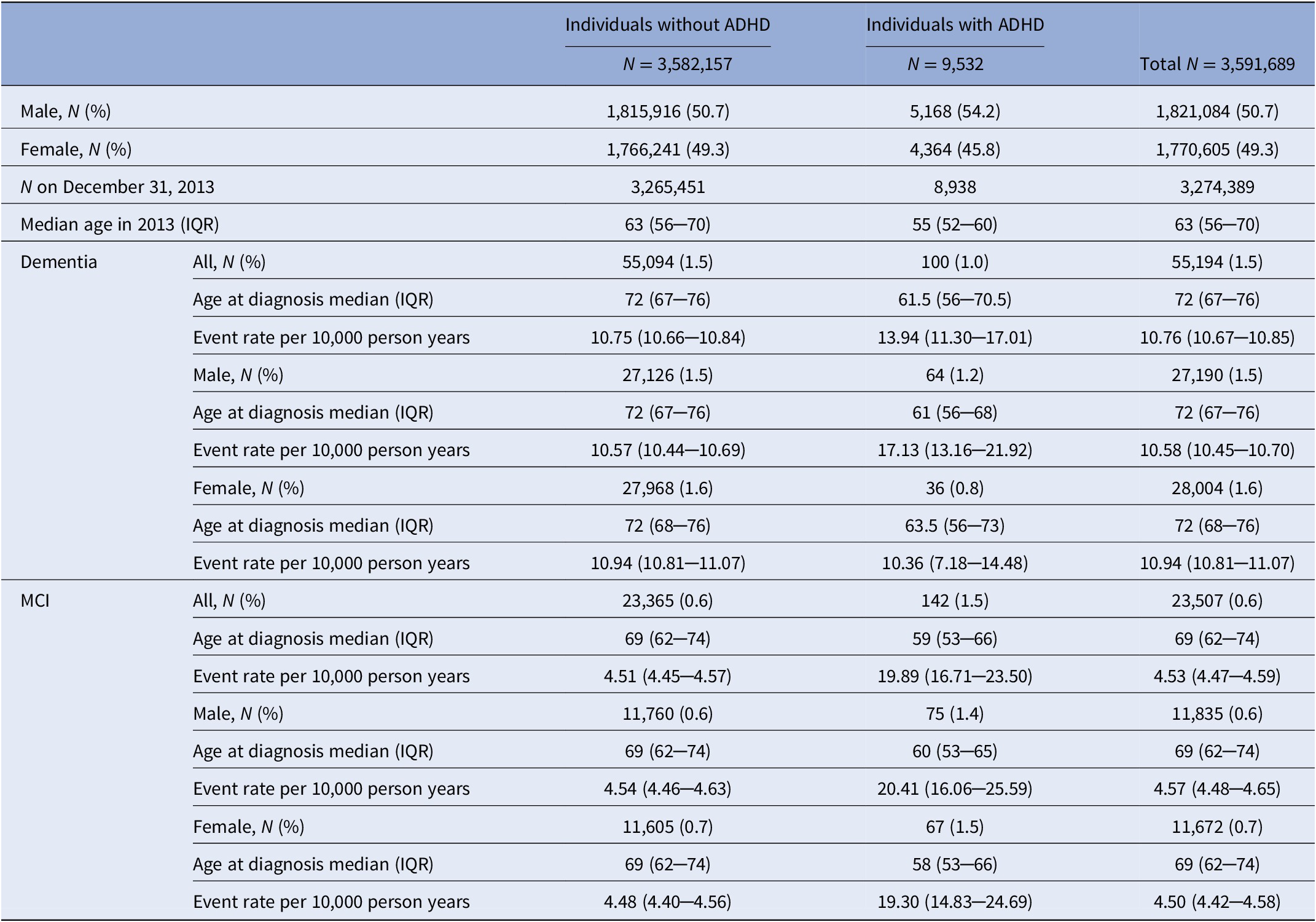
Abbreviations: ADHD, Attention-deficit/hyperactivity disorder; MCI, Mild cognitive impairment.
Table 2. Descriptive characteristics of the study population with regard to educational attainment, metabolic and sleep disorders, head injuries, psychiatric disorders, and other developmental disorders, and the associations with ADHD, presented as odds ratios (OR) with 95% confidence intervals (CI), adjusted for sex and birth year.

a Missing values for educational attainment: 212,649. These cases were deleted from the main analysis adjusted for educational attainment.
b Reference category is compulsory educational attainment.
Main findings
We found an increased risk of having both dementia (HR = 2.92, 95% CI = 2.40─3.57) and MCI (HR = 6.21, 95% CI = 5.25─7.35) in individuals with ADHD compared with individuals without ADHD, adjusting for sex and birth year (Table 3). After separate adjustments of the analysis for each considered covariate set, we observed that educational attainment, metabolic disorders, sleep disorders, head injuries and other developmental disorders had a minimal impact on the associations of ADHD with dementia/MCI (Table 3). In contrast, the adjustment for psychiatric disorders significantly attenuated the observed associations with HR = 1.62, 95% CI = 1.32─1.98, for dementia, and HR = 2.54, 95% CI = 2.14─3.01, for MCI (Table 3).
Table 3. Association between ADHD and dementia and mild cognitive impairment (MCI) as hazard ratios (HR) with 95% confidence intervals (CI) adjusted for covariates, collapsed across sex, and stratified by sex.
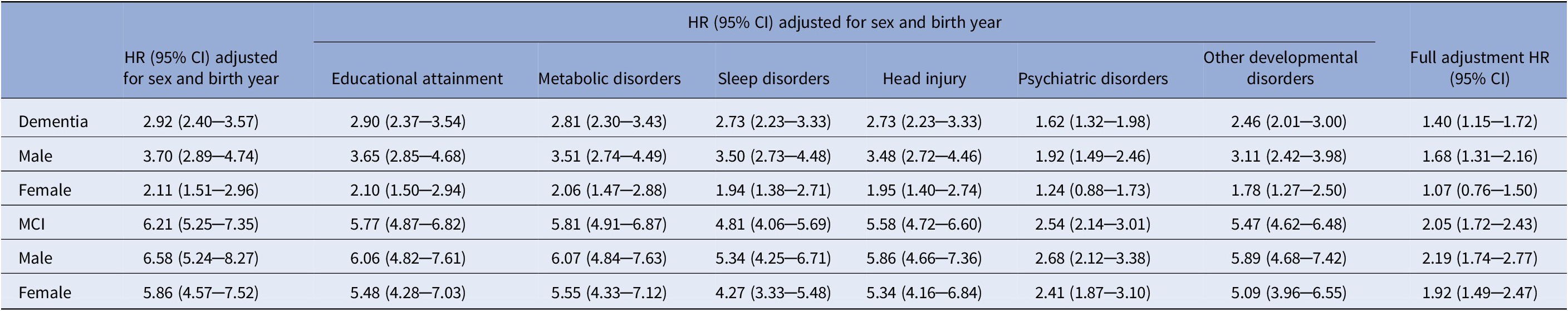
Analyses stratified by sex were not additionally adjusted for sex.
The association between ADHD and dementia across all levels of adjustments was stronger in men compared to women (Table 3), with a significant ADHD by sex interaction coefficient, 0.58, 95% CI = 0.38─0.88, p = 0.01, meaning that the risk of having dementia in women with ADHD was 42% lower than in men with ADHD. An event rate per 10,000 person years for dementia was lower in women (10.36, 95% CI = 7.18─14.48) than men (17.13, 95% CI = 13.16─21.92) with ADHD, while the event rates for men and women without ADHD were equal (Table 1). On the other hand, the risk of having MCI was not significantly different between men and women with ADHD (ADHD by sex interaction coefficient of 0.97, 95% CI = 0.69─1.35, p = 0.84).
Sensitivity analyses
We performed a sensitivity analysis to investigate whether the results were affected by the case identification for ADHD that revealed the same pattern of results across adjustments for all included covariate sets with overlapping confidence intervals, but somewhat lower associations of ADHD with dementia/MCI compared to the main analysis (Supplementary Table S2). When case identification of ADHD was based on a diagnosis only, point estimates for dementia attenuated towards the null and became nonsignificant after adjusting for psychiatric disorders.
Since the median age of ADHD and MCI/dementia diagnoses were relatively close in time and to provide further confirmation of the clinical validity of ADHD diagnoses, we conducted two post hoc sensitivity analyses, adjusted for sex and birth year, based on more sctrict case defitions of ADHD by including (a) only cases with at least two confirmed diagnoses of ADHD and (b) only cases with a primary diagnosis of ADHD. Both post hoc sensitivity analyses yielded results consistent with the main analysis (Supplementary Material, p. 4).
Discussion
In this Swedish population-based register study, we found that ADHD was associated with an increased risk for both dementia and MCI. Psychiatric disorders substantially attenuated the associations of ADHD with dementia and MCI. Additionally, we demonstrated that the association between ADHD and dementia was stronger in men than in women.
Our findings support previous studies that identified an increased risk for dementia in people with antecedent ADHD [Reference Tzeng, Chung, Lin, Yeh and Huang5–Reference Golimstok, Rojas, Romano, Zurru, Doctorovich and Cristiano7]. One study, which did not find an association [Reference Ivanchak, Abner, Carr, Freeman, Seybert and Ranseen8], applied a restrospective assessment of childhood ADHD sympoms in a small geriatric sample, while the current study used population-based, large scale data and ICD-diagnoses of ADHD. Furthermore, a recently published study that used Swedish health registers [Reference Zhang, Du Rietz, Kuja‐Halkola, Dobrosavljevic, Johnell and Pedersen28] identified an association between ADHD and Alzheimer’s disease and any dementia across generations.
Additionally, findings of the current study support a hypothesis that cumulative health-compromising factors of ADHD may affect the association between ADHD and dementia [Reference Callahan, Bierstone, Stuss and Black12]. Specifically, we found that psychiatric disorders (i.e., anxiety, depression, SUD, and bipolar disorder), highly comorbid with ADHD [Reference Fayyad, Sampson, Hwang, Adamowski, Aguilar-Gaxiola and Al-Hamzawi1,Reference Nigg2], may partially explain the associations. Midlife psychiatric disorders present significant risk factors for dementia, independently from physical comorbidities [Reference Norton, Matthews, Barnes, Yaffe and Brayne13,Reference Zilkens, G Bruce, Duke, Spilsbury and B Semmens14]. It has been proposed that ADHD symptoms could affect cognitive functioning in older age indirectly through depressive symptoms. [Reference Das, Cherbuin, Easteal and Anstey29,Reference Semeijn, Korten, Comijs, Michielsen, Deeg and Beekman30] Plausible underlying mechanisms, such as increased oxidative stress and inflammation, linked to both psychiatric disorders and dementia [Reference Kim, Nunes, Oliveira, Young and Lafer31], should be considered in future research. Future longitudinal studies are required to elucidate the timeline between the onset of ADHD, other psychiatric disorders, and dementia.
Our finding that educational attainment had a limited influence on the association between ADHD and dementia needs to be interpreted carefully. Individuals with ADHD were more likely to complete secondary education, but less likely to complete postsecondary/postgraduate education compared to those without ADHD. This is somewhat inconsistent with previous studies that have identified an association between ADHD and lower educational levels [Reference Fayyad, Sampson, Hwang, Adamowski, Aguilar-Gaxiola and Al-Hamzawi1]. A potential explanation is that older adults with low education remain undiagnosed for ADHD in Sweden. Additionally, individuals with more severe ADHD and low education may not live to an older age, due to increased mortality rates associated with ADHD [Reference Dalsgaard, Østergaard, Leckman, Mortensen and Pedersen32]. Future studies need to explore whether our findings generalize to other settings.
We found an increased risk for common metabolic (i.e., hypertension, T2D, obesity) and sleep disorders, and head injuries in individuals with ADHD. Still, these covariates only minimally affected the associations of ADHD with dementia/MCI. One study [Reference Fluegge and Fluegge6] found that metabolic dysregulation mediated the association between ADHD and dementia. However, metabolic disorders might have been underdiagnosed in our cohort, which could have attenuated their effect on the association between ADHD and dementia/MCI. Individuals with ADHD have low rates of seeking medical treatment, despite having an increased risk for overall health problems [Reference Fayyad, Sampson, Hwang, Adamowski, Aguilar-Gaxiola and Al-Hamzawi1]. Additionally, individuals with ADHD in our cohort were significantly younger at the end of the follow-up period and had lower crude prevalence estimates for T2D and hypertension, than those without ADHD. Since the rates of T2D and hypertension increase with age [Reference Nigg2], the group with ADHD in this population might have not developed these disorders to the full extent due to younger age.
Further, our findings suggest that the association between ADHD and dementia/MCI may be independent from other developmental disorders. We were unable to address other early-life and neurobiological/genetic explanations to the observed associations [Reference Callahan, Bierstone, Stuss and Black12]. Early-life adverse factors, both prenatal (i.e., fetal stress and low birth weight) and postnatal (i.e., psychosocial adversity) have been recognized as risk factors for ADHD [Reference Banerjee, Middleton and Faraone33], as well as MCI/dementia [Reference Seifan, Schelke, Obeng-Aduasare and Isaacson34]. A large genome-wide, cross-disorder study failed to find statistically significant genetic correlations between ADHD and dementia [Reference Anttila, Bulik-Sullivan, Finucane, Walters, Bras and Duncan35], but the findings of this study are inconclusive as only the overlap between common genetic variants was explored, explaining a small proportion of the heritability. Further, the current study design did not allow us to differentiate between the effect of ADHD and ADHD medication on dementia/MCI. Future research is needed to investigate early-life and neurobiological/genetic mechanisms underlying the link between ADHD and dementia, and a potential role of medication.
The association between ADHD and dementia was somewhat stronger in men, while previous studies did not address the effect of sex on the association [Reference Fluegge and Fluegge6–Reference Ivanchak, Abner, Carr, Freeman, Seybert and Ranseen8], or they found a stronger association in women [Reference Tzeng, Chung, Lin, Yeh and Huang5]. We also found higher rates of dementia in men than women with ADHD. It is possible that dementia is underdiagnosed in women with ADHD. Alternatively, as ADHD is potentially underdiagnosed in females [Reference Fayyad, Sampson, Hwang, Adamowski, Aguilar-Gaxiola and Al-Hamzawi1], it is plausible that preceding symptoms of ADHD were not recognized in some women who developed dementia.
The current study identified a prevalence of clinically diagnosed ADHD of 0.3%. This is notably lower compared to a pooled prevalence estimate based on validated scales of 2.5% in adults [Reference Simon, Czobor, Balint, Meszaros and Bitter36] and 2.2% in older adults [Reference Dobrosavljevic, Solares, Cortese, Andershed and Larsson3], but consistent with the pooled prevalence of 0.2% based on clinically diagnosed ADHD in adults older than 50 [Reference Dobrosavljevic, Solares, Cortese, Andershed and Larsson3]. Although the prevalence of ADHD declines with age [Reference Simon, Czobor, Balint, Meszaros and Bitter36], it may be underdiagnosed in older age due to (a) ADHD being misdiagnosed as age-related cognitive decline or other psychiatric conditions [Reference Pollak25], (b) inadequate diagnostic criteria for this age group [Reference Simon, Czobor, Balint, Meszaros and Bitter36], and (c) only the most severe forms of ADHD being diagnosed, and/or (4) premature mortality associated with ADHD [Reference Sun, Kuja-Halkola, Faraone, D’Onofrio, Dalsgaard and Chang37]. Further, a study from the Netherlands reported a population weighted prevalence of ADHD of 2.8% in older adults based on an ADHD diagnostic interview [Reference Michielsen, Semeijn, Comijs, van de Ven, Beekman and Deeg38], and may additionally indicate a substantial underestimation of the prevalence in the current study. Future studies using validated instruments for assessment of ADHD in large community samples of older adults are therefore needed to replicate the current findings.
Limitations
Some limitations of the current study should be addressed when interpreting its findings. The risk for MCI in people with ADHD was larger than the risk for dementia, and the median age of diagnosis for ADHD and MCI/dementia were close in time. In older adults, symptoms of ADHD, such as difficulty to organize activities, inability to sustain attention, memory problems, behavioral and psychiatric symptoms (e.g., sleep disturbances, anxiety, and depression) may resemble prodromal dementia/MCI [Reference Pollak25]. Additionally, other psychiatric conditions with similar clinical presentation may be misdiagnosed as ADHD, or vice versa [Reference Pollak25]. The question of misdiagnosis could not be fully addressed due to limited information on dementia/MCI diagnoses provided in the registers. We partially addressed this issue by re-running the analyses including only cases with at least two established diagnoses and only cases with a primary diagnosis of ADHD, which confirmed our main findings. Future validation studies of MCI and adult ADHD classification criteria in Swedish registries are warranted.
We could not investigate separate associations between ADHD and dementia subtypes (i.e., AD, vascular dementia, dementia with Lewy bodies, frontotemporal dementia) due to a small number of individuals with ADHD diagnosed with each subtype. It is plausible that different subtypes of dementia, having distinct etiological pathways, are differentially associated with ADHD and comorbid disorders. Additionally, ADHD and some subtypes of dementia might only share similar clinical presentation; however, underlying mechanisms could be distinct and unrelated [Reference Callahan, Bierstone, Stuss and Black12]. For instance, previous research has found no or very little evidence for an association between AD and ADHD [Reference Tzeng, Chung, Lin, Yeh and Huang5–Reference Ivanchak, Abner, Carr, Freeman, Seybert and Ranseen8]. Future studies should focus on building a more comprehensive model of possible associations between ADHD and different subtypes of dementia, and on investigating the role of specific comorbid disorders for each association.
We did not cover behavioral outcomes (i.e., smoking, diet, exercise, and treatment-seeking behaviors) of ADHD that could affect the association between ADHD and dementia [Reference Callahan, Bierstone, Stuss and Black12], since this information was not available in the registers. Future studies should aim to include relevant behavioral variables.
The median age of the study population at the end of the follow-up was 63 years, while the risk for dementia substantially increases after the age of 65 [Reference Ruitenberg, Ott, van Swieten, Hofman and Breteler11], thus, we might have mostly captured early-onset cases of dementia. Further follow-up of these individuals to a more advanced older age would be necessary to explore the association with later-onset dementia. The use of a relatively young cohort may explain our finding of similar event rates of dementia for men and women without ADHD, which is inconsistent with reported higher rates of dementia in women in the general population, since potential sex differences could be present after age 90 [Reference Ruitenberg, Ott, van Swieten, Hofman and Breteler11]. Further, the number of dementia cases were almost twice the number of MCI cases and only 9,908 individuals had both MCI and dementia. It is plausible that some individuals tend to seek medical help only when they experience more severe cognitive symptoms due to social stigma [Reference Phillipson, Magee, Jones, Reis and Skaldzien39]. Because of the younger age of the cohort, the remaining individuals with MCI might develop dementia in the future.
Conclusions
In conclusion, the present study suggests that there is an increased risk for dementia and MCI in individuals with ADHD, with the association between ADHD and dementia being stronger in men than in women. These associations substantially attenuate after controlling for comorbid psychiatric disorders, while common metabolic disorders and sleep disorders, head injuries, educational attainment, and other developmental disorders, have a limited impact. Since this is a notably understudied topic, more research is of crucial importance to confirm our findings and investigate underlying mechanisms. Specifically, future neurobiological and epidemiological studies are needed, which should cover different subtypes of dementia, behavioral outcomes of ADHD, and a broader range of socioeconomic variables and comorbid health conditions.
Supplementary Materials
To view supplementary material for this article, please visit http://doi.org/10.1192/j.eurpsy.2021.2261.
Data Availability Statement
The Public Access to Information and Secrecy Act in Sweden prohibits individual level data to be publicly available. Researchers who are interested in replicating this study can apply for individual level data at Statistics Sweden: www.scb.se/en/services/guidance-for-researchers-and-universities/.
Acknowledgments
This project has received funding from the European Union’s Horizon 2020 research and innovation program under the Marie Skłodowska-Curie grant agreement No 754285. E.D.R. was supported by grant 2019-01172 from the Swedish Research Council for Health, Working Life, and Welfare, and by grant 2019-00482 from Fredrik & Ingrid Thurings Stiftelse. Z.C. was supported by grants from the Swedish Council for Health, Working Life and Welfare (2019-00176) and the Karolinska Institutet Research Foundation. H.L. acknowledge financial support from the Swedish Research Council (2018-02599) and the Swedish Brain Foundation (FO2018-0273).
Author Contributions
M.D. and H.L. conceptualized and designed the study. M.D. conducted literature search, analyzed the data, and drafted the manuscript. L.Z., E.D.R., and M.G.A. assisted with the study design and data analysis. H.L. provided supervision. All authors contributed to the interpretation of results, reviewing, and editing of the final manuscript, and had responsibility in deciding to submit the manuscript for publication. M.D. attests that all listed authors meet authorship criteria and that no other individuals meeting the criteria have been omitted.
Ethical Standards
This study was approved by the regional ethics review board in Stockholm, Sweden (2013/862-31/5).
Conflicts of Interest
E.D.R. has served as a consultant for Shire Sweden AB (fully owned subsidiary of Takeda Pharmaceutical Company Limited) outside the submitted work. In the past year, Dr. Faraone received income, potential income, travel expenses continuing education support and/or research support from Takeda, OnDosis, Tris, Otsuka, Arbor, Ironshore, Rhodes, Akili Interactive Labs, Sunovion, Supernus, and Genomind. With his institution, he has U.S. patent US20130217707 A1 for the use of sodium-hydrogen exchange inhibitors in the treatment of ADHD. He also receives royalties from books published by Guilford Press: Straight Talk about Your Child’s Mental Health, Oxford University Press: Schizophrenia: The Facts and Elsevier: ADHD: Non-Pharmacologic Interventions. He is Program Director of www.adhdinadults.com. H.L. has served as a speaker for Evolan Pharma and Shire/Takeda and has received research grants from Shire/Takeda; all outside the submitted work. All other authors have no competing interests.







Comments
No Comments have been published for this article.