Introduction
Maternal malnutrition during pregnancy adversely affects maternal health and fetal development, which may result in intrauterine growth restriction (IUGR) and may also be associated with an increased risk of health complications in the offspring during adulthood. Reference Belkacemi, Nelson, Desai and Ross1,Reference King2 In underdeveloped countries, malnutrition typically results from low- or poor-quality protein dietary content, leading to overall sufficient calorie intake but inadequate nutrient consumption. In a nonhuman primate (NHP) model of gestational protein restriction (PR), we previously reported the adverse effects of a 50% reduction in maternal dietary protein on pregnancy outcomes. Our data demonstrated a decrease in placental perfusion, no change in amniotic fluid volume, but an approximately 20% decrease in total protein levels in the amniotic fluid of protein-restricted animals. Additionally, although there was no adverse impact of PR on conception, we demonstrated an increased risk of early pregnancy loss. Reference Roberts, Lo and Lewandowski3 Upon establishment of this model, maternal consumption, assessed by daily food weighing and monitoring quantified throughout, and for 2 weeks following, the transition from control to 50% protein-reduced chow, showed a maintenance of consumed calories. Reference Kirigiti, Frazee and Bennett4 This NHP model mimics the human scenario of reasonable total intake but significant protein deficiency. It provides the opportunity to examine the consequences of maternal malnutrition on placental nutrient transport and regulation of fetal supply and developmental cues.
The mechanisms linking maternal nutrition and fetal outcomes are multifactorial and complex, with the placenta playing a central role as the mediator of maternal-fetal exchange. Over the course of gestation, inadequacy in maternal nutrient supply undoubtedly alters the ability to meet fetal growth demands. Importantly, timing of maternal dietary deficiency is a key determinant of pregnancy outcome. Many animal studies modify diet for a specific portion of gestation, which provides insight into vulnerable periods of fetal development, Reference Coan, Vaughan and Sekita5–Reference Pantham, Rosario and Weintraub11 but is less reflective of the human situation where mothers diet typically remains relatively similar prior to and throughout pregnancy, especially if food is scarce. This was the basis for our model design of pre-conception and chronic gestational PR. An additional consideration is the changing fetal requirements across gestation due to increasing growth needs, with placental resource allocation influenced by both fetal demand cues and substrate availability in the maternal supply. Reference Sferruzzi-Perri, Lopez-Tello, Fowden and Constancia12 Unsurprisingly, human and animal model studies of IUGR demonstrate a decrease in the activity and expression of nutrient transporters in the placenta Reference Pantham, Rosario and Weintraub11,Reference Malandro, Beveridge, Kilberg and Novak13,Reference Norberg, Powell and Jansson14 with evidence of altered fetal nutrient transport capabilities across the placenta. However, there are many aspects to consider, and the complexity of placental regulation makes in vivo functional studies challenging. Post-delivery in vitro assessments of placental tissue can help to address this knowledge gap, and various functional assays have been developed for this purpose. Reference Greenwood, Sibley, Soares and Hunt15 Taurine (2-aminoethanesulfonic acid) is an essential amino acid in pregnancy as the fetus and placenta lack the synthetic enzymes to generate its precursor, cysteine. Reference Gaull, Sturman and Raiha16 Thus, placental taurine transfer from the maternal blood supply must meet fetal demand Reference Ditchfield, Desforges and Mills17 , and for many years, taurine uptake has been used as an in vitro assessment of placental transport capacity. Reference Greenwood, Sibley, Soares and Hunt15,Reference Desforges, Ditchfield and Hirst18–Reference Desforges, Whittaker, Farmer, Sibley and Greenwood20 Taurine has various physiological functions in the human placenta, including regulation of trophoblast cell volume, proliferation, apoptosis, and cytoprotection. Reference Desforges, Parsons, Westwood, Sibley and Greenwood19 Additionally, sufficient taurine availability is essential for normal fetal development, with deficiency resulting in compromised fetal growth and dysfunction within several major organ systems. Reference Sturman21,Reference Sturman22 Thus, this important amino acid provides a good functional readout of the capacity of the placenta to adapt to protein deficiency in our model.
The placenta is a highly metabolically active endocrine organ that receives developmental and functional cues from both the maternal environment and the fetus, in addition to producing its own growth hormones and regulators. One key signaling molecule is the angiogenic stimulator, placental growth factor (PLGF). PLGF is highly expressed in pregnancy, and aberrant concentrations are associated with poor obstetric outcomes such as fetal growth restriction and preeclampsia in women. Reference Hayes Ryan, McCarthy, O’Donoghue and Kenny23 The insulin/insulin-like growth factor (IGF) system is another central growth regulator with the peptide hormones IGF-1 and IGF-2 mediating a range of metabolic functions in the developing fetus and placenta. Reference Hiden, Glitzner, Hartmann and Desoye24 IGF plays an important signaling role in placental growth, transport, trophoblast invasion, and placental angiogenesis. Reference Nayak and Giudice25 Perturbations in the IGF signaling system are reported in several experimental animal models of protein deficiency. Reference Kavitha, Rosario and Nijland8,Reference Constancia, Hemberger and Hughes26,Reference Muaku, Beauloye and Thissen27
Given the importance of blood flow to placental function, and the suggestion of reduced substrate availability for protein synthesis, we sought to investigate the impact of maternal PR on placental function and regulation of development. In our initial studies with the NHP model of gestational PR Reference Roberts, Lo and Lewandowski3 , animals were delivered naturally at term (~168 d in the rhesus macaque) and therefore placental tissue studies were not feasible. In the current animal cohort, we examine placental function in tissue obtained in the mid-third trimester (at gestational day 135) at a time when the placenta should be fully functional and supporting the exponential fetal growth phase, and prior to any early pregnancy loss that may have resulted from reduced protein availability. We performed an in vitro functional uptake assay to assess placental taurine transport in protein-deficient animals. Additionally, we quantified the expression of PLGF, IGF-1, and IGF-2 in placental protein homogenate from control and PR-fed animals. As blood flow is critical to maternal-fetal exchange, we hypothesized that maternal PR during pregnancy would lead to impaired placental taurine uptake and altered growth factor concentrations.
Method
Animal model
All protocols were approved by the Institutional Animal Care and Use Committee of the Oregon National Primate Research Center (ONPRC), and guidelines for humane animal care were followed. Adult rhesus macaques (Macaca mulatta) were maintained on one of three diet regimes: 1) control chow diet consisting of 26% protein (CON), 2) a protein-modified diet containing 17% protein (a 33% reduction from control; PR33), or 3) 13% protein content (a 50% reduction from control; PR50). All diets were matched for vitamin and micronutrient content, with the calorie content deficit made up with additional carbohydrates in the two PR diets (manufactured by TestDiet, St. Louis, MO). Reference Kirigiti, Frazee and Bennett4 This model was established 2 years prior to the current study with animals chronically maintained on their respective diets throughout. Breeding occurred each year, and this was the third round of pregnancies in this animal cohort. Males used for breeding were maintained on the same diet as females. Animals were indoor, pair housed with pregnancies generated by time-mated breeding. In brief, hormone levels were monitored by daily blood sampling, and females were paired with a male several days prior to the estradiol surge and then separated the day after peak levels were observed. Day 1 of gestation was considered to be 48 h post-estradiol surge. This timed-mating scheme allows gestational age to be determined within 24 to 48 h of conception.
Tissue collection
To avoid pregnancy loss due to early delivery, animals were delivered by cesarean section in the mid-third trimester, on gestational day 135. Standard hysterotomy surgery was performed by trained personnel in the ONPRC surgical unit with animals maintained under general anesthesia, and post procedural care guidelines were observed. At delivery, placental tissue was collected, weights and measures recorded, with villous tissue dissected and processed for protein extraction, and fresh tissue explant studies. Full thickness tissue sections were formalin-fixed and paraffin-embedded for histological analysis. Fetal weights and measures were recorded.
3H taurine uptake
Uptake of 3H-taurine into fresh villous explants was measured in control and Na+-free Tyrode’s buffer over a 60 min time course as previously described in detail. Reference Greenwood, Sibley, Soares and Hunt15 In brief, ~2 mm3 villous tissue pieces were tied to specially designed hooks that facilitate consistency with replicates and quick transfer between incubation solutions. After an initial pre-incubation at 37°C, fragments were washed prior to incubation with radiolabeled 3H-taurine for a time course of 5 to 60 min, washed, and lysed overnight in H2O to release 3H-taurine taken up by the tissue, followed by overnight incubation in 0.3 M NaOH to lyse the tissue for protein determination. Taurine activity was determined as the Na-dependent fraction of 3H-taurine uptake expressed as fmol/mg tissue.
Placental protein preparation
Protein was extracted from villous tissue following homogenization in lysis buffer containing 20 mM Tris (pH 7.5), 1 mM ethylenediaminetetraacetic acid, 1 mM ethylene glycol tetraacetic acid, 20 mM sodium fluoride, 0.15 M sodium chloride, 0.5% Nonidet P-40, 0.5% Triton X-100, 200 µM sodium orthovanadate, 2 µM leupeptin, 5.8 µM pepstatin, 200 µM 4-(2-aminoethyl) benzenesulfonyl fluoride hydrochloride, and 5 µM N-tosyl-L-lysine chloromethyl ketone. Homogenate was centrifuged for 5 min at 20,000 × g to remove cell debris from the placental samples. The pellet was discarded, and supernatant was stored at −80°C until use. Protein quantification was determined by bicinchoninic acid assay (BioRad Laboratories, Hercules, CA).
Protein analysis by western blotting
Protein samples were heat-denatured at 95°C for 5 min in Laemmli buffer (125 M Tris, 4.1% sodium dodecyl sulfate, 40 mM urea, 20% glycerol, 0.002% bromophenol blue) and separated on 4–20% Tris-glycine pre-cast gels (Invitrogen) using a mini-gel electrophoresis system (Bio-Rad Laboratories) at 35 mA per gel for 80 min. Protein was transferred to nitrocellulose membranes using the iBlot2 system (Life Technologies). Equal protein loading and transfer efficiency were assessed using Ponceau S staining (Sigma-Aldrich). Following transfer, membranes were blocked for 2 h with Tris-buffered saline/0.1% Tween 20 (polyoxyethylene sorbitan monolaurate, TBST) containing non-fat milk protein (Mid-America farms).
Nitrocellulose membranes were incubated with a rabbit anti-TauT antibody (Abcam ab196821), overnight at 4°C in 1% BSA prepared in TBST. Following primary antibody incubation, the membranes were washed four times for 5 min each in TBST prior to incubation with a horseradish peroxidase (HRP)-conjugated goat anti-rabbit secondary antibody (Abcam ab97051) diluted 1:10,000 in TBST containing 5% non-fat milk protein for 1 h at room temperature. Signal was detected using enhanced chemiluminescence detection (Pierce), and images were acquired on a Fluorchem M digital darkroom system (ProteinSimple, San Jose, CA). To normalize protein loading, membranes were later stripped using Re-blot solution (Chemicon International) and incubated with anti-α-actin antibody (1:1000; Abcam ab8224) in TBST containing 5% non-fat milk protein, followed by a HRP-conjugated donkey anti-mouse secondary antibody (Santa Cruz Biotech sc-2314). Protein band intensity was determined using scanning densitometry.
Protein analysis by enzyme-linked immunosorbent assay
Commercially available ELISA kits for PLGF (Elabscience E-EL-MK0082), IGF-1 (MyBioSource, MBS700305), and IGF-2 (MyBioSource, MBS2503887) were used according to manufacturers’ instructions. Placental protein homogenate prepared as described above was diluted by volume, and data were corrected for protein concentration.
Histological analysis of placental tissue
Formalin-fixed, paraffin-embedded tissue blocks representative of the placenta (8–11 separate 0.5 cm3 samples/placenta harvested from individual cotyledons of the primary and secondary placental lobes) were hematoxylin and eosins stained (H&E) to generate histologic sections (5 µm thickness) for review by an experienced placental pathologist (TKM) similar to our previously published studies. Reference Frias, Morgan and Evans28 Sections were scored while blinded to diet group for the presence or absence of placental infarctions, accelerated villous maturation (AVM), villous stromal calcifications, chorangiosis, and acute chorioamnionitis.
Statistical analysis
All data were analyzed using GraphPad Prism, version 8 (GraphPad Software, San Diego, CA). One-way analysis of variance (ANOVA) was used to compare continuous data among groups, and a p value of <0.05 was used to indicate statistical significance. For statistical significant complex interactions, Tukey’s multiple comparison test was used to compare individual groups. Categorical data were analyzed with Fisher Chi exact test.
Results
Maternal and fetal outcomes
Dams maintained on either a PR33 or PR50 diet had significantly lower body weights than control animals (Fig. 1A and B). Fetal body weights and crown rump length at G135 were not significantly reduced compared to controls (Fig. 1C and D). Similarly, placental weights were comparable across groups leading to no difference in the fetal:placental ratio (Fig. 1E and F). At the time of delivery, fetal blood gases were measured by iSTAT in umbilical artery cord blood samples. Markers of fetal metabolic condition (Table 1) demonstrated variability within the groups, but were within normal range per published human reference literature. Reference Thorp, Dildy, Yeomans, Meyer and Parisi29,Reference Thorp and Rushing30 Within the small sample size, we report a modest decrease in blood urea nitrogen (BUN) expression which is a characteristic of protein deficiency (Table 1). Circulating fetal blood glucose levels were not significantly different between the three animal cohorts.

Fig. 1. Weight characteristics. Maternal body weight pre-pregnancy (A) and at G135 (B) in CON, PR33-, and PR50-fed dams. Fetal body weight (C) and crown rump length (D) at G135. Placental weight (E) and the fetal:placental weight ratio (F). Data are mean + SEM. *p < 0.05, PR50 vs. CON one-way ANOVA and Tukey’s post-hoc test.
Table 1. Fetal metabolic parameters
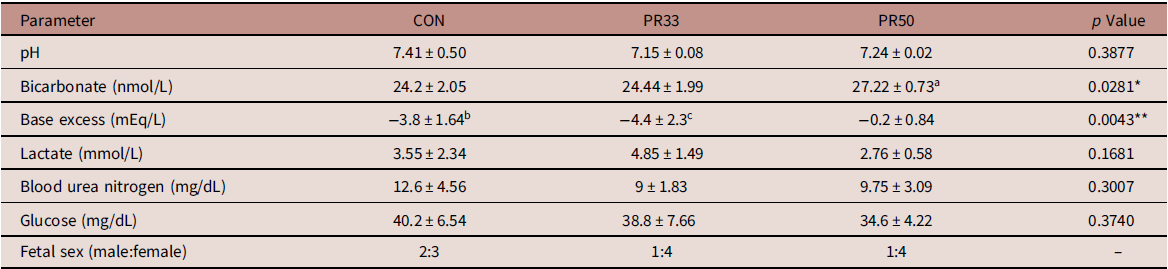
a p < 0.05 PR50 vs. CON and PR33.
b p < 0.05 CON vs. PR50.
c p < 0.01 PR33 vs. PR50.
* p < 0.05, **p < 0.01 one-way ANOVA.
Taurine transporter activity and expression
Uptake of 3H-Taurine was measured in ex vivo placental villous explants from control and PR-fed animals. We demonstrate variability within animal groups across the 60 min time course, with an overall reduction in taurine uptake in both the PR33 and PR50 cohorts compared to control (Fig. 2A and B, p < 0.05, one-way ANOVA). Protein expression of the taurine transporter (TauT) was assessed by Western blot. Quantitative densitometry of TauT normalized to actin showed no difference in TauT expression in PR-fed animals compared to control animals (Fig. 2C).

Fig. 2. Taurine uptake and receptor expression. Placental 3H-Taurine uptake over the 60 min experimental time course (A) in CON (open circles), PR33 (closed squares), and PR50 (closed triangles) animals in fmol/mg, and displayed as the area under the curve (B). Data are mean + SEM, p < 0.05 One-way ANOVA. (C) Protein expression of the taurine transporter in placental homogenate from CON, PR33-, and PR50-fed animals (upper image). The lower image shows the same blot re-probed for actin expression, and the graph displays densitometry data of TauT expression normalized to actin.
Growth factor expression
PLGF expression was significantly elevated in PR50 animals compared to controls (Fig. 3A, p < 0.05, one-way ANOVA). There was a trend toward a stepwise increase in expression of IGF-1 with increasing severity of PR (Fig. 3B), but this finding was not statistically significant with our cohort sizes of five animals per diet group. IGF-2 expression was unaltered across the three groups (Fig. 3C).
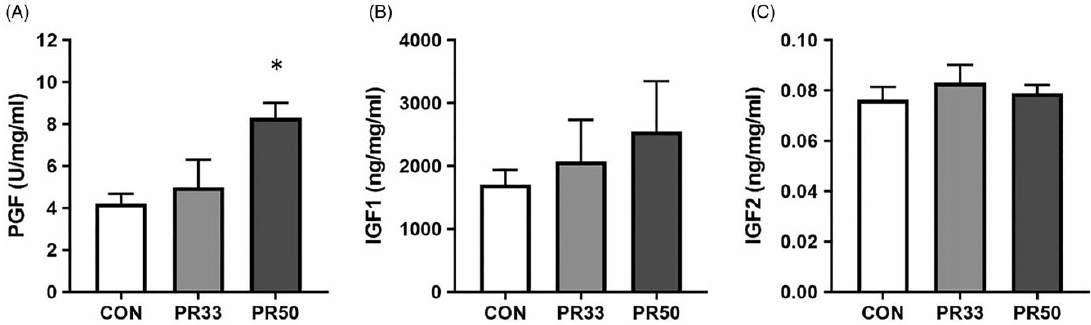
Fig. 3. Growth factor expression. Protein expression of placental growth factor (A), insulin-like growth factor 1 (B), and insulin-like growth factor 2 (C) in placental homogenate from CON, PR33-, and PR50-fed animals. Data are mean + SEM. *p < 0.05, PR50 vs. CON one-way ANOVA and Tukey’s post-hoc test.
Placental histology
Placental sections were negative for acute chorioamnionitis, chorangiosis, and villous stromal calcifications in all three groups. AVM was present in all five cases of PR50 and in three out of five cases with PR33. All five controls were negative for AVM with X2 of 10.2 and p = 0.006 (Fisher Chi test). Microscopic placental infarctions were present in all three groups (20%–40% of cases; p = 0.74). Representative photomicrographs are shown in Fig. 4.

Fig. 4. Placental accelerated villous maturation in cases of maternal protein restriction. Compared with gestational age-matched negative controls on normal diet (A, B), placental sections from cases fed PR33 (C) and PR50 (D) were more likely to have accelerated villous maturation, which was defined as a premature hyper-maturation with a predominance of terminal villi, conspicuous syncytial knotting (arrow), and perivillous fibrin deposition (arrowhead). Reference Khong, Mooney and Ariel42 Scale bar is 100 µm.
Discussion
In this study, we find evidence to suggest that when maternal PR occurs prior to conception and continues throughout pregnancy, the placenta adapts to the in utero environment and conserves its own growth and that of the fetus. Although our animal cohorts have a small sample size and we find variability in some data parameters, at G135, we demonstrate overall maintenance of fetal growth with conservation of placental size, resulting in comparable fetal:placental ratios even in the most severely protein-restricted animals. This ratio is used as a crude determinant of placental efficiency with placental size, morphology, and transport capacity determining prenatal growth. However, an important consideration of the adaptive capacity of the placenta is timing; both of the onset and duration of the insult, and of gestational age at the time of delivery.
Numerous experimental models of maternal nutrient manipulation in a variety of species such as mice, Reference Coan, Vaughan and Sekita5,Reference Gonzalez, Gasperowicz, Barbeito-Andres, Klenin, Cross and Hallgrimsson31 rats, Reference Merezak, Reusens and Renard32 guinea pigs, Reference Elias, Maki, Matushewski, Nygard, Regnault and Richardson6 sheep, Reference Edwards, McKnight, Askelson, McKnight, Dunlap and Satterfield33 pigs Reference Wu, Pond, Ott and Bazer34 , and NHPs Reference McDonald, Wu, Nijland, Jenkins, Nathanielsz and Jansson10 have demonstrated that one key determinant of fetal growth and placental adaptations is the timing of the insult and the portion of gestation impacted by nutrient restriction. Additionally, our group has used a surgical manipulation model, in which the bridging vessels that supply the fetal side circulation to the secondary lobe of the NHP placenta are ligated, to demonstrate that the ability of the placenta to compensate and adapt to an in utero insult is dependent on timing in gestation, and the reserve capacity available. Reference Roberts, Rasanen and Novy35 For mechanistic studies, the manipulation of timing can aid our understanding of vulnerable periods in development and the capacity of the placenta to buffer the potential impact by adapting its growth trajectory. Here, our experimental design modeled the more likely human circumstances of pre-conception protein deficiency, and our reported findings suggest adaptations made by the placenta, to optimize fetal growth, similar to recently reported work from an ovine model of maternal nutrient restriction. Reference Edwards, McKnight, Askelson, McKnight, Dunlap and Satterfield33
Timing of delivery is also important for the assessment of placental adaptations and fetal outcomes to an adverse in utero environment. In a baboon model of maternal nutrient restriction (MNR) where animals were fed 70% of normal control diet intake (i.e., no specific nutrient alterations, just a decrease in total calories from reduced food availability) from G30 to G120, pregnancy outcomes differed depending on gestational age at delivery. Specifically, these studies demonstrate that at G120, there is no evidence of fetal growth restriction, Reference Kavitha, Rosario and Nijland8 whereas at G165 (term), there was a 13% decrease in fetal weight Reference Pantham, Rosario and Weintraub11 even though MNR ended at G120 with a switch back to control diet for the remainder of gestation. In our study, pregnancies were delivered at G135, which is in the mid-third trimester. If allowed to continue for one additional month to full term, a period in which the fetus grows exponentially, we may yield differing results as conserved placental size may conserve fetal weight earlier in gestation, but fetal growth demands could outweigh these capabilities later in gestation. Whether or not this would occur remains to be determined.
One of our initial objectives was to assess placental taurine transport capacity as a functional readout of gestational PR. By in vitro assay, we found a decrease in taurine uptake in the placenta from both PR33- and PR50-fed animals at G135, yet expression of the TauT was unchanged across the three diet groups. This finding is consistent with the human literature from full-term studies that demonstrate decreased taurine uptake with maternal obesity but unchanged placental expression of the TauT receptor. Reference Ditchfield, Desforges and Mills17 Taurine has many functions and is regulated by a wide variety of factors. Reference Desforges, Ditchfield and Hirst18,Reference Desforges, Parsons, Westwood, Sibley and Greenwood19,Reference Roos, Powell and Jansson36 Mechanistic understanding was not the purpose of this study and is beyond the scope of our current analysis, yet the use of this in vitro assay demonstrates that the placenta appears to have some ability to adapt to an adverse in utero environment and modify maternal to fetal nutrient transfer. Importantly, although we report a slight decrease in taurine activity, the overall impact on fetal growth appears not to be significantly detrimental at G135.
We measured fetal blood gases at the time of delivery as an assessment of fetal metabolic health. For the most part, umbilical artery pH, bicarbonate, and base excess values were within the normal range reported in human assessments of fetal blood gases. Reference Thorp, Dildy, Yeomans, Meyer and Parisi29,Reference Thorp and Rushing30 However, there appears to be some discrepancy in the lower base excess and elevated bicarbonate measured in the cord blood of PR50 animals. In an expanded data set from 15 fetal deliveries that occurred between G140 and G155 in control animal pregnancies, we found a wider span of these two parameters compared to human literature, with bicarbonate ranging from 21.4 to 31.3 nmol/L and base excess ranging from 3 to −6 mEq/L (unpublished observations). The explanation for this discordance in the PR50 cohort is unknown but may be a species-specific issue, or it may once again reflect the variability within the small sample set. Decreased BUN levels are a known consequence of low-protein malnutrition. Reference Klahr and Tripathy37 In the baboon model of MNR, mean fetal BUN levels were reported to be 110 mg/L in control and 80 mg/L with MNR. Reference McDonald, Wu, Nijland, Jenkins, Nathanielsz and Jansson10 Although the numbers reported in our cohort would be considered to be within the lower limits of the normal range, BUN expression in the PR-fed animals was comparable with those reported in the baboon. Reference McDonald, Wu, Nijland, Jenkins, Nathanielsz and Jansson10 We do not find significant differences in circulating fetal glucose levels with chronic maternal consumption of a PR diet. It has previously been postulated that small for gestational age offspring can be attributed to decreased maternal glucose supply, rather than increased fetal consumption of glucose as an energy source utilized for growth. Reference Economides and Nicolaides38 Although we do not report the maternal phenotypic characteristics of this animal cohort during the current pregnancy, data from the first round of pregnancies in control and PR50-fed animals were recently published. Reference Kirigiti, Frazee and Bennett4 Those data demonstrate that despite the chronic consumption of a severely protein-deficient diet, the dams appear to make physiological adaptations to their insulin and glucagon levels to maintain comparable circulating glucose at G120. Reference Kirigiti, Frazee and Bennett4 If these data are extrapolated to our current study, they may offer an explanation for the conserved fetal growth we observed. It is also plausible to suggest that additional adaptations are made to mobilize and reallocate maternal nutrient resources to support fetal growth needs. Further assessments would be required to investigate these theories.
One of the hallmark characteristics of placental insufficiency is AVM, which is an adaptation that results in expansion of the syncytiotrophoblast surface area of maternal-fetal exchange and is thought to occur in response to maternal malperfusion. Reference Ernst39 Human studies have reported improved neonatal outcomes including higher birth weight for gestational age associated with AVM. Reference Christians and Grynspan40 We report significant AVM in PR-fed animals, which further demonstrates placental compensation to the adverse in utero environment. To continue the characterization of placental adaptations, we quantified PLGF expression in protein homogenates and found increased levels in PR-fed animals. In human studies, maternal circulating levels of PLGF are found to be decreased in growth-restricted infants Reference Benton, McCowan and Heazell41 ; this may reflect the exceeded capacity of the placenta to compensate to the underlying cause of the insufficiency. In our model, we maintain overall fetal growth and have increased PLGF expression. However, we report no difference in expression of two growth factors that plays a key role in fetal growth, Reference Hiden, Glitzner, Hartmann and Desoye24 IGF-1 and IGF-2, across the three diet groups.
In conclusion, we have used a translational animal model to study the effect of gestational PR on placental development and function. Our data demonstrate that in pregnancies delivered in the mid-third trimester, fetal growth is largely maintained despite a severe restriction of 50% protein. Within some data parameters, we observe a stepwise effect which suggests that the severity of PR is an important determinant of outcome, with PR33 being better tolerated than PR50; this finding seems logical and suggests that there may be a placental reserve capacity threshold. Similarly, we acknowledge that later in gestation, the ability of the placenta to support fetal growth and development with no detrimental outcomes might be challenged by exponentially rising fetal demands toward the end of pregnancy. This may, in part, explain our earlier outcomes from this model in which significant pregnancy loss occurred (50%), and our in vivo functional assessments indicated inadequate placental perfusion and perturbed markers of fetal health in amniotic fluid analysis. Reference Roberts, Lo and Lewandowski3
Acknowledgments
None.
Financial Support
Funding for this project was provided by The Bill & Melinda Gates Foundation (OPP1110865 to AEF) and NIH R01 HD086331 (Frias). In addition, research reported in this publication was supported by the Office of the Director of the National Institutes of Health under Award Number P51OD011092 to the Oregon National Primate Research Center.
Conflict of Interest
None.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national guides on the care and use of laboratory animals from the Animal Welfare Act and enforced by the USDA, and this work was approved by the Institutional Animal Care and Use Committee at the ONPRC.







