Diabetes has recently become a major public health issue in China, and its prevalence continues to increase(Reference Pan, Yang and Li1, Reference Gu, Reynolds and Duan2). According to the latest national study in China, the age-standardised prevalence of total diabetes and impaired glucose regulation (IGR) was 9·7 and 15·5 %, respectively(Reference Yang, Lu and Weng3). IGR refers to a metabolic status between normal glucose homeostasis and diabetes(Reference Alberti and Zimmet4), and is associated with an increased risk of type 2 diabetes(Reference Nathan, Davidson and DeFronzo5, Reference Kim, Pavkov and Looker6) and CVD(Reference Levitzky, Pencina and D'Agostino7–Reference Bonora, Kiechl and Willeit9). Therefore, it is necessary to initiate effective interventions for IGR patients to promote the restoration of normal glucose tolerance and prevent diabetes.
Lifestyle changes in diet, moderately intense exercise and modest weight loss have consistently demonstrated benefits in preventing or delaying the progression of IGR to diabetes(Reference Knowler, Barrett-Connor and Fowler10–Reference Eriksson and Lindgarde13). Meal replacement is a relatively new strategy for weight loss and glycaemic control in diabetic patients(Reference Sun, Wang and Chen14–Reference Wadden, West and Neiberg16). However, limited information is available on the impact of interventions that seek to return those with IGR to normal glucose regulation (NGR).
In the present study, we performed a randomised, prospective study to assess the impact of a structured intervention, which included a daily low-glycaemic index meal replacement and lifestyle intervention, on glycaemic and body-weight control among subjects with IGR in China.
Methods
Subjects
Through health-screening examinees, eighty-eight IGR subjects were recruited from two community health service centres in urban districts of Shanghai. Eligibility criteria were as follows: ≥ 25 years old; a BMI ≥ 18·5 kg/m2; a plasma glucose concentration of 5·6–6·9 mmol/l in the fasting state, or 7·8–11·0 mmol/l 2 h after a 75 g glucose load, based on the 2003 American Diabetes Association criteria for IGR(Reference Genuth, Alberti and Bennett17). Subjects were excluded if they had a history of stroke, CHD, malignancies in the past 5 years, or suspected disease of the liver, pancreas or kidney. In addition, it should be noted that all enrollees had not taken insulin, hypoglycaemic or weight-reduction agents at any time in the 3 months previous to the beginning of the study. The present study was conducted according to the guidelines of the Declaration of Helsinki, and all procedures were approved by the ethics committee of Huadong Hospital, Fudan University, Shanghai, China. Written informed consent was obtained from all subjects.
Study protocol
This was a 12-month randomised prospective study. Subjects were evaluated in the Clinical Nutrition Center of Huadong Hospital. Eligible subjects were randomly assigned to either the intervention (n 46) or control (n 42) group using a random allocation schedule. Randomisation was stratified by age and the level of fasting plasma glucose.
At baseline, subjects in both groups received an educational lecture on balanced diet, regular exercise and behavioural strategies to control blood glucose. All subjects were encouraged to follow the ‘2007 Chinese guidelines for the management of type 2 diabetes’(18) and ‘Dietary Guidelines for Chinese’(19) to maintain healthy diet habits, and to increase physical activity.
Subjects in the intervention group received a daily meal replacement and intensive lifestyle intervention during the first 3 months of the study. The low-glycaemic meal replacement product (Pure Grain Company) provided 950 kJ (227 kcal)/d. It was used to replace breakfast food items such as milk, soyamilk, rice soup or congee at the morning meal. About 75 % of breakfast was replaced by the meal replacement. The primary components of the product were soyabean, oat and flaxseed in a powdered mixture. The nutrient profile of the meal replacement is outlined in Table 1. Compliance to the product intake was assessed weekly by the return of empty formula containers and review of the product intake forms by the study dietitians at weekly follow-up.
Table 1 The composition of the meal replacement product

The intensive lifestyle intervention consisted of: (1) individualised eating instructions covering concepts, food exchanges and low-glycaemic foods; (2) an assessment of the current physical activity of the subjects, and recommended moderate exercise, such as walking 30–40 min/d. Subjects met with a study physician every week for a medical evaluation that included an assessment of adverse events, and a review of blood glucose measurements. After 3 months, follow-up visits were scheduled monthly to obtain data on body weight, blood glucose, blood pressure and diet.
Analytical techniques
Overnight (10–12 h) fasting venous blood samples were collected at baseline and at 12 months post-treatment. A standard 75 g oral glucose tolerance test was also performed, and 2 h post-load glucose samples were obtained. Fasting plasma total cholesterol, HDL- and LDL-cholesterol, TAG, fasting plasma glucose, and 2 h plasma glucose were measured by an enzymatic immunoassay (Roche Automatic Analyzer modular P800; Roche Diagnostics GmbH). Glycated Hb (HbA1c) was assessed with an immunosuppressive turbidimetric assay (Hitachi Automatic Analyzer 7600-010; Hittachi High Technologies Corp.).
Body weight, height, waist and hip circumference, and blood pressure were measured according to standard protocols. Body composition was assessed by direct segmental multi-frequency bioelectrical impedance analysis using a body composition analyser (Inbody720; Biospace Corporation). BMI was calculated as weight divided by height squared (kg/m2).
Statistical analyses
Data were analysed according to an intention-to-treat principle and included all eighty-eight subjects who were randomly assigned. Primary outcomes were 12-month changes in body weight and 2 h plasma glucose. Data from baseline and 12-month changes from baseline were compared between the treatment groups using Student's t test or the Wilcoxon rank-sum test for two independent samples. A χ2 test was used to compare categorical data. The relationship between body-weight loss and changes in 2 h plasma glucose was examined by least-squares linear regression. P values ≤ 0·05 were considered significant. Analyses were performed with SAS 9.1.3 (SAS Institute Inc.) and STATA 10.0 (Stata Corp) software.
Results
Of the eighty-eight subjects enrolled in this 1-year study, seven (two in the control and five in the intervention groups) discontinued participation before completion for various reasons, including interference with work schedule, travel abroad, moved away and personal reasons. Finally, a total of eighty-one subjects (92 %) completed the study. Table 2 shows the characteristics of each group at baseline. The numbers of subjects taking hypertension and lipid medications at baseline were not different between the groups (data not shown).
Table 2 Baseline characteristics of the subjects in the intervention and control groups (Mean values and standard deviations)

HbA1c, glycated Hb.
* Difference assessed using Student's t test.
† χ2 test.
Body-weight loss
At 12 months, the decrease in body weight, BMI, body fat, and waist and hip circumference of the intervention group was significantly higher than that of the control group (P< 0·05; Table 3). In the intervention group, 74 % of the weight loss was due to the loss of fat mass.
Table 3 Comparison of endpoints at 1 year expressed as changes from baseline (Mean values with their standard errors)

HbA1c, glycated Hb.
* Changes from baseline to 1 year were analysed between the treatment groups using Student's t test.
† χ2 test.
‡ Wilcoxon rank-sum test.
Blood pressure and lipids
Systolic and diastolic blood pressures were similar between these two groups at baseline and did not change significantly over the 12-month period (Table 3). At the end of the study, LDL-cholesterol decreased (0·18 (sem 0·12) mmol/l) in the intervention group, but increased (0·33 (sem 0·13) mmol/l) in the control group (P< 0·05). There were no significant differences in total cholesterol, TAG or HDL-cholesterol between these two groups (Table 3). In addition, no differences were detected with respect to hypertension and hypolipidaemic medications between these two groups at 1 year.
Glycaemic control
At the end of the study, glucose and HbA1c levels were significantly reduced in the intervention group compared with the control group (P≤ 0·02 for both; Table 3). We further evaluated the relationship between long-term body-weight loss and changes in 2 h plasma glucose: each subject's 12-month body-weight change was plotted against the corresponding 12-month change in 2 h plasma glucose. For the observed range of body-weight changes (4·3 to − 9·0 kg), there was a significant positive linear association between change in body weight at 1 year and change in 2 h plasma glucose (r 0·32, P< 0·01; Fig. 1). A 5 kg decrease in body fat at 1 year was associated with a decrease of 1·49 mmol/l in 2 h plasma glucose. Subjects in the two groups were then divided into three subgroups according to body-weight change. The incidence of weight loss (body-weight decrease ≥ 1·0 kg) was significantly greater in the intervention group than in the control group (P= 0·02; Table 4). After 1 year, the rate of improvement in glucose tolerance (2 h plasma glucose < 7·8 mmol/l) was 56·1 % in the intervention group and 15·0 % in the control group (P< 0·001; Table 4).
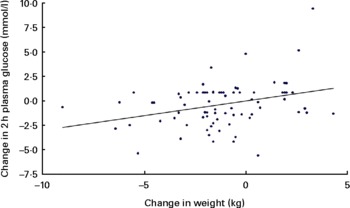
Fig. 1 Plot of change in body weight v. change in 2 h plasma glucose at 1 year. The fitted regression equation was (2 h plasma glucose change in mmol/l) = 0·30 (body-weight change in kg) − 0·01; r 0·32, P= 0·004.
Table 4 Incidence of normal glucose regulation (NGR) and type 2 diabetes, changes in body weight, and improvement in glucose tolerance in two groups during 1-year follow-up

OGTT, oral glucose tolerance test.
* χ2 test.
† Fisher's exact test.
‡ Body weight decreased: decreased by 1·0 kg or more; unchanged: within 1·0 kg change; increased: increased by 1·0 kg or more.
§ 2 h plasma glucose < 7·8 mmol/l.
Changes in the incidence of normal glucose regulation and type 2 diabetes
Subjects in the intervention group reverted to NGR at a rate of 39·0 % during the 1-year period, and 14·6 % progressed to diabetes. In the control group, the incidence of NGR and diabetes during 1 year was 7·5 and 17·5 %, respectively. The incidence of NGR in these two groups was significantly different (P= 0·001; Table 4).
Discussion
The present study was performed to evaluate the effect of a lifestyle intervention programme and a daily low-glycaemic index meal replacement on glycaemic and body-weight control in subjects at risk of developing type 2 diabetes mellitus. The main finding of the present study was that glucose tolerance and body weight were significantly controlled in the intervention group after a 1-year intervention. Consistent with these improvements, the incidence of regression from IGR to NGR in the intervention group was significantly higher than that in the control group.
Weight control effort was an important component of the clinical management of diabetes. Previous studies have suggested that multiple strategies, including lifestyle intervention(Reference Unick, Beavers and Jakicic20), meal replacements(Reference Heymsfield, van Mierlo and van der Knaap21) and contact with investigators(Reference Svetkey, Stevens and Brantley22), may contribute to successful weight loss. In the present study, average weight loss was − 1·75 kg in the intervention group and − 0·55 kg in the control group. This is somewhat less than that found in the Diabetes Prevention Program (DPP)(Reference Knowler, Barrett-Connor and Fowler10) and the Diabetes Prevention Study (DPS)(Reference Tuomilehto, Lindstrom and Eriksson12). However, our population was less obese (BMI 26·3 kg/m2) than the populations in the DPS and DPP, in which the mean BMI was 31·2 and 33·9 kg/m2, respectively. As of this writing, it has been 9 months since the end of the meal replacement intervention, and it is inevitable that many subjects would have regained weight after their initially successful weight loss. There were about 17·1 % subjects who regained weight in the intervention group, and 37·5 % subjects in the control group (P= 0·04). It should be noted that interventions in the present study demonstrated benefits in preventing subjects regain the weight they lost on an intensive intervention phase. A previous study has suggested that, even with weight regain, losing weight still has long-lasting benefits in type 2 diabetes(Reference Feldstein, Nichols and Smith23). Few studies have quantified the effect of sustained weight loss on glycaemic control(Reference Redmon, Raatz and Reck24). Based on the analysis of IGR subjects in the present study, a 1 kg weight loss at 1 year would be expected to result in a considerable decrease in 2 h blood glucose concentration.
Besides the weight reduction, there were significant reductions in 2 h plasma glucose and HbA1c. Subjects in the intervention group achieved a mean reduction in 2 h plasma glucose (1·24 mmol/l) and HbA1c (0·12 %) after 1 year. The incidence of type 2 diabetes increases in a graded manner with increasing 2 h blood glucose concentrations(Reference Edelstein, Knowler and Bain25). In the DPP, every kg of body-weight loss was associated with a 16 % reduction in diabetes risk(Reference Hamman, Wing and Edelstein26). Thus, the decrease in blood glucose and body weight found in the present study should lead to a decreased risk of progression to diabetes. However, the incidence of diabetes at the end of the present study between the two groups was similar. The difference was that subjects in the intervention group had an increase rate of progression from IGR to NGR.
The incidence of diabetes at the end of the study in our IGR patients in the control group was 17·5 % – a much higher incidence than suggested by epidemiological data (11·74 %)(Reference Jia, Pang and Chen27) or the DPS (6 %)(Reference Tuomilehto, Lindstrom and Eriksson12), but similar to the Da Qing study (17·2 %)(Reference Pan, Li and Hu28). However, the incidence of diabetes in the intervention group was higher than the Da Qing study (14·6 v. 11·4 %, respectively). These discrepancies may result from the long-term follow-up after the intensive intervention in the present study and the difference in intervention strategies.
Research data on the regression rate from IGR to NGR are limited. In diabetes prevention studies with IGR patients, less often recognised were the 20–50 % of participants who returned to NGR(Reference Knowler, Barrett-Connor and Fowler10, Reference Eriksson and Lindgarde13, Reference Chiasson, Josse and Gomis29, Reference Gerstein, Yusuf and Bosch30). Therefore, the risk reduction for type 2 diabetes may reside in restoring NGR rather than in the maintenance of IGR(Reference Perreault, Kahn and Christophi31). NGR may be attained through weight loss and additional aspects of intensive lifestyle modifications. In the DPP, about 25 % of participants returned to NGR in the lifestyle intervention group during the year of the study. In our programme, about 39·0 % of patients were restored to NGR in the intervention group, which was significantly higher than that in the control group. In addition, subjects in the intervention group had a higher rate of body-weight decrease and 2 h plasma glucose improvement. Thus, restoration of NGR should be attributed to the decrease in 2 h glucose concentration and body weight.
The contribution that each component (regular contact, meal replacement and lifestyle advice) of this programme made to the outcome was not independent of each other. The major research limitation of the present study was the failure to assess the true effect of meal replacement in the progression to NGR. Future studies should include sub-studies in order to be able to fully explain the findings.
In conclusion, the present study has illustrated that treatment with regular contact, lifestyle advice and low-glycaemic index meal replacements was an effective means of improving weight loss and glycaemic control, and promoting the conversion from IGR to NGR in IGR patients over a 1-year period.
Acknowledgements
The present study was funded by a research project of the Science and Technology Commission of Shanghai Municipality (07ZR14036) and the Public Health Bureau of Shanghai (2007168). The authors acknowledge the assistance of all volunteers in the present study, plus the support of the Shanghai Caojiadu Community Medical Care Center and Jing'an Temple Community Medical Care Center. J.-Q. S., M. C. and Y.-Q. C. discussed the core ideas of the protocol design and/or handled the execution of the study. H. X., W.-J. S., Y.-F. L., J.-J. J., W. S., A.-F. C. and Q.-R. T. were responsible for patient recruitment, visit and/or sample collection. D.-F. X. and J.-Q. S. were involved in the data analysis and interpretation, literature review and drafting of the manuscript. All authors read, edited and approved the final manuscript. The authors declare that there are no conflicts of interest.







