Obesity and related metabolic disorders have emerged as serious global health problems( Reference Haidar and Cosman 1 , Reference Jing, Binkley and Suever 2 ). Unfortunately, many health problems in adulthood, such as type 2 diabetes, hyperlipaemia, obesity and other metabolic diseases, are associated with early-life nutrition( Reference Ojha, Saroha and Symonds 3 ). Nutritional programming is defined as the process by which exposure to an abnormal nutritional environment during critical periods could permanently influence organ structure, function and genomic expression in the brain, adipose tissue, liver, pancreas and other organs( Reference Koletzko, Symonds and Olsen 4 – Reference Armitage, Khan and Taylor 6 ). Abnormal nutrition in early fetal life, infancy and adolescence can influence adipocyte proliferation, differentiation and energy homoeostasis in adulthood( Reference Mostyn and Symonds 7 , Reference Habbout, Li and Rochette 8 ). Therefore, early intervention for childhood obesity is considered the optimal strategy by clinicians.
Glucocorticoid (GC) play an essential role in adipocyte differentiation, lipolysis and insulin action( Reference Peckett, Wrigh and Riddell 9 – Reference Lee, Pramyothin and Karastergiou 11 ), and dysregulated GC action in tissues has been implicated in obesity, type II diabetes and other related diseases( Reference Bujalska, Kumar and Stewart 12 ). 11β-Hydroxysteroid dehydrogenase type 1 (11β-HSD1) is highly expressed in adipose tissue, the liver and the brain, and it converts inactive cortisone into active cortisol( Reference Stomby, Andrew and Walker 13 ). The overexpression of 11β-HSD1 in visceral adipose tissue is positively correlated with obesity, dyslipidaemia, glucose intolerance and other metabolic disorders in rodents( Reference Masuzaki, Paterson and Shinyama 14 , Reference Masuzaki, Yamamoto and Kenyon 15 ) and humans( Reference Johansson, Andrew and Forsberg 16 , Reference Dube, Norby and Pattan 17 ). Similarly, elevated hepatic 11β-HSD1 levels are correlated with insulin resistance and dyslipidaemia( Reference Chapagain, Caton and Kieswich 18 ). Notably, postnatal overfeeding induced by small litter (SL) rearing in rats can persistently increase 11β-HSD1 expression in peripheral tissues and aggravate the development of obesity and metabolic disorders in adults( Reference Hou, Liu and Zhu 19 ). In addition, 11β-HSD1-inhibited or 11β-HSD1-knockout mice show resistance to diet-induced insulin resistance and hyperglycaemia( Reference Seckl and Walker 20 , Reference Harno, Cottrell and Keevil 21 ). Therefore, 11β-HSD1 is a potential contributor to the development of obesity and other metabolic diseases and may be a therapeutic target for the metabolic syndrome.
n-3 PUFA, which are particularly rich in fish oil, mainly include EPA (C20 : 5n-3) and DHA (C22 : 6n-3)( Reference Poudyal, Panchal and Diwan 22 ). They are important dietary elements that can modify the expressions of genes involved in obesity, hypertension, diabetes and other inflammatory conditions( Reference Rafiee, Sotoideh and Djalali 23 , Reference Simopoilos 24 ). The decreasing n-3:n-6 PUFA ratio in modern diets contributes to the development of obesity, diabetes and other metabolic syndromes( Reference Patterson, Wall and Fitzgerald 25 , Reference Ailhaud, Massiera and Weill 26 ). Conversely, increasing the consumption of n-3 PUFA can decrease adiposity, hypertriacylglycerolaemia and fatty liver disease, and improve insulin sensibility and glucose homoeostasis in rodents( Reference Buettner, Parhofer and Woenckhaus 27 – Reference Jelenik, Rossmeisl and Kuda 31 ) and humans( Reference Su, Lee and Cheng 32 ). 11β-HSD1, PPARγ and CCAAT/enhancer-binding protein α (C/EBPα) are involved in adipogenesis and lipogenesis and seem to be the key targets of n-3 PUFA( Reference Martínez-Fernández, Laiglesia and Huerta 33 – Reference Stimson and Walker 35 ). However, the dose-dependent effect of the n-3 PUFA dietary intervention that accounts for the decreased risk of obesity and other metabolic disorders induced by overfeeding in early life remains unclear.
In addition, it is well known that childhood and adolescence are important periods for the development of adipose tissue( Reference Mostyn and Symonds 7 , Reference Budge, Sebert and Sharkey 36 , Reference Symonds, Pope and Sharkey 37 ), and increased dietary n-3 PUFA levels during early critical windows of fat cell development limit adipose tissue growth, which may be a novel strategy for the prevention of childhood obesity( Reference Ailhaud and Guesnet 38 ). A number of studies in humans have shown that early-life interventions may be beneficial for improving subsequent weight gain, depression disorder and some respiratory diseases in later life( Reference Lloyd, Langley-Evans and McMullen 39 – Reference Khan, McCormack and Bolger 43 ). The aim of this study was to elucidate the effects of the doses and timing of a fish oil dietary intervention on reversing the adverse metabolic outcomes and expression of the GC-activated enzyme 11β-HSD1 in postnatal overfed rats.
Methods
Animals
The animal protocols used in this study were approved by the University Committee on the Use and Care of Animals and were overseen by the Unit for Laboratory Animal Medicine at Nanjing Medical University (ID:20130102-01). Male Sprague–Dawley rats were used in this study. All animals were maintained on a 12 h light–12 h dark cycle under normal temperature (22±2°C) with free access to chow and tap water. The animals used in this study were housed in cages with three rats per cage after weaning.
Experimental design
On postnatal day 3, male pups were randomly assigned to normal litters (NL) or SL. The litter size in the NL group was adjusted to ten male pups to imitate normal feeding pattern, whereas the litter size in the SL group was adjusted to three male pups to simulate early postnatal overfeeding( Reference Plagemann, Harder and Schellong 44 ). After postnatal day 21, rats from the NL or the SL were fed a standard diet and represented the control group (6 % dietary fat was soyabean oil, NL group or SL group). The SL rats were fed a low-dose fish oil diet (2 % dietary fat was fish oil, SL-LFO group) or a high-dose fish oil diet (6 % dietary fat was fish oil, SL-HFO group) and represented the intervention groups. The dietary nutrient compositions are shown in Table 1, and the diets (Slac, Shanghai, China) and the fatty acid compositions of the diets are shown in Table 2. The SL rats were fed the HFO or the LFO diet until postnatal week 13 (W13).
Table 1 Purified diet formula and composition (weight (%))

Table 2 Fatty acid profile of the diets (mg/100 mg)
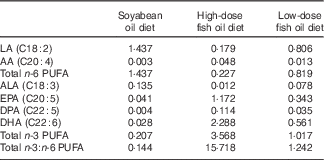
LA, linoleic acid; AA, arachidonic acid; ALA, α-linolenic acid; DPA, docosapentaenoic acid.
At the beginning of the interventional experiment (Fig. 1), the SL rats were fed the HFO diet from postnatal week 3 (SL-HFOW3), week 6 (SL-HFOW6) or week 8 (SL-HFOW8) for 10 weeks. For male rats, postnatal week 3 is designated as the weaning period, postnatal week 6 is the puberty period and postnatal week 8 is the post-puberty period( Reference Tirelli, Laviola and Adriani 45 ). Body weight and food intake were monitored weekly throughout life. The rats were killed at W13, postnatal week 16 (W16) or postnatal week 18 (W18) after an overnight fast.

Fig. 1 Schematic overview of the experimental study design. Effects of the different doses of fish oil diets and timing of intervention on postnatal overfed rats. The small litter rats were fed the high-dose fish oil dietary intervention from postnatal week 3 (SL-HFOW3), week 6 (SL-HFOW6) or week 8 (SL-HFOW8) for 10 weeks. Postnatal week 3 is designated as the weaning period, postnatal week 6 is the puberty period and postnatal week 8 is the post-puberty period. NL, normal litter; SL, small litter; SL-LFO, SL rats fed low-dose fish oil; W3, week 3; W6, week 6; W8, week 8; W13, week 13; W16, week 16; W18, week 18.
Collection of blood and tissue samples
Rats were anaesthetised by an intraperitoneal injection of chloral hydrate (300 mg/kg body weight) after an overnight fast (12 h). Blood samples were collected from the right ventricle and centrifuged (2000 g for 15 min) to obtain serum fractions, which were promptly stored at −70°C until use. The epididymal and retroperitoneal white adipose tissues were dissected and weighed, and all tissue samples were snap-frozen in liquid N2 and stored at −70°C.
Analysis of adipose tissue histology
Adipose tissues were fixed in 10 % formaldehyde in PBS, pH 7·4, for 24–48 h at room temperature. After fixation, the tissues were dehydrated, cleared, embedded in paraffin blocks, cut into 8-μm-thick sections and stained with haematoxylin–eosin. The cross-sectional adipocyte area of rats was determined in at least three slices per adipose tissue sample and ten fields of vision per slice and was analysed with imaging software.
Intraperitoneal glucose tolerance test
The intraperitoneal glucose tolerance test (IPGTT) was performed as previously described( Reference Chen, Simar and Lambert 46 ). In brief, rats were fasted overnight at W13, W16 and W18. A blood sample was then collected from the tail vein, and 2·0 g of d-glucose (50 % stock solution in saline)/kg of body weight was injected intraperitoneally. Blood samples were collected from the tail vein at 30-, 60- and 120-min intervals after glucose injection, and glucose levels were measured using a glucose meter (Accu-Chek; Roche).
Biochemical analysis
Total TAG, total cholesterol (TC) and HDL-cholesterol levels in the serum were measured using enzymatic colorimetric assays according to the protocols of the commercial clinical diagnostic kits (TCHOD-PAP reagent kit 20090 and GPO-PAP reagent kit 20080; BIOSINO BIO) using the Olympus AU400 analyser.
Serum fatty acid composition
Fatty acid profiles were determined by gas chromatography using the general technique reported by Bligh & Dyer( Reference Bligh and Dyer 47 ). In brief, fatty acid methyl esters were prepared using a 14 % boron trifluoride (BF3)/methanol reagent (Sigma). Lipid samples were heated at 90°C in glass tubes in a metal block for 30 min and allowed to stand for 10 min. The fatty acid methyl esters were analysed using an Agilent 7890Agas chromatography system. The peaks were identified by comparison with fatty acid standards (Nu-Chek-Prep), and the percentages of the areas for all resolved peaks were analysed using GC ChemStation software.
Quantitative real-time PCR
Total RNA was isolated from the liver and adipose tissue using TRIzol (Invitrogen), according to the manufacturer’s instructions, and quantified spectrophotometrically by OD260. Total RNA integrity was assessed using agarose gel electrophoresis, and complementary DNA were synthesised from 1·0 μg of the RNA sample using M-MLV RT (Promega) according to the manufacturer’s recommendations. The genes of interest, 11β-HSD1, C/EBPα and PPARγ, were analysed by real-time PCR using the SYBR Green ABI Prism 7500 sequence detector (Table 3). The expressions of the target genes were normalised to the expression of glyceraldehyde-3-phosphate dehydrogenase.
Table 3 Primer sequences used for mRNA quantification by real-time PCR

HSD1, hydroxysteroid dehydrogenase type 1; C/EBPα, CCAAT/enhancer-binding protein α; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Statistical analysis
Data are expressed as mean values with their standard error of mean. Significant differences among the groups of rats were analysed using one-way ANOVA. Serum glucose levels during the IPGTT were analysed by one-way ANOVA with repeated measures. Statistical significance was accepted at P<0·05.
Results
Body weight and adipose tissue weight
Body weight and fat pad weight of SL rats were higher compared with NL rats at W13, W16 and W18 (P<0·05). After the 10-week intervention with the fish oil diet, body weight and fat pad weight of SL-HFOW3 rats were decreased compared with SL rats (P<0·05) and were similar to those of NL rats. Body weight and fat pad weight of SL-LFOW3 rats were not significantly different from SL rats (Table 4).
Table 4 Effects of the dose of the fish oil diet on body weight and adiposity (Mean values with their standard errors of mean, n 6 in each group)Footnote *
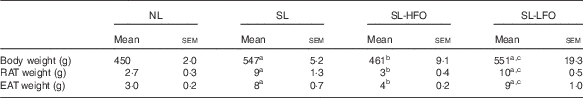
NL, normal litter; SL, small litter; SL-HFO, SL rats fed high-dose fish oil; SL-LFO, SL rats fed low-dose fish oil; RAT, retroperitoneal adipose tissue; EAT, epididymal adipose tissue.
a P<0·05 v. NL, b P<0·05 v. SL, c P<0·05 v. SL-HFO.
* Body weight, RAT weight and EAT weight were measured in rats fed the high-dose fish oil diet, low-dose fish oil diet or a standard diet at week 13. Data were analysed by one-way ANOVA using the least square difference approach, and P<0·05 was considered to be statistically significant.
Body weight of SL-HFOW6 rats was less than that of SL rats (P<0·05), but higher than that of NL rats (P<0·05). Compared with SL rats, fat pad weight of SL-HFOW6 rats was slightly reduced. Body and fat pad weights of SL-HFOW8 rats were not significantly different from those of SL rats (Table 5).
Table 5 Effects of the timing of the fish oil diet on body weight and adiposity (Mean values with their standard errors of mean, n 6 in each group)Footnote *
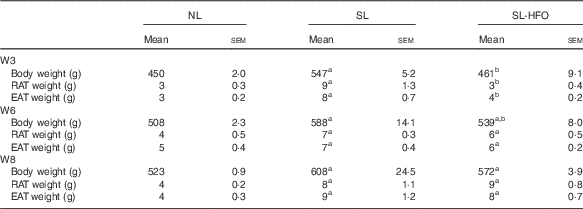
NL, normal litter; SL, small litter; SL-HFO, SL rats fed high-dose fish oil; W3, postnatal week 3; RAT, retroperitoneal adipose tissue; EAT, epididymal adipose tissue; W6, postnatal week 6; W8, postnatal week 8.
a P<0·05 v. NL, b P<0·05 v. SL.
* Body weight, RAT weight and EAT weight were measured in rats fed the HFO diet for 10 weeks during different time points, and the starting points for the HFO intervention were W3, W6 and W8. Data were analysed by one-way ANOVA using the least square difference approach, and P<0·05 was considered to be statistically significant.
Haematoxylin–eosin staining
As shown in Fig. 2, the average cross-sectional adipocyte area of SL rats in each field was larger than that of NL rats at W13, W16 and W18 (P<0·05, Fig. 2). The average cross-sectional adipocyte area in SL-HFOW3 rats was smaller than that of SL rats (P<0·05), and not different from that of NL rats. However, the average cross-sectional adipocyte area in SL-LFOw3 rats was not different from that of SL rats (Fig. 2(B)).

Fig. 2 Effects of the dose and timing of the fish oil dietary intervention on haematoxylin–eosin-stained sections (200×) and average cross-sectional adipocyte area in rat adipose tissue. Effects of different doses on haematoxylin–eosin-stained sections (A) and average cross-sectional adipocyte area (B) at week 13 (W13). Effects of different intervention start points on haematoxylin–eosin-stained sections (C) and average cross-sectional adipocyte area (D) at W13, week 16 (W16) and week 18 (W18). The starting points for the high-dose fish oil (HFO) intervention were week 3 (W3), week 6 (W6) and week 8 (W8), and the end points were W13, W16 and W18, separately. Values are means (n 3 in each group), with standard errors of mean. Data were analysed by one-way ANOVA using the least square difference approach, and P<0·05 was considered to be statistically significant. a
P<0·05 v. normal litter (NL), b
P<0·05 v. small litter (SL), c
P<0·05 v. SL rats fed high-dose fish oil (SL-HFO). SL-LFO, SL rats fed low-dose fish oil.
![]() , NL;
, NL; ![]() , SL;
, SL; ![]() , SL-HFO;
, SL-HFO; ![]() , SL-LFO.
, SL-LFO.
Moreover, the average cross-sectional adipocyte area in SL-HFOW6 rats was smaller than that in corresponding SL rats (P<0·05), but larger than that in NL rats (P<0·05). However, the average cross-sectional adipocyte area in SL-HFOW8 rats did not show any change compared with SL rats (Fig. 2(D)).
Glucose homoeostasis
The AUC for plasma glucose levels in SL rats was increased at W13, W16 and W18 compared with NL rats (P<0·05, Fig. 3(B) and (F)), indicating that glucose tolerance was impaired in SL rats. At W13, the AUC of SL-HFOW3 rats was decreased compared with SL rats (P<0·05) and recovered to a normal level. Glucose intolerance in SL-HFOW6 and SL-HFOW8 rats was also improved to the normal state (P<0·05, Fig. 3(F)). However, glucose intolerance was not altered in SL-LFOW3 rats (Fig. 3(B)).

Fig. 3 Effects of the dose and timing of the fish oil dietary intervention on intraperitoneal glucose tolerance test and AUC. Effects of different doses on intraperitoneal glucose tolerance test (A) and AUC (B) at week 13 (W13). Effects of different intervention start points on glucose homoeostasis (C, D, E) and AUC (F) at W13, week 16 (W16) and week 18 (W18). The starting points for the HFO intervention were week 3 (W3), week 6 (W6) and week 8 (W8), and the end points were, W13, W16 and W18, separately. Values are means (n 6 in each group), with their standard error of mean. Data were analysed by one-way ANOVA using the least square difference approach, and P<0·05 was considered to be statistically significant. a
P<0·05 v. normal litter (NL), b
P<0·05 v. small litter (SL), c
P<0·05 v. SL rats fed high-dose fish oil (SL-HFO). a: ![]() , NL;
, NL; ![]() , SL;
, SL; ![]() , SL-HFO;
, SL-HFO; ![]() , SL-LFO; b, f:
, SL-LFO; b, f: ![]() , NL;
, NL; ![]() , SL;
, SL; ![]() , SL-HFO;
, SL-HFO; ![]() , SL-LFO; c:
, SL-LFO; c: ![]() , NL;
, NL; ![]() , SL;
, SL; ![]() , SL-HFOW3; d:
, SL-HFOW3; d: ![]() , NL;
, NL; ![]() , SL;
, SL; ![]() , SL-HFOW6; e:
, SL-HFOW6; e: ![]() , NL;
, NL; ![]() , SL;
, SL; ![]() , SL-HFOW8.
, SL-HFOW8.
Serum lipids
Total TAG levels in SL rats were higher compared with NL rats (P<0·05); total TAG levels in SL-HFOW3 rats were decreased (P<0·05), but not in SL-LFOW3 rats (Fig. 4(A)). Total TAG levels in SL rats with the HFO dietary intervention at all points were reduced to the normal levels (P<0·05, Fig. 4(D)). TC levels in SL-HFOW3 rats were not changed (P>0·05), but HDL-cholesterol levels were increased compared with NL and SL rats (P<0·05, Fig. 4(C)). TC levels in SL-HFOW6 rats were reduced compared with NL and SL rats (P<0·05, Fig. 4(E)). At W18, TC levels in SL rats were increased compared with NL rats, but TC levels in SL-HFOW8 rats were reduced to normal levels (P<0·05, Fig. 4(E)).

Fig. 4 Effects of the dose and timing of the fish oil dietary intervention on serum biological parameters in rats. Effects of different doses on serum levels of total TAG (A), total cholesterol (B) and HDL-cholesterol (C) at week 13 (W13). Effects of different intervention start points on serum levels of total TAG (D), total cholesterol (E) and HDL-cholesterol (F) at W13, week 16 (W16) and week 18 (W18). The starting points for the high-dose fish oil (HFO) intervention were week 3 (W3), week 6 (W6) and week 8 (W8), and the end points were W13, W16 and W18, separately. Values are means (n 6 in each group), with standard errors of mean. Data were analysed by one-way ANOVA using the least square difference approach, and P<0·05 was considered to be statistically significant. a
P<0·05 v. normal litter (NL), b
P<0·05 v. small litter (SL), c
P<0·05 v. SL rats fed high-dose fish oil (SL-HFO). ![]() , NL;
, NL; ![]() , SL;
, SL; ![]() , SL-HFO;
, SL-HFO; ![]() , SL-LFO.
, SL-LFO.
Serum fatty acid profile
As shown in Fig. 5, serum EPA and DHA levels were significantly increased in SL-HFOW3 rats compared with NL and SL rats (P<0·05) as well as SL-LFOW3 rats (P<0·05). Interestingly, SL rats that were fed the HFO or LFO diet exhibited a significant decrease in serum n-6 PUFA levels compared with NL and SL rats that were fed the standard diet (P<0·05).

Fig. 5 Effects of the three diets on serum fatty acid composition of rats at week 13 (W13). LA, linoleic acid; AA, arachidonic acid; ALA, α-linolenic acid; DPA, docosapentaenoic acid. The n-3 PUFA contain ALA, EPA, DPA and DHA, and n-6 PUFA contain LA and AA. Values are means (n 6 in each group), with their standard errors of mean. Data were analysed by one-way ANOVA using the least square difference approach, and P<0·05 was considered to be statistically significant. a
P<0·05 v. normal litter (NL), b
P<0·05 v. small litter (SL), c
P<0·05 v. (SL-HFO). ![]() , NL;
, NL; ![]() , SL;
, SL; ![]() , SL-HFO;
, SL-HFO; ![]() , SL-LFO.
, SL-LFO.
11β-Hydroxysteroid dehydrogenase type 1, CCAAT/enhancer-binding protein α and PPARγ mRNA expressions in adipose tissue
The expression of 11β-HSD1 mRNA in the retroperitoneal adipose tissue of SL rats was increased compared with NL rats at W13 and W16 (P<0·05), although this difference was not statistically significant at W18. Similar to 11β-HSD1, the expressions of C/EBPα and PPARγ mRNA in the retroperitoneal adipose tissue were increased in SL rats at W13 and W16 (P<0·05). The expressions of these mRNA were reduced to normal levels in SL-HFOW3 rats but not in SL-HFOW6 rats. The expressions of 11β-HSD1 and PPARγ mRNA in SL-HFOW8 rats were increased compared with NL or SL rats (P<0·05, Fig. 6(A) and (C)).

Fig. 6 Effect of the timing of the fish oil dietary intervention on 11β-hydroxysteroid dehydrogenase type 1 (HSD1) (A), CCAAT/enhancer-binding protein α (C/EBPα) (B) and PPARγ (C) mRNA expressions in the adipose tissue. The starting points for the high-dose fish oil (HFO) intervention were week 3 (W3), week 6 (W6) and week 8 (W8), and the end points were week 13, week 16 and week 18, separately. Values are means (n 6 in each group), with standard errors of mean. Data were analysed by one-way ANOVA using the least square difference approach, and P<0·05 was considered to be statistically significant. a
P<0·05 v. normal litter (NL), b
P<0·05 v. small litter (SL). ![]() , NL;
, NL; ![]() , SL;
, SL; ![]() , SL-HFO.
, SL-HFO.
11β-Hydroxysteroid dehydrogenase type 1 and CCAAT/enhancer-binding protein α mRNA expression in hepatic tissue
In hepatic tissue, the expression of 11β-HSD1 mRNA in SL rats was increased compared with NL rats (P<0·05), although this difference was not statistically significant at W16. The C/EBPα mRNA expression levels among the groups were similar to 11β-HSD1 expression levels. Importantly, the HFO dietary intervention was effective in reducing the liver expressions of 11β-HSD1 and C/EBPα at all time points, except at puberty (W6), where 11β-HSD1 expression was significantly lower than NL (Fig. 7(A)).

Fig. 7 Effect of the timing of the fish oil dietary intervention on 11β-hydroxysteroid dehydrogenase type 1 (HSD1) (A) and CCAAT/enhancer-binding protein α (C/EBP) (B) mRNA expressions in the liver. The starting points for the high-dose fish oil (HFO) intervention were week 3 (W3), week 6 (W6) and week 8 (W8), and the end points were week 13, week 16 and week 18, separately. Values are means (n 6 in each group), with standard errors of mean. Data were analysed by one-way ANOVA using the least square difference approach, and P<0·05 was considered to be statistically significant. a
P<0·05 v. normal litter (NL), b
P<0·05 v. small litter (SL). ![]() , NL;
, NL; ![]() , SL;
, SL; ![]() , SL-HFO.
, SL-HFO.
Discussion
Early postnatal life is critical for long-term programming of health, which might provide an opportunity to limit obesity and its metabolic consequences in later life( Reference Patel and Srinivasan 48 ). In the present study, we explored the effects of the doses and timing of a fish oil dietary intervention on the adverse effects induced by postnatal overfeeding. We found that the HFO, but not LFO, dietary intervention could reduce weight gain and improve glucose intolerance, dyslipidaemia and local tissue 11β-HSD1 expression in postnatal overfed rats. A novel finding of this study was that the HFO prevented weight gain in SL rats and was more pronounced in the post-weaning period compared with an intervention that was implemented at puberty or post-puberty. These data suggest that dietary fatty acid composition and intervention timing may potentially interact with weight gain and metabolic regulation, and these effects may be partly involved in regulating tissue 11β-HSD1 expression levels.
It has been established that n-3 PUFA are not only a source of energy but also have protective effects against some metabolic diseases( Reference Kremmyda, Tvrzicka and Stankova 49 , Reference Flachs, Horakova and Brauner 50 ). Some studies have postulated that the balance of dietary n-3:n-6 PUFA plays an important role in reducing the risk factors of the metabolic syndrome( Reference Russo 51 , Reference Simopoulos 52 ). In adult Sprague–Dawley rats, an increase in the dietary ratio of n-3:n-6 PUFA to 1:1 may decrease body weight, hyperlipaemia and type II diabetes, whereas a decrease in the ratio of n-3:n-6 PUFA to 1:4 failed to induce these metabolic changes( Reference Liu, Qiu and Mu 53 ). Therefore, we increased the dietary ratio of n-3:n-6 PUFA by replacing soyabean oil with fish oil without changing the energy content of the diets used in the dietary intervention for postnatal overfed rats. We found that a diet containing a higher ratio of n-3:n-6 PUFA effectively reduced body weight, visceral fat gain, adipocyte volume and blood lipids and improved insulin sensitivity to normal levels in SL rats. However, we did not observe any metabolic changes in SL rats that were fed a diet containing a lower ratio of n-3:n-6 PUFA. The serum fatty acid profile mirrors the dietary lipid composition and reflects the endogenous fatty acid metabolism to a certain extent( Reference Bertrand, Pignalosa and Wanecq 54 ). As the main components of n-3 PUFA, EPA and DHA inhibited adipocyte differentiation, lipid droplet formation and improved glucose homoeostasis and insulin sensitivity( Reference Pereira, Leonard and Huang 55 – Reference Siriwardhana, Kalupahana and Fletcher 58 ). Notably, the EPA and DHA levels in the circulation of SL-HFOW3 rats were increased compared with NL, SL and SL-LFOW3 rats, strengthening the beneficial anti-obesity effects of EPA and DHA on SL rats.
Developmental plasticity plays an important role in the aetiology of chronic diseases and is supported by the ‘developmental origins of health and disease’ hypothesis( Reference Victora, Adair and Fall 59 , Reference Gluckman, Hanson and Cooper 60 ). Thus, interventions that are implemented during developmental plasticity are considered the optimal strategy for treating chronic diseases. Perinatal mice fed a normal diet were resistant to high-fat diet-induced hyperphagia, obesity and type II diabetes( Reference Gallou-Kabani, Vigé and Gross 61 ), but postnatal overfed rats still developed obesity in adulthood, even after being fed regular chow diet after weaning( Reference Hou, Liu and Zhu 19 ). These observations indicate that the perinatal period represents a critical time frame during which metabolic regulatory set points may be modified( Reference Hou, Ji and Wang 34 ). Interestingly, a post-weaning n-3 PUFA diet could prevent adiposity, dyslipidaemia and other programmed outcomes in rats induced by a maternal and post-weaning, sucrose-rich diet( Reference Chicco, Creus and lllesca 62 ). In this study, we provided the HFO diet to SL rats at post-weaning, puberty or post-puberty periods, separately. We found that the most effective intervention was at weaning, and body weight and other metabolic indices of SL-HFOW3 rats were effectively recovered to normal levels, although the dietary intervention at puberty or post-puberty was useful in improving glucose intolerance and hyperlipaemia. In has been confirmed that the accumulation of adipose tissue includes increase in both adipose cell number and size( Reference Mostyn and Symonds 7 , Reference Spalding, Arner and Westermark 63 ). In the present study, we found that the HFO diet at weaning was effective in reducing adipocyte size, and this effect was present partly at puberty and it disappeared completely at post-puberty. Therefore, the timing of the dietary intervention might be critical for regulating adipose tissue growth, particularly in postnatal overfed rats.
Furthermore, we observed 11β-HSD1 mRNA expression in the adipose tissues and the liver of rats. The results showed that the post-weaning HFO dietary intervention could decrease 11β-HSD1 mRNA expression levels in the adipose tissue of SL rats, which was consistent with the changes in the cross-sectional adipocyte area and adipose tissue weight. Many studies have indicated that increased 11β-HSD1 expression amplifies GC action and then promotes the differentiation of adipose stromal cell to mature adipocytes( Reference Bujalska, Kumar and Hewison 64 ) and increases visceral fat accumulation( Reference Shively, Register and Clarkson 65 ), as well as the development of the metabolic syndrome( Reference Tomlinson and Stewart 66 ). In contrast, decreased 11β-HSD1 expression reduced fat accumulation and improved some metabolic diseases in rodent models and human clinical trials( Reference Chapman, Holmes and Seckl 67 ). In addition, the expressions of C/EBPα and PPARγ, which are involved in pre-adipocyte differentiation( Reference Sun, Wang and Li 68 , Reference Hollenberg, Susulic and Madura 69 ), and the modulation of 11β-HSD1 expression( Reference Bruley, Lyons and Worsley 70 – Reference Vagnerová, Loukotová and Ergang 72 ) were also decreased to normal levels in SL-HFOW3 rats. All the genes studied, 11β-HSD1, PPARγ and CEBPα, were consistently expressed in the retroperitoneal adipose tissue, which could indicate their potential relevance in the regulation of GC metabolism in adipose tissue. Taken together, these results show that the post-weaning-to-puberty period could be a critical time to implement a dietary intervention to regulate GC activity, which may be an attractive therapeutic target in early nutrition programming.
Unlike 11β-HSD1 expression in the adipose tissue, the HFO dietary intervention could effectively reduce the expression of this mRNA in the liver of SL rats at all time points. 11β-HSD1 is expressed at high levels in the liver, and the high concentrations of cortisol in the liver could have important effects on hepatic insulin action( Reference Dube, Norby and Pattan 73 ) and lipid metabolism( Reference Paterson, Morton and Fievet 74 ). An intervention with 11β-HSD1 inhibition led to attenuated GC action in the mouse liver, as well as improved insulin sensitivity and dyslipidaemia( Reference Harno, Cottrell and Keevil 75 , Reference Park, Rhee and Jung 76 ). C/EBPα is an activator of 11β-HSD1 in the liver, and C/EBPα-deficient mice exhibit reduced 11β-HSD1 expression in hepatic tissue( Reference Williams, Lyons and MacLeod 77 ). Similarly, the HFO diet decreased the expressions of both 11β-HSD1 and C/EBPα in the hepatic tissue of SL rats. These results might explain why hepatic 11β-HSD1 expression was reduced by the HFO dietary intervention, which contributes to the improvements in glucose intolerance and dyslipidaemia.
In conclusion, we have shown that the HFO, but not LFO, dietary intervention could reduce weight gain and improve glucose intolerance and dyslipidaemia in postnatal overfed rats. Moreover, the implementation of the HFO dietary intervention at the beginning of the post-weaning, puberty or post-puberty periods could improve glucose utilisation and dyslipidaemia in postnatal overfed rats, but only the intervention during the post-weaning period could significantly reverse obesity and down-regulate 11β-HSD1 expression both in adipose tissue and hepatic tissue. These characteristics may have potential therapeutic implications for appropriate dietary fatty acid compositions and interventional timing with respect to the development of adipose tissue and the prevention of obesity and the metabolic syndrome induced by postnatal overfeeding.
Acknowledgements
This study was supported by the Natural Science Foundation of China (81273064), the National Program on Key Basic Research Project (2013CB530604), the Key Program of Nanjing Public Health Bureau (ZKX14011) and the Jiangsu province Research Project (BE2015607).
The authors’ contributions are as follows: Y. D. and X. L. conceived and designed the experiment. Y. D., F. Y., N. Z., L. S., S. Z. and J. W. performed the experiments. Y. D. and X. L. analysed the data. Y. D. and X. L. wrote the manuscript. All the authors read and approved the final version of the manuscript.
The authors state that there is no conflicts of interest.















