An overwhelming body of evidence indicates that non-enzymic glycation of proteins is implicated in a number of biochemical abnormalities associated with ageing and diabetes. The glycation reaction occurs between the carbonyl group of sugars and the amino group of proteins (predominantly the ɛ-amino group of lysine and the guanidine group of arginine), which finally results in the formation of advanced glycation endproducts (AGE)(Reference Baynes, Watkins and Fisher1–Reference Sing, Barden and Mori3). Glycosylated Hb (HbA1c) is one of the abundant glycated proteins, used most widely in monitoring the glycaemic status of individuals with diabetes(Reference Bry, Chen and Sacks2). Although AGE formation takes place during the ageing process, it is accelerated in hyperglycaemic conditions. Glycation alters protein conformation and stability and induces protein aggregation and cross-linking(Reference Kumar, Reddy and Kumar4, Reference Kumar, Mrudula and Mitra5). It has been shown that the formation of AGE in vivo contributes to several pathophysiologies associated with ageing and diabetes mellitus, such as arthritis, atherosclerosis, chronic renal insufficiency, Alzheimer's disease, nephropathy, neuropathy and cataract(Reference Sing, Barden and Mori3, Reference Vlassara6–Reference Brownlee9). Many cells possess receptors for AGE (RAGE). Interaction of AGE with RAGE leads to activation of NF-κB, which stimulates the generation of pro-inflammatory and adhesion molecules that underlie the pathology of diabetic vascular complications(Reference Sing, Barden and Mori3, Reference Yan, Schmidt and Anderson10–Reference Bengmark12). The degree of accumulation of AGE is related to the severity of diabetic complications such as nephropathy, neuropathy, retinopathy and cataract(Reference Sing, Barden and Mori3, Reference Brownlee9).
Since studies have shown the role of AGE in promoting diabetic complications, it is considered that inhibition of AGE formation may prevent the progression of diabetic complications. However, designing a drug having anti-AGE activity is a challenge due to the complexity of reactions involved in the formation of AGE. A nucleophilic hydrazine compound, aminoguanidine, has shown promising results in vitro and in animal models in terms of inhibition of AGE formation and has entered into phase three clinical trials(Reference Sing, Barden and Mori3, Reference Edelstein and Brownlee13, Reference Freedman, Wuerth and Cartwright14). However, the trial was terminated due to various safety concerns(Reference Sing, Barden and Mori3, Reference Freedman, Wuerth and Cartwright14). A number of other agents such as pyridoxamine, carnosine, taurine, 2-isopropylidenehydrazono-4-oxo-thiazolidin-5-ylacetanilide (OPB-9195) and phenyl thiazolium bromide have been investigated in several in vitro and in vivo studies and have shown promising results(Reference Sing, Barden and Mori3, Reference Stitt, Gardiner and Alderson15, Reference Vasan, Zhang and Zhang16). However, except pyridoxamine, none has progressed, as yet, to the stage of clinical trials. Thus, there is a need for developing new antiglycating agents combining higher levels of efficacy, selectivity and safety in humans. Many dietary agents, particularly spices, are a major part of traditional medicine that has been practised to control many chronic ailments including diabetes(Reference Swanston-Flatt, Flatt and Day17–Reference Bailey and Day20). Hence, in the present study we investigated the antiglycating activity of aqueous extracts of plant materials that are commonly used in the diet and/or traditional medicine. An in vitro protein glycation system was utilised to evaluate the antiglycating potential of these agents with a multi-pronged approach.
Materials and methods
Materials
Fructose, bovine serum albumin (BSA), keyhole limpet haemocyanin (KLH), ribonuclease (RNase), methylglyoxal (MGO), Freund's complete and incomplete adjuvant, m-aminophenyl boronic acid and horseradish peroxidase-conjugated goat anti-rabbit antibody were obtained from Sigma-Aldrich (St Louis, MO, USA). Glyoxylic acid and sodium cyanogen bromide were purchased from ICN Biochemicals (Aurora, OH, USA). All other chemicals and solvents used were of analytical grade.
Preparation of aqueous extracts of dietary agents
Dietary material was obtained fresh from the local market. The edible parts of the material were freeze-dried and powdered. The following dietary materials were used: fruits of Malus pumila (apple), Momordica charantia (bitter gourd), Psidium guajava (guava), Citrus limettoides (lemon), Cucumis sativus (cucumber) and Citrus sinensis aurantium (orange); seeds of Cuminum cyminum (cumin), Brassica juncea (mustard), Piper nigrum (black pepper) and Trachyspermum ammi (omum); leaves of Ocimum sanctum (basil), Camellia sinensis (green tea) and Spinaceae oleracea (spinach); bulb of Allium sativum (garlic) and A. sepa (onion); bark of Cinamomum zeylencium (cinnamon); root of Zingiber officinalis (ginger). Aqueous extracts (5 %) were prepared from these materials, stirring at room temperature for 3 h. Insoluble material was removed by centrifugation followed by filtration. The water extracts were freeze-dried and stored under desiccation at 4°C. The selection criteria of plants relied on their hypoglycaemic activity(Reference Swanston-Flatt, Flatt and Day17–Reference Bailey and Day20) and also on the fact that they are commonly consumed in the diet or used as herbal remedies in various pathological conditions including diabetes.
Preparation of advanced glycation endproduct antigens
AGE-RNase and AGE-BSA were prepared as described previously(Reference Makita, Vlassara and Cerami21). Briefly, RNase (25 mg/ml) or BSA (50 mg/ml) was incubated with 1 m-glucose in 0·2 m-phosphate buffer (pH 7·4) containing 0·05 % sodium azide at 37°C for 90 d. Low-molecular-weight reactants and unbound sugars were removed by extensive dialysis. Carboxymethyllysine (CML)-KLH was prepared as described previously(Reference Kumar, Reddy and Srinivas22). MGO-BSA was prepared as described previously(Reference Kumar, Reddy and Kumar4).
Production of polyclonal anti-advanced glycation endproduct antibodies
Antibodies were produced against AGE-RNase, CML-KLH and MGO-BSA by immunising female New Zealand white rabbits as described previously(Reference Kumar, Reddy and Kumar4, Reference Kumar, Reddy and Srinivas22). Rabbits aged 8–12 weeks were immunised at multiple sites on their backs by subcutaneous administration of a 1 ml solution containing 1 mg/ml AGE-protein antigen in Freund's complete adjuvant and subsequently three boosters were given at 3-week intervals in Freund's incomplete adjuvant. For testing the titres, rabbits were bled intermittently from the ear vein and the titre was checked by immunoblot analysis. The rabbits were bled after the last booster, and the serum was collected by centrifugation. Animal care and protocols were followed in accordance with and approved by the Institutional Animal Ethics Committee. Antiserum was partially purified by ammonium sulfate fractionation followed by diethylaminoethyl-sepharose anion exchange chromatography to obtain the IgG-rich fraction.
In vitro glycation of proteins
Eye lens soluble protein was prepared from 6-month-old goat lenses as described previously(Reference Kumar, Kumar and Reddy23). A 10 % homogenate of goat lenses was prepared in PBS (pH 7·4) and centrifuged at 10 000 g for 30 min at 4°C. The supernatant fraction (lens soluble protein) was used for in vitro glycation. Each 1 ml reaction mixture contained 10 mg of lens soluble protein, 0·2 m-phosphate buffer (pH 7·4), 0·1 m-fructose, 50 μg of penicillin and streptomycin and 0·01 % sodium azide. Reactions tubes were incubated in the dark at 37°C for 3 weeks. At the end of the incubation, unbound sugars were removed by dialysis against the same buffer. Protein concentration was determined by Lowry's method using BSA as standard. Stock solutions of all the reaction contents were filtered through 0·20 μm syringe filters. The rationale behind using the lens protein as a model protein was its longevity and susceptibility to extensive accumulation of AGE, which is accelerated in diabetes-associated pathologies(Reference Kumar, Reddy and Kumar4, Reference Kumar, Mrudula and Mitra5, Reference Kumar, Reddy and Srinivas22, Reference Kumar, Kumar and Reddy23).
Inhibition studies with dietary extracts
For inhibition studies, concentrated stocks of the aqueous extracts of the dietary agents were prepared in water. Stock solutions of the dietary extracts were filtered through 0·20 μm syringe filters. Various concentrations of these extracts (typically 0·01, 0·1, 1·0 and 10 mg/ml) were added to the in vitro protein glycation assay mixture and incubated in the dark at 37°C for 3 weeks as described above. At the end of the incubation, unbound reactants were removed by dialysis and protein concentration was determined as described above. The extent of protein glycation in the absence and presence of dietary aqueous extracts was evaluated by monitoring protein cross-linking on SDS-PAGE, AGE-related non-tryptophan fluorescence, protein carbonyl content, phenyl boronate affinity chromatography and immunoblotting as described below. The percentage of inhibition with extracts was calculated, considering the extent of glycation in the absence of extract as 100 %.
Fluorescence measurements
Non-tryptophan AGE fluorescence was monitored using 0·15 mg/ml protein in 20 mm-sodium phosphate buffer (pH 7·4) by exciting at 370 nm; emission was recorded between 400 and 500 nm using a spectrofluorometer (Jasco FP-6500; Jasco, Tokyo, Japan). The formation of high-molecular-weight (HMW) aggregates and cross-linked peptides obtained as a result of protein glycation of eye lens soluble protein was monitored by SDS-PAGE using 12 % gels.
Glyco-oxidative damage
Non-enzymic glycation-mediated glyco-oxidative damage of lens proteins was monitored by estimating total protein carbonyls according to the method of Uchida et al. (Reference Uchida, Kanematsu and Sakai24).
Immunodetection of advanced glycation endproducts
The formation of specific AGE products was detected with immunoblotting using anti-CML-KLH, anti-MGO-BSA and anti-AGE-RNase antibodies. Glycated proteins were resolved on a 12 % SDS-PAGE. After SDS-PAGE, proteins were transferred onto nitrocellulose membrane. The membrane was incubated for 2 h in blocking buffer containing 5 % skimmed milk powder. Subsequently, it was incubated with the respective primary antibodies (AGE-RNase, 1:1000; CML-KLH, 1:2000; MGO-BSA, 1:2000) separately. The membrane was then incubated with horseradish peroxidase-conjugated goat anti-rabbit antibody (1:5000), and substrate buffer containing diaminobenzidine and H2O2 was used for detection.
Affinity chromatography
The extent of glycation of lens proteins in the absence and presence of dietary agents was determined using phenyl boronate affinity chromatography(Reference Kumar, Reddy and Srinivas22, Reference Kumar, Kumar and Reddy23). In the present study, 5 mg of glycated lens protein was passed through a phenyl boronate affinity column (8 × 1 cm) equilibrated with 0·25 m-ammonium acetate buffer (pH 8·5) containing 0·05 m-MgCl2. The unbound fraction containing non-glycated protein was washed with the above buffer, while bound glycated protein was eluted using 0·1 m-2-amino-2-hydroxymethyl-propane-1,3-diol-HCl (pH 7·5) containing 0·2 m-sorbitol.
Statistical analysis
Results were expressed as mean values and standard deviations. Data were analysed using SPSS software (version 15·0; SPSS, Inc., Chicago, IL, USA). Mean values were compared by one-way ANOVA with post hoc tests of the least significant difference method. Heterogeneity of variance was tested by the non-parametric Mann–Whitney test. Differences between comparisons of groups were considered to be significant at P < 0·05.
Results
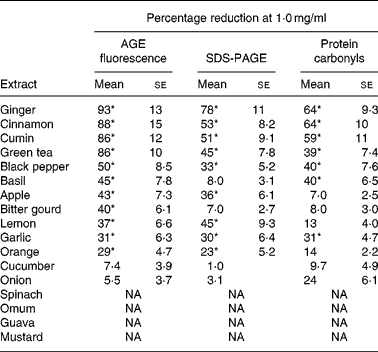
Out of the seventeen dietary materials tested, the aqueous extracts of five or six agents showed significant antiglycating activity. Though the extracts showed a dose-dependent inhibition of protein glycation with concentrations ranging from 0·01 to 10 mg/ml, the response varied with the extract and with the method of detection of glycation. For example, while the aqueous extracts of ginger, cinnamon, cumin and green tea decreased AGE fluorescence by 80–90 % at the concentration of 1 mg/ml, black pepper, basil, apple, bitter gourd, lemon, orange and garlic extract showed 30–50 % inhibition at the same concentration (Fig. 1 and Table 1). Despite their heterogeneity, propensity to form covalent cross-links is the common consequence of AGE, which leads to the formation of HMW aggregates on proteins. We monitored the fructose-induced cross-links of lens proteins (above 30 kDa) and also HMW aggregates above 200 kDa by SDS-PAGE (not shown). In terms of preventing protein cross-links and HMW aggregates of proteins, the aqueous extracts of ginger, cinnamon, cumin, green tea, lemon, apple, garlic and black pepper showed effects of 30–80 % at the concentration of 1 mg/ml (Fig. 2 and Table 1). The extracts of cinnamon, ginger, cumin, black pepper, basil, green tea and garlic were effective against glyco-oxidative damage as they could prevent the formation of protein carbonyl by 30–65 % at the concentration of 1 mg/ml (Fig. 3 and Table 1).
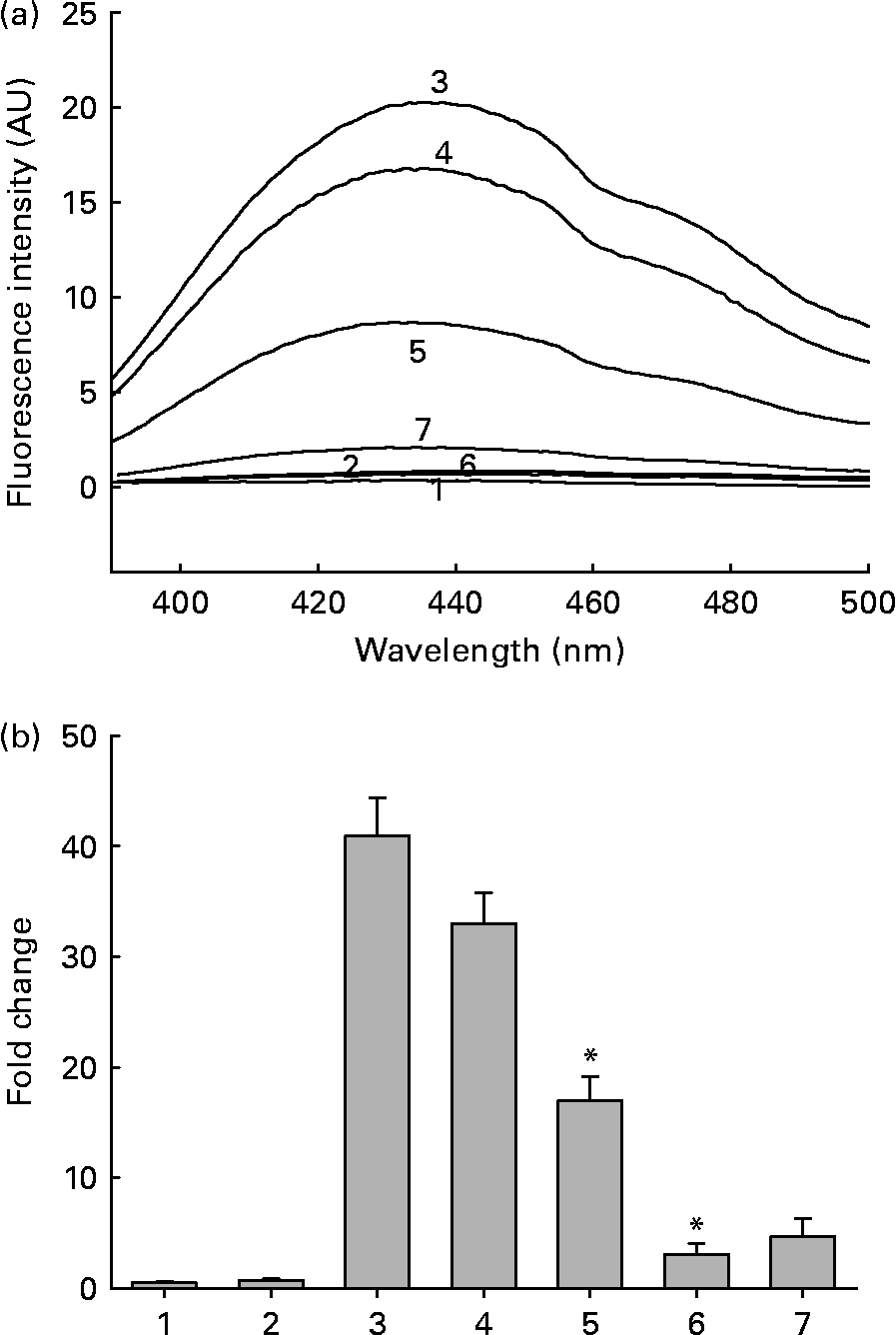
Fig. 1 (a) Representative non-tryptophan advanced glycation endproduct (AGE)-related fluorescence of soluble lens protein upon in vitro glycation in the absence and presence of aqueous extracts of ginger. Trace 1, protein alone; trace 2, protein+1·0 mg/ml extract; trace 3, protein+100 mm-fructose; trace 4, protein+fructose+0·01 mg/ml extract; trace 5, protein+fructose+0·1 mg/ml extract; trace 6, protein+fructose+1·0 mg/ml extract; trace 7, 1·0 mg/ml extract alone; AU, arbitrary units. (b) Fold change in non-tryptophan AGE fluorescence was calculated considering the emission intensity (at 440 nm) of protein alone as one-fold. Bars 1–7 correspond to traces 1–7 in (a). Values are the means of three independent experiments, with standard errors represented by vertical bars. * Mean value was significantly different from that of bar 3 (P < 0·05).
Table 1 Antiglycating activity of aqueous extracts of dietary agents against in vitro glycation of lens protein†
(Mean values with their standard errors of three independent experiments)
AGE, advanced glycation endproducts; NA, no activity.
* Statistically significant (P < 0·05).
† Percentage reduction of AGE by aqueous extracts was determined considering the formation of AGE in the absence of extracts as 100 % as evaluated by AGE fluorescence (Fig. 1), SDS-PAGE (Fig. 2) and protein carbonyl content (Fig. 3). The relative ability of the extracts to reduce AGE formation was determined at 1·0 mg/ml.

Fig. 2 Densitometry quantification of cross-linked and aggregated proteins upon in vitro glycation in the absence and presence of aqueous extracts of ginger based on SDS-PAGE analysis (not shown). Intensity of protein bands above 31 kDa was quantified considering the intensity of protein alone as 100 %. Bar 1, protein alone; bar 2, protein+100 mm-fructose; bar 3, protein+fructose+0·01 mg/ml extract; bar 4, protein+fructose+0·1 mg/ml extract; bar 5, protein+fructose+1·0 mg/ml; bar 6, protein+1·0 mg/ml extract; bar 7, 1·0 mg/ml extract alone; AU, arbitrary units. Values are the means of three independent experiments, with standard errors represented by vertical bars. * Mean value was significantly different from that of bar 2 (P < 0·05).
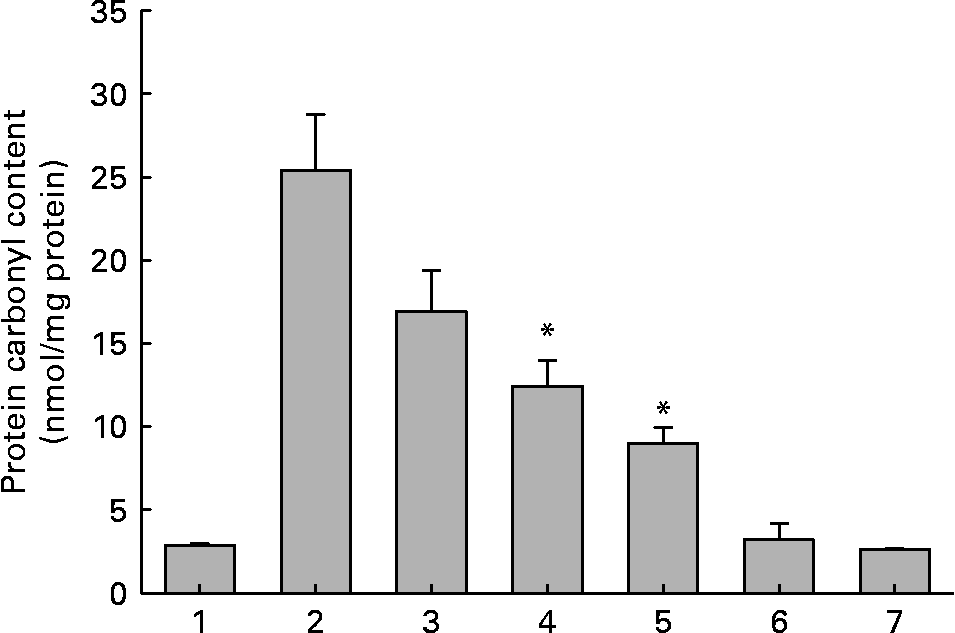
Fig. 3 Protein carbonyl content of soluble lens protein upon in vitro glycation in the absence and presence of aqueous extracts of ginger. Trace 1, protein alone; trace 2, protein+100 mm-fructose; trace 3, protein+fructose+0·01 mg/ml extract; trace 4, protein+fructose+0·1 mg/ml extract; trace 5, protein+fructose+1·0 mg/ml extract; trace 6, protein+1·0 mg/ml extract; trace 7, 1·0 mg/ml extract alone. Values are the means of three independent experiments, with standard errors represented by vertical bars. * Mean value was significantly different from that of bar 2 (P < 0·05).
Based on relative ability to reduce protein glycation with three complementary methods, i.e. AGE fluorescence, protein cross-linking and carbonyl content of the protein, the antiglycating activity of ginger, cinnamon and cumin was noteworthy, as these three agents prevented AGE formation by more than 50 % at 1·0 mg/ml in all of the three complementary methods. While black pepper, green tea, apple, basil, lemon and garlic exhibited moderate but significant antiglycating activity, the rest of the agents were not significant in reducing the in vitro protein glycation. Further, we investigated the impact of the inhibition of protein glycation by ginger, cinnamon and cumin using boronate affinity chromatography. The proportion of glycated protein was 46 % upon incubation of lens soluble proteins with fructose for 21 d (Fig. 4). Reduction in the amount of glycated protein in the presence of extract was evaluated considering the amount of glycated protein in the absence of extracts as 100 %. Aqueous extracts of ginger, cinnamon and cumin reduced the amount of glycated protein in a dose-dependent manner. For example, extracts of ginger, cinnamon and cumin reduced glycated protein by 63, 54 and 44 %, respectively, at the concentration of 1·0 mg/ml, whereas at the concentration of 0·1 mg/ml the reduction was found to be 45, 42 and 30 %, respectively.

Fig. 4 The effect of aqueous extracts of ginger on the amount of glycated protein in soluble lens protein upon in vitro glycation as analysed by phenyl boronate affinity chromatography. (—), Protein alone; (–··–··–), protein+100 mm-fructose; (····), protein+fructose+0·01 mg/ml ginger; (–·–·–), protein+fructose+0·1 mg/ml ginger; (– – –), protein+fructose +1·0 mg/ml ginger. OD, optical density.
Although the above-described results showed the ability of dietary extracts to inhibit or prevent AGE formation in an empirical manner, these chemical methods might not be able to help in bringing about the specific inhibition or prevention of particular AGE products by these extracts. Hence, we investigated the ability of ginger, cinnamon and cumin to decrease specific AGE formation by immunodetection. We used three different anti-sera raised against CML-KLH, MGO-BSA, AGE-RNase and AGE-BSA. Data obtained with immunoblotting demonstrated the presence of diverse antigenic determinants over the protein (Fig. 5). For example, anti-CML-KLH detected HMW aggregates >118 kDa along with some cross-linked species of approximately 45 and 20–30 kDa. Anti-MGO-BSA detected cross-linked species between 20–30 and 36–45 kDa along with HMW aggregates >118 kDa. Anti-AGE-RNase demonstrated the presence of HMW aggregates >118 kDa and also cross-linked species at about 85 kDa. Results with anti-AGE-BSA were similar to that of anti-AGE-RNase (data not shown). Densitometry analysis indicated that AGE antigens detected by CML and MGO antisera were more prominent than those detected by glucose antisera (Fig. 5). Nonetheless, the aqueous extracts of cinnamon, ginger and cumin reduced the formation of all the above-mentioned antigenic AGE structures on lens proteins in a dose-dependent manner (Fig. 5 and Table 2). At 1·0 mg/ml, while these three extracts could reduce CML- and MGO-derived AGE by approximately 70–80 %, glucose-derived AGE products were reduced by approximately 60 % (Table 2). Since these AGE are the main protagonists of diabetic complications, their reduction by ginger, cinnamon and cumin highlights their antiglycating ability and in turn potential in ameliorating diabetic complications.

Fig. 5 Immunodetection of advanced glycation endproducts (AGE) in soluble lens protein. (a) Representative Western blot profile of soluble lens protein upon in vitro glycation in the absence and presence of aqueous extracts of cinnamon. Blots were probed with anti-carboxymethyllysine-keyhole limpet haemocyanin (top), anti-methylglyoxal-bovine serum albumin (middle) and anti-AGE-ribonuclease antibodies (bottom). Lane 1, molecular-weight markers; lane 2, protein alone; lane 3, protein+100 mm-fructose; lane 4, protein+fructose+0·01 mg/ml extract; lane 5, protein+fructose+0·1 mg/ml extract; lane 6, protein+fructose+1·0 mg/ml extract. (b) Densitometry analysis of AGE. Intensity of AGE signals was quantified considering the intensity of lane 2 as 100 %. Bars 1–5 correspond to lanes 2–6 in (a). Values are the means of three independent experiments, with standard errors represented by vertical bars. * Mean value was significantly different from that of bar 2 (P < 0·05).
Table 2 Reduction of antigenic advanced glycation endproduct (AGE) structures by aqueous extracts of ginger, cinnamon and cumin as evaluated by immunoblotting†
(Mean values with their standard errors of three independent experiments)

CML, carboxymethyllysine; KLH, keyhole limpet haemocyanin; MGO, methylglyoxal; BSA, bovine serum albumin; RNase, ribonuclease.
* Mean value was significantly different from that in the absence of dietary extracts (P < 0·05).
† Degree of antigenic AGE was assessed by Western blot (Fig. 5 (a)) and quantified by densitometry analysis (Fig. 5 (b)). Percentage of reduction in AGE formation in the presence of the extracts was estimated using densitometry analysis considering the density of AGE in the absence of extract as 100 %.
Discussion
Prolonged exposure to uncontrolled chronic hyperglycaemia in diabetes can lead to various complications, both of macro- and microvascular nature, such as cardiovascular, renal, neurological and ocular(Reference Gabir, Hanson and Dabelea25, Reference Brownlee26). In view of the widespread prevalence of diabetes in Southeast Asian countries(Reference Mohan, Sandeep and Deepa27–Reference Zimmet30), secondary complications of diabetes may pose major health problems in this part of the world. Non-enzymic glycation (the Maillard reaction) is a complex series of reactions between reducing sugars and free amino groups of lysine residues of proteins, which leads to browning and cross-linking of the proteins. The reaction is initiated by the reversible formation of a Schiff's base, which undergoes a rearrangement to form a relatively stable Amadori product. The Amadori product further undergoes a series of reactions through dicarbonyl intermediates to form AGE(Reference Baynes, Watkins and Fisher1–Reference Sing, Barden and Mori3). AGE is a collective term referred to a heterogeneous group of chemical structures such as protein–AGE adducts (CML), protein–protein cross-links (pentosidine) and glyoxal- or MGO-derived–lysine dimers(Reference Bry, Chen and Sacks2, Reference Sing, Barden and Mori3, Reference Yan, Schmidt and Anderson10, Reference Huebschmann, Regensteiner and Vlassara31). Further, food-derived AGE (from dairy products, bread, meat, tobacco and many processed foods), as a result of the Maillard reaction, have become an unrecognised risk factor for diabetic complications(Reference Sing, Barden and Mori3, Reference Bengmark12). In view of the ever-expanding role of AGE in diabetes and age-associated pathologies, it has been suggested that inhibition of the formation of AGE may prevent the progression of diabetic complications and slow down the ageing process(Reference Strippoli, Di Paolo and Cincione32). Although some recent studies highlighted the antiglycating potential of a few natural sources, namely garlic(Reference Ahmad and Ahmed33), green tea(Reference Nakagawa, Yokozawa and Terasawa34) and tomato(Reference Kiho, Usui and Hirano35), adequate work has not yet been done.
The results of the present study showing the inhibition of protein glycation by dietary agents merit attention in many respects. Most of these plant and spice materials are expected to be largely free from adverse effects as they are either consumed as dietary components routinely or used in traditional medicines. Glycation and AGE-induced toxicity are known to be associated with increased free radical production. Recent studies have demonstrated the benefits of using compounds with combined antiglycation and antioxidant properties(Reference Ahmad and Ahmed33, Reference Nakagawa, Yokozawa and Terasawa34). Such compounds not only prevent AGE formation but also reduce free radical-mediated toxicity. In this context, most of the antiglycating agents described in the present study are expected to have antioxidant potential. Furthermore, the low-molecular-weight compounds such as polyphenols, phenolic acids or flavonoids, which are known to have antioxidant activity, are likely to be present in aqueous extracts(Reference Cai, Luo and Sun36, Reference Balasundram, Sundram and Samman37). Hence it is the cumulative effect of antioxidant and antiglycating activities that might contribute to effective action. However, the in vitro results may not reflect the effects of these agents in vivo, as they undergo some cooking process followed by the liver first-pass effect, which invariably affect the content, activity and bioavailability of bioactive compounds; hence further investigations are needed to address these issues. Thus we have investigated the impact of the antiglycating potential of a few prominent agents such as cumin(Reference Kumar, Reddy and Srinivas22) and ginger(Reference Saraswat, Reddy and Suryanarayana38) and dietary agents with other properties(Reference Suryanarayana, Saraswat and Mrudula39, Reference Suryanarayana, Saraswat and Petrash40) under in vivo conditions using the streptozotocin-induced diabetic cataract rat model. Irrespective of the effect of cooking and digestion on content and bioavailability, beneficial effects observed with these agents in animal models (under in vivo conditions)(Reference Kumar, Reddy and Srinivas22, Reference Saraswat, Reddy and Suryanarayana38–Reference Suryanarayana, Saraswat and Petrash40) provide an indication of their potential utility for the management of diabetic complications.
Activation of the polyol pathway due to increased aldose reductase (ALR)-2 activity per se and/or along with protein glycation has also been implicated in the development of diabetic complications(Reference Brownlee26). Several studies suggest that the compounds that inhibit ALR2 could be effective in the prevention of certain complications(Reference Raskin and Rosenstock41). In addition to the antiglycating property described in the present study, previously we have demonstrated that many of these agents including cinnamon, cumin, basil, and black pepper inhibited ALR2 (M Saraswat and GB Reddy, unpublished results). Therefore, multiple properties of these agents might make them more potent to prevent diabetic complications. In addition to the above-described dietary agents, we have previously shown that curcumin from turmeric(Reference Suryanarayana, Saraswat and Mrudula39) and tannoids of Emblica officinalis (Reference Suryanarayana, Saraswat and Petrash40) delay diabetic cataract in rats mainly through inhibition of eye lens ALR2. Attempts are underway to identify the active principles responsible for inhibition of protein glycation by the dietary agents used in the study and to investigate their ability to delay or prevent diabetic complications.
Nutritional intervention has been shown to have an important role in the management of diabetes and its complications. Current dietary strategies are centred on nutrients, energy restriction, and antioxidant and hypoglycaemic effects but are not focused on the antiglycating activity of and ALR2 inhibition by dietary components. Further, food- (or exogenously) derived AGE have become an unrecognised risk factor for diabetic complications(Reference Bengmark12). Therefore, effective restriction of those dietary components and/or modulation of food-derived AGE by blending those foods with the diet sources having antiglycating potential should be considered for the optimal management of AGE-mediated pathologies, particularly for those who are at risk of developing diabetic complications.
Acknowledgements
Part of this work was presented at the Annual Meeting of the Association for Research in Vision and Ophthalmology (ARVO) held at Fort Lauderdale (FL, USA) during 27 April to 1 May 2008.
M. S. received a research fellowship from the Indian Council of Medical Research, Government of India; P. M. received a research fellowship from the University Grants Commission, Government of India; G. B. R. received grants from the Indian Council of Medical Research and the Department of Science and Technology, Government of India.
M. S. was involved in data collection, data analysis, data interpretation, literature search and manuscript preparation. P. Y. R. and P. M. were involved in data collection, data analysis and data interpretation. G. B. R. was involved in study design, data analysis, data interpretation, literature search, manuscript preparation and review of the manuscript.
There were no conflicts of interest.









