Dinosaurs first evolved in the Late Triassic, but remained a relatively sparse component of ecosystems until after the end-Triassic mass extinction. During the Middle Jurassic, the group underwent a significant evolutionary radiation and they became the dominant vertebrates on land for the subsequent 100 million years (Benson et al. Reference Benson, Campione, Carrano, Mannion, Sullivan, Upchurch and Evans2014; Benson Reference Benson2018). However, our understanding of the mode, tempo, and evolutionary drivers of this radiation are hindered by the globally sparse fossil record for dinosaurs at this time. For example, the Paleobiology Database (www.paleobiodb.org) records just 430 occurrences of Middle Jurassic dinosaurian body fossils globally. In contrast, the much better-known Late Jurassic record preserves 2100 occurrences of Dinosauria (data downloaded June 2020). This makes all contributions to the Middle Jurassic dinosaur fossil record significant.
The Inner Hebrides of Scotland yield rare Middle Jurassic dinosaur remains, but until now these have been exclusively from the Isle of Skye. The Bajocian–Bathonian Great Estuarine Group on Skye provides a vivid picture of the diverse Middle Jurassic ecosystem of the Inner Hebrides. It comprises a series of lagoonal and deltaic sedimentary rocks (Andrews Reference Andrews1985) that have yielded a wealth of vertebrate material, including marine, terrestrial and flying archosaurs, turtles, squamates, lissamphibians, tritylodontids, and mammaliaforms (e.g., Evans et al. Reference Evans, Barrett, Hilton, Butler, Jones, Liang, Parrish, Rayfield, Sigogneau-Russell, Underwood, Barrett and Evans2006; Anquetin et al. Reference Anquetin, Barrett, Jones, Moore-Fay and Evans2009; Anquetin Reference Anquetin2010; Wills et al. Reference Wills, Barrett and Walker2014; Young et al. Reference Young, Tennant, Brusatte, Challands, Fraser, Clark and Ross2016a; Panciroli Reference Panciroli, Benson and Walsh2017a, Reference Panciroli, Walsh, Fraser, Brusatte and Corfeb, Reference Panciroli, Benson and Butler2018, Reference Panciroli, Benson and Luo2019, Reference Panciroli, Benson, Walsh, Butler, Castro, Jones and Evans2020; Yi et al. Reference Yi, Tennant, Young, Challands, Foffa, Hudson, Ross and Brusatte2017).
Dinosaur body fossils in the Great Estuarine Group remain exceptionally rare and are often fragmentary (see review in Clark Reference Clark2018). From the Bathonian Valtos Sandstone Formation, they include a sauropod limb bone (Clark et al. Reference Clark, Boyd, Dixon and Ross1995; Liston Reference Liston2004), a sauropod tooth (Clark & Gavin Reference Clark and Gavin2016), two theropod teeth (Brusatte & Clark Reference Brusatte and Clark2015; Young et al. Reference Young, Hendrickx, Challands, Foffa, Ross, Butler and Brusatte2019), and a possible basal coelurosaurian theropod caudal vertebra (Brusatte & Clark Reference Brusatte and Clark2015). Finds from other formations within the Great Estuarine Group and underlying units include a theropod limb bone (Benton et al. Reference Benton, Martill and Taylor1995), an isolated theropod tooth (Young et al. Reference Young, Hendrickx, Challands, Foffa, Ross, Butler and Brusatte2019), isolated sauropod teeth (Barrett Reference Barrett2006), and a thyreophoran proximal ulna and radius (Clark Reference Clark2001). Dinosaur ichnofossil tracks range from isolated tracks on loose boulders (from the Valtos Sandstone Formation, Clark & Gavin Reference Clark and Gavin2016, from other formations in the Great Estuarine Group, Andrews & Hudson Reference Andrews and Hudson1984; Clark et al. Reference Clark, Ross and Booth2005), to extensive in situ trackway sites (from the Valtos Sandstone Formation, Marshall Reference Marshall2005, and from other formations in the Great Estuarine Group, Clark et al. Reference Clark, Booth, Booth and Ross2004; Marshall Reference Marshall2005; Brusatte et al. Reference Brusatte, Challands, Ross and Wilkinson2016; dePolo et al. Reference dePolo, Brusatte, Challands, Foffa, Ross, Wilkinson and Yi2018, Reference dePolo, Brusatte, Challands, Foffa, Wilkinson, Clark, Hoad, da Pereira, Ross and Wade2020).
The Isle of Eigg has long been recognised for its fossils, particularly the ‘Hugh Miller Reptile Bed’, named for the prolific Victorian stonemason turned palaeontologist, geologist, and writer, Hugh Miller (1802–1856) who discovered it (Miller Reference Miller1858). The reptile bed is part of the Bathonian Lealt Shale Formation (formerly ‘Estheria Shales’; Hudson Reference Hudson1962, Reference Hudson1963), which underlies the Valtos Sandstone Formation (Andrews Reference Andrews1985; Barron et al. Reference Barron, Lott and Riding2012). Vertebrate fossils from the Lealt Shale Formation mainly comprise isolated skeletal and dental remains of sharks, marine turtles, crocodylomorphs, and plesiosaurs (Hudson Reference Hudson1966; Benton Reference Benton, Spencer, Benton and Spencer1995). A single purported dinosaur tooth from Eigg was mentioned by Rees & Underwood (Reference Rees and Underwood2005), but this specimen was not figured and has subsequently been lost, so the identification cannot be confirmed. Despite extensive explorations of the island by Miller and contemporaries, and subsequent attention from geologists and palaeontologists in the latter half of the 20th Century (e.g., Hudson Reference Hudson1962, Reference Hudson1963, Reference Hudson1966; Harris & Hudson Reference Harris and Hudson1980; Andrews Reference Andrews1985), no archosaur material has been discovered in any of the other exposed sections of the Great Estuarine Group on Eigg until now.
Herein, we describe the first unequivocal Mesozoic dinosaur specimen to be found in Scotland outside of Skye. The specimen is an indeterminate limb bone – probably from a stegosaur – that was found in shoreline exposures of the Valtos Sandstone Formation on the Isle of Eigg. The specimen described here is poorly preserved, hindering higher-level taxonomic assignment, but this limitation does not negate the significance of this fossil both in the context of the Scottish dinosaur body fossil record, and for our knowledge of Middle Jurassic dinosaur palaeo-distribution.
1. Geological setting
The Great Estuarine Group (Harris & Hudson Reference Harris and Hudson1980; ‘Great Estuarine Series’ of Judd Reference Judd1878, p. 722) crops out in the Scottish Inner Hebridean islands of Skye, Muck, Eigg, and Raasay (though contemporaneity of formations between the isles is by no means certain) (Fig. 1). It comprises six formations (the Cullaidh Shale Formation, Elgol Sandstone Formation, Lealt Shale Formation, Valtos Sandstone Formation, Duntulm Formation, and the Kilmaluag Formation) of Bajocian–Bathonian (Middle Jurassic) age, consisting of sedimentary rocks dominated by sandstone and mudstone, with subordinate shelly, algal, and dolomitic limestone beds (Harris & Hudson Reference Harris and Hudson1980; Barron et al. Reference Barron, Lott and Riding2012). Environments represented include shallow marine, saline, and freshwater lagoons, with tidally influenced littoral lagoons, fluvial delta lobes, and alluvial floodplains and mudflats (Barron et al. Reference Barron, Lott and Riding2012).
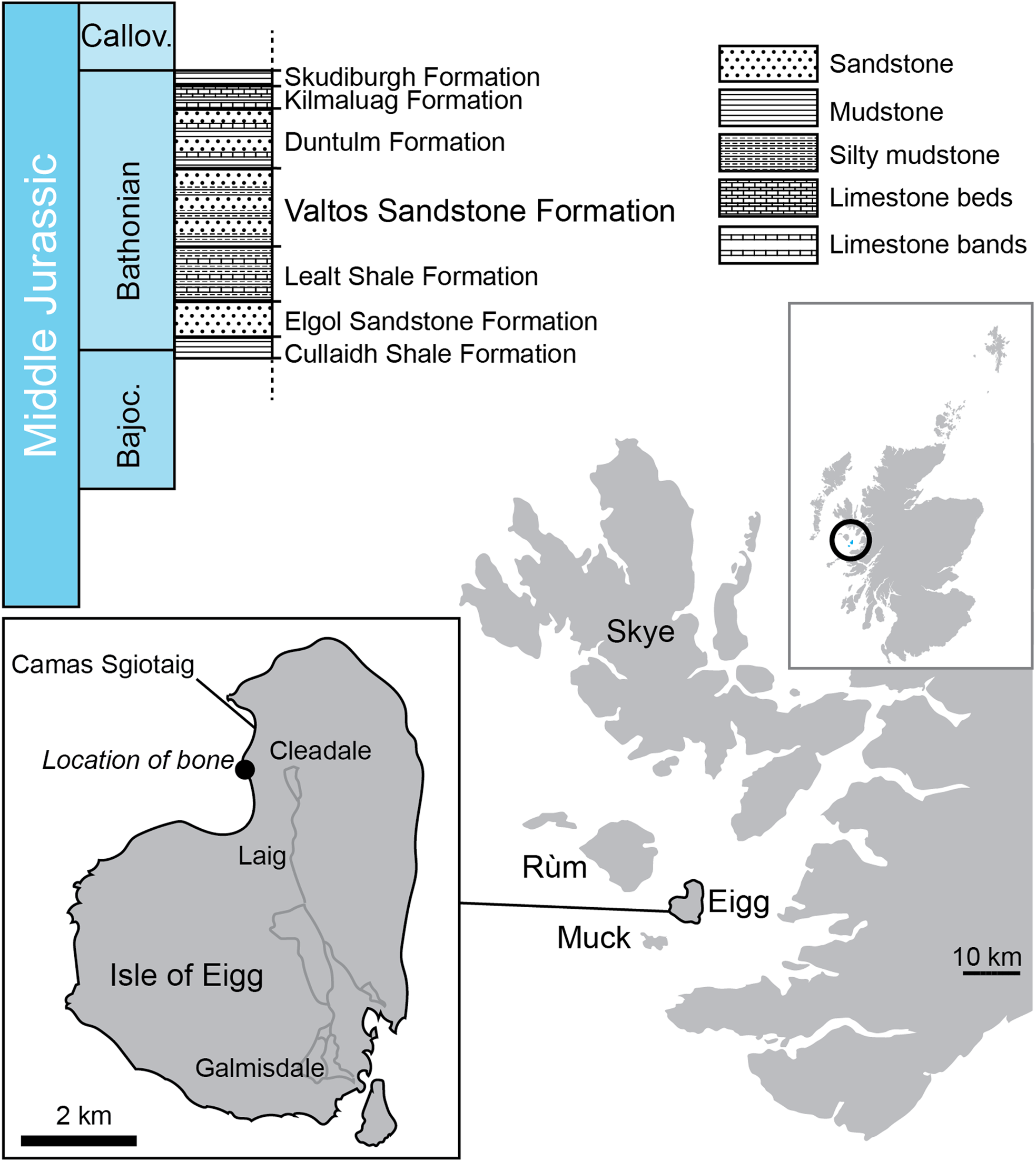
Figure 1 The lithostratigraphy of the Great Estuarine Group and location at Camas Sgiotaig on the Isle of Eigg where NMS G.2020.10.1 was found.
The Valtos Sandstone Formation is named after the village of Valtos on the Trotternish Peninsula on the Isle of Skye, near the type section [NG 517 638 to NG 509 653] (Harris & Hudson Reference Harris and Hudson1980, pp. 240–243). It is underlain by the Lealt Shale Formation and overlain by the Duntulm Formation. Fossils found include: invertebrates such as the bivalve Neomiodon and gastropod Viviparus; trace fossils Lockeia, Monocraterion, Planolites, Thalassinoides, and tridactyl and ovoid footprints; coniferous wood; and fragmentary dinosaur and crocodyliform body fossils (Hudson & Harris Reference Hudson and Harris1979; Andrews & Hudson Reference Andrews and Hudson1984; Clark et al. Reference Clark, Boyd, Dixon and Ross1995; Barron et al. Reference Barron, Lott and Riding2012; Brusatte & Clark Reference Brusatte and Clark2015; Clark & Gavin Reference Clark and Gavin2016; Young et al. Reference Young, Tennant, Brusatte, Challands, Fraser, Clark and Ross2016a). The Valtos Formation represents a tidally influenced shallow littoral lagoon, frequently inundated by fluvial delta lobes, and with evidence of periodic emergence (Barron et al. Reference Barron, Lott and Riding2012). This interpretation is supported by brackish to freshwater palynomophs such as Botryococcus (Riding et al. Reference Riding, Walton and Shaw1991).
A section of the Valtos Sandstone Formation is exposed on the north-western shore of the Isle of Eigg at Camas Sgiotaig (the ‘singing sands’) and the bay of Laig [NM 468 905 to NM 472 885]. The limb bone NMS G.2020.10.1 was found in a loose block within Camas Sgiotaig, in a broken, but originally sub-spherical, calcite-cemented sandstone concretion (sensu Wilkinson Reference Wilkinson1992). On the Isle of Eigg, such concretions are only known from the Valtos Sandstone Formation, confirming the provenance of the specimen.
2. Materials and methods
The limb bone NMS G.2020.10.1 is part of the collection at National Museums Scotland (NMS), Edinburgh, UK. It was found by EP on a loose boulder below the high tide line south of Camas Sgiotaig on the Isle of Eigg in May 2017, during fieldwork funded by the National Geographic Society, including team members SLB, EP, TJC, PEdP, DF, and MW. It was subsequently collected under permit by SLB, DR, and DG, using a rock saw to extract the specimen. Preparation was carried out by NL: the bone was consolidated using Paraloid B72 at 5–10% in acetone, then pneumatic circular saws followed by pneumatic pens were used to remove surrounding matrix. A small <1 cm section of the bone was accidentally removed during removal of excess matrix (Fig. 2c). The natural mould of the missing mid-section of the bone was filled in with Jesmonite acrylic resin with some fibreglass matting with grey pigment.

Figure 2 NMS G.2020.10.1, a probable thyreophoran limb bone from the Isle of Eigg, Scotland. (A) NMS G.2020.10.1 in matrix after initial prep. (B–E) NMS G.2020.10.1 removed from matrix and partially reconstructed: (B) the eroded ‘upper’ surface; (C) the surface that was downwards into the matrix; (D) and (E) side views of NMS G.2020.10.1. Scale bar = 100 mm.
A photogrammetric model of NMS G.2020.10.1 was created by MH using photographs taken on a Nikon D5300 and uploaded and reconstructed in Agisoft Photoscan Professional version 1.4.5. The resulting mesh was repaired and optimised in Blender 2.8.1 and then exported in .fpx format. This can be accessed freely on Sketchfab at https://sketchfab.com/3d-models/eigg-dinosaur-bone-6e670b8f91e947d6860f14016540c7bb.
A portion of the mid-shaft was removed at a natural break for osteohistological analysis by GFF, and a transverse thin section made following a modified petrographic sectioning procedure (Lamm Reference Lamm, Padian and Lamm2013). The piece was embedded in Buehler Epothin II epoxy resin under a vacuum (−1 bar) and left to cure at room temperature for 24 h. The block was sectioned in a transverse plane using a Buehler Isomet 1000 precision saw with a table saw attachment and a diamond-tipped wafering blade. The cut billet was mounted to a polycarbonate plastic slide using Buehler Epothin II epoxy, which was left to cure at room temperature for 12 h. The mounted billet was resectioned to a thickness of 0.7 mm using a Buehler Isomet 1000 precision saw. The resectioned slide was hand-ground on a glass plate using a sequence of 220-grit, 600-grit, and 1200-grit silicon carbide abrasive powders, until the desired optical contrast was achieved. The final slide thickness was ~180 μm. The slide was polished on a short nap cloth and on a nap cloth with mineral oil to improve optical clarity.
The slide was photographed using a Nikon D7200 DSLR camera with a Nikkor 60-mm micro lens and a Nikon SB-600 Speedlight to produce transmitted light. Detailed images were taken using a Leica DMLP transmitted-light polarising microscope under normal light using Leica Application Suite 4. The polycarbonate sheet used for the slide is anisotropic, which interferes with cross-polarisation of the thin-section, so only images under normal light were taken. Images were stitched together using Adobe Photoshop 2020. Where adjustments to contrast, brightness, or colour balance were required, these modifications were applied to the entire image. Osteocyte lacunar density was calculated using the method of Cullen et al. (Reference Cullen, Evans, Ryan, Currie and Kobayashi2014). Histological terminology follows Francillon-Vieillot et al. (Reference Francillon-Vieillot, de Buffrenil, Castanet, Gkraudie, Meunier, Sire, Zylberberg, de Ricqlès and Carter1990) and Padian & Lamm (Reference Padian and Lamm2013).
Measurements of NMS G.2020.10.1 were checked using photogrammetry models. Measurements and some figures for the comparative taxa were taken from Benson (Reference Benson2010), Holwerda et al. (Reference Holwerda, Rauhut and Polin press), and Remes et al. (Reference Remes, Ortega, Fierro, Joger, Kosma, Ferrer, Ide and Maga2009) in combination with the authors' (EP, FH, SCRM) own photographs of specimens.
Institutional abbreviations. CGP = Council for Geosciences, Pretoria, South Africa; ISIR = Indian Statistical Institute, Kolkata, India; MACN = Museo Argentino de Ciencias Naturales, Buenos Aires, Argentina; MOR = Museum of the Rockies, Montana, USA; NHMUK (previously BMNH) = Natural History Museum, London, UK; NMS = National Museums Scotland, Edinburgh, UK; OUMNH = Oxford University Museum of Natural History, Oxford, UK; PVL = Paleontologia de Vertebrados Lillo, Universidad Nacional de Tucuman, Tucman, Argentina; QMF = Queensland Museum, Brisbane, Australia.
3. Systematic palaeontology
Superorder Dinosauria Owen, Reference Owen1842 Order Ornithischia Seeley, Reference Seeley1887 Suborder Thyreophora Nopcsa, Reference Nopcsa1915 (sensu Norman, Reference Norman, Reif and Westphal1984)
Material. NMS G.2020.10.1, an isolated hind limb bone (Fig. 2).
Locality. Bathonian (Middle Jurassic) Valtos Sandstone Formation, Great Estuarine Group. Found on a loose boulder on the shoreline S of Camas Sgiotaig, Isle of Eigg, Scotland.
Description. NMS G.2020.10.1 is badly eroded along most of its length where exposed to weathering by the sea. It is also missing the proximal and distal ends, and is slightly compressed along its length. The total preserved length is 64 cm, and the bone is broken into two halves. Without a definitive identification, it is not possible to say which is the proximal or distal end. One half of the bone has been worn longitudinally, leaving a depth of ~5 cm of bone and exposing the internal structure (Fig. 2). The centre of the shaft is missing, but there was a natural mould of the shaft in the rock, which was used to make a reconstruction (Fig. 2) The moulded section is approximately 14 cm in length and 7.3 cm at the narrowest transverse width. There is a longitudinal ridge on the bone shaft, beginning near the break and extending to the margin of one of the broken ends (Fig. 2c), where the bone flares laterally on one side. The extent of this lateral projection is unclear because the rest of the bone is broken and missing. At least two potential tooth marks are visible on the opposite end of the bone, measuring ~2 cm in length and ~0.2 cm deep (Fig. 3a). A layer of small molluscs (probably Neomiodon) are present on the underside of the bone (Fig. 3b).

Figure 3 Possible bite marks (indicated by arrows) (A) and molluscs (B) on underside of NMS G.2020.10.1. Scale bars = 50 mm.
In the absence of the epiphyses and without a complete shaft of the bone, identification presents a challenge. Based on dimensions, comparative anatomy, and histology (see below), we suggest that NMS G.2020.10.1 is probably a stegosaur fibula.
4. Possible identity of NMS G.2020.10.1
4.1. Marine reptiles and crocodylomorphs
A variety of marine reptile (ichthyosaurs and plesiosaurs) and various crocodylomorph fossils are known from the Middle Jurassic formations of the Inner Hebrides, mostly from the Isle of Skye (Lee & Buckman Reference Lee and Buckman1920; Arkell Reference Arkell1933; Hudson Reference Hudson1966; Martill Reference Martill1985; Clark et al. Reference Clark, Nimmo and Nicholas1993; Benton et al. Reference Benton, Martill and Taylor1995; Brusatte et al. Reference Brusatte, Challands, Ross and Wilkinson2016; Young et al. Reference Young, Tennant, Brusatte, Challands, Fraser, Clark and Ross2016a; Yi et al. Reference Yi, Tennant, Young, Challands, Foffa, Hudson, Ross and Brusatte2017). Marine reptile remains from the Isle of Eigg comprise disarticulated plesiosaurian bones from the ‘Hugh Miller Bonebed’ (Miller Reference Miller1858; Hudson Reference Hudson1966).
Despite the presence of marine reptiles and crocodylomorphs in these outcrops, we do not consider NMS G.2020.10.1 to belong to any of these groups. The large size of NMS G.2020.10.1 excludes it from belonging to any of the small-bodied crocodylomorph taxa found in the Middle Jurassic of the Hebrides (e.g., Young et al. Reference Young, Tennant, Brusatte, Challands, Fraser, Clark and Ross2016a; Yi et al. Reference Yi, Tennant, Young, Challands, Foffa, Hudson, Ross and Brusatte2017). Although larger-bodied thalattosuchian crocodylomorphs (teleosauroids and metriorhynchoids) have been recovered from contemporaneous Middle Jurassic formations elsewhere (Mannion et al. Reference Mannion, Benson, Carrano, Tennant, Judd and Butler2015; Wilberg Reference Wilberg2015; Johnson et al. 2019), no thalattosuchians have yet been reported from Scotland. Even the largest thalattosuchian femora are much smaller than NMS G.2020.10.1 (e.g., ~45 cm in Lemmysuchus obtusidens and Machimosaurus mosae; Hua Reference Hua1999; Young et al. Reference Young, Márton, Bell, Foffa, Steel, Sachs and Peyer2016b; Johnson et al. Reference Johnson, Young, Steel, Foffa, Smith, Hua, Havlik, Howlett and Dyke2017). The fibula/tibia is shortened compared to their femur – a modification linked to their aquatic lifestyle (Foffa et al. Reference Foffa, Johnson, Young, Steel and Brusatte2019). In addition, NMS G.2020.10.1 has several features that make it unlikely to belong to this clade. For example, thalattosuchian femora have a sigmoidal profile with an oval cross-section, which is not seen in NMS G.2020.10.1 (Andrews Reference Andrews1913; Hua & De Buffrenil Reference Hua and De Buffrenil1996), although compression and poor preservation make the cross-sectional geometry difficult to assess. In large thalattosuchians, the cranial bones attained a length comparable to NMS G.2020.10.1, but the absence of articular facets, alveoli, or dermal ornamentation that characterise most thalattosuchian cranial bones (Andrews Reference Andrews1913) make this identification incompatible with the morphology seen here. NMS G.2020.10.1 differs histologically from crocodylomorphs in the predominance of fibrolamellar bone with abundant osteons and the absence of parallel-fibered bone in the cortex. Whereas fibrolamellar or woven bone is occasionally present in some crocodylomorphs (Woodward et al. Reference Woodward, Horner and Farlow2014; Cubo et al. Reference Cubo, Köhler and de Buffrénil2017), their cortices are usually formed exclusively of parallel-fibered or lamellar bone with simple vascular canals or sparse primary osteons (Hua & De Buffrénil Reference Hua and De Buffrenil1996; de Andrade & Sayão Reference de Andrade and Sayão2014; Sayão et al. Reference Sayão, Bantim, Andrade, Lima, Saraiva, Figueiredo and Kellner2016; Cubo et al. Reference Cubo, Köhler and de Buffrénil2017). For these reasons, we preclude this bone from being identified as that of a crocodylomorph.
The large size of NMS G.2020.10.1 also rules out attribution to an ichthyosaur or plesiosaur. Furthermore, the overall shape of NMS G.2020.10.1 does not match that of marine reptiles. The limbs of the latter are highly modified for underwater propulsion, being reduced or absent compared to terrestrial animals – as in all fully marine tetrapods (Andrews Reference Andrews1910). Plesiosaur and ichthyosaur limbs have short, robust humeri and femora with flared proximal and/or distal ends. The propodeal bones (radius, ulna, tibia, fibula) of these taxa are highly modified into short, often polygonal elements in the paddle (McGowan & Motani Reference McGowan and Motani2003; Benson Reference Benson, MacLeod, Archibald and Levin2013). The microstructure of marine reptile bones is also significantly different from that of terrestrial animals, typically showing either osteoporotic or pachyostotic textures (Hua & De Buffrenil Reference Hua and De Buffrenil1996; Houssaye Reference Houssaye2013) and does not match that found in the histological section of NMS G.2020.10.1 (see 5. Osteohistology).
4.2. Theropoda
Theropod dinosaurs were the primary terrestrial carnivores during the Middle Jurassic. They ranged from those with small body masses similar to many extant birds, to medium- to large-sized genera like Megalosaurus bucklandii (Benson Reference Benson2010), which reached body masses of ~1.4 tons (Benson et al. Reference Benson, Campione, Carrano, Mannion, Sullivan, Upchurch and Evans2014) and lengths of ~8–9 m, and Eustreptospondylus oxoniensis (Sadlier et al. Reference Sadlier, Barrett and Powell2008). Both of these taxa are well-known basal tetanurans from the Middle Jurassic of England. Medium to large ceratosaurians and potentially mid-sized basal coelurosaurs (tyrannosauroids) were also present globally during this time (see review by Hendrickx et al. Reference Hendrickx, Hartman and Mateus2015).
If NMS G.2020.10.1 is a theropod dinosaur, it would belong to a mid-to-large-sized taxon. Based on size and proportions, the only theropod skeletal element NMS G.2020.10.1 could be is a femur. The length-to-width ratio is similar to the femur of Middle Jurassic M. bucklandii (Benson Reference Benson2010, fig. 16) and E. oxoniensis (Sadlier et al. Reference Sadlier, Barrett and Powell2008, fig. 19) (Table 1; Fig. 4a–d). The lateral projection at one end of NMS G.2020.10.1 may correspond to the neck of the femoral head (with the head missing), and the opposite end may represent the distal end of a femur with the beginning of an epicondylar (flexor or extensor) groove (Fig. 4). However, the bone lacks the prominent lesser (= anterior) and fourth trochanters that characterise theropod femora. The longitudinal ridge on one half of NMS G.2020.10.1 may be the base of a lesser trochanter, but the lesser trochanters of mid-to-large-sized theropods project strongly from the anterior surface of the bone, whereas this ridge is less prominent in NMS G.2020.10.1. Even the less pronounced lesser trochanter of Eustreptospondylus (Fig. 3c, d) is more pronounced than in NMS G.2020.10.1. We consider it unlikely that the ridge is a damaged remnant of a more pronounced trochanter, as its surface is smooth and unbroken. It is also unlikely that a more prominent lesser trochanter was present in life but not observable because the bone has broken distal to it. If NMS G.2020.10.1 is a theropod femur, the lateral projection of the presumed proximal end indicates that only a moderate portion of the head is missing proximally. Therefore, the region that would have included the trochanter is preserved, but lacks this predominant feature. The fourth trochanter should also be visible along the posterior surface of the shaft, but appears to be absent. Although there is a large portion of the mid-shaft missing – meaning we cannot completely rule out the presence of a fourth trochanter – overall, we consider the identification of NMS G.2020.10.1 as a theropod femur unlikely.

Figure 4 Postcranial elements of theropod dinosaurs Megalosaurus and Eustreoptospondylus. Megalosaurus bucklandii NHMUK PV OR31806 femur 31806 in anterior (A) and posterior (B) view; Eustreptospondylus oxoniensis OUMNH J.13558 femur in anterior (C) and posterior (D) view; Megalosaurus bucklandii OUMNH J.13575 humerus in anterior view (E); Megalosaurus bucklandii NHMUK PV OR31809 tibia in anterior (F) and posterior (G) view. Scale bar = 100 mm.
Table 1 Measurements of NMS G.2020.10.1 and other Middle Jurassic dinosaur limb bones. Data from Benson (Reference Bensonin press), Holwerda et al. (Reference Holwerda, Rauhut and Polin press), Remes et al. (Reference Remes, Ortega, Fierro, Joger, Kosma, Ferrer, Ide and Maga2009), and authors own photographs of specimens.

1 Measurements are estimated due to missing proximal and distal ends of NMS G.2020.10.1 and compression and erosion of mid-shaft, and should be considered with caution.
NMS G.2020.10.1 is much larger, longer, and more slender than the humerus, radius, or ulna of M. bucklandii (Benson Reference Benson2010, figs 12, 13) (Fig. 4e), Eustreptospondylus (Sadlier et al. Reference Sadlier, Barrett and Powell2008, fig. 16), and other mid- to large-bodied theropods, which were all bipedal animals with much shorter forelimbs than hind limbs. Although NMS G.2020.10.1 has a similar length and width as the tibia of Megalosaurus, NHMUK PV OR31809 (Fig. 4f, g), it lacks the twist of the shaft from proximal to distal ends observed in the tibia of Megalosaurus, Eustreptospondylus (Sadlier et al. Reference Sadlier, Barrett and Powell2008, fig. 20), or other theropods, and also lacks any sign of the prominent cnemial and fibular crests. NMS G.2020.10.1 also does not match the gracile and distally tapering morphology of theropod fibulae.
Histological analysis does not support the identification of NMS G.2020.10.1 as belonging to a theropod. The cancellous medullary cavity of NMS G.2020.10.1 is unlike the limb bones of most theropod dinosaurs, which are hollow. The pectoral and pelvic girdle elements of theropods have a cancellous medullary cavity, but it is difficult to reconcile the gross morphology of NMS G.2020.10.1 with these bones: theropod scapulae are flat, strap-like bones, which is not the case in NMS G.2020.10.1, and there is no evidence of a pubic apron, pubic boot, or obturator process in NMS G.2020.10.1, which eliminates a pubis or ischium as a candidate.
4.3. Sauropoda
The body fossil record for Middle Jurassic Sauropoda is relatively scarce compared to that of the Late Jurassic or Cretaceous. Material is known from China, India, North Africa, Argentina, and the UK. From the Bajocian–Bathonian of Oxfordshire and Gloucestershire, sauropods are represented by Cetiosaurus oxoniensis (Upchurch & Martin Reference Upchurch and Martin2002, Reference Upchurch and Martin2003). Finds from the NW of Scotland provide additional indeterminate sauropod material, comprising incomplete limb elements and single teeth (Clark et al. Reference Clark, Boyd, Dixon and Ross1995; Liston Reference Liston2004; Barrett Reference Barrett2006; Clark & Gavin Reference Clark and Gavin2016; Clark Reference Clark2018).
The small size of NMS G.2020.10.1 makes it likely that if it is a sauropod limb bone, it represents a juvenile animal. The femora of contemporaneous sauropods such as Cetiosaurus and Patagosaurus fariasi are more robust than NMS G.2020.10.1, with a lower length-to-width ratio (Fig. 5a; Table 1). Cetiosaurid femora, even in juveniles, are usually anteroposteriorly flattened and mediolaterally wide, creating an elliptical cross-section (Holwerda et al. Reference Holwerda, Rauhut and Polin press). This shape contrasts with NMS G.2020.10.1, which has a more gracile and rounded mid-shaft (Fig. 2). However, features congruent with a sauropod femur include the curved, lateral projection at one end of NMS G.2020.10.1, which may correspond to the base of the greater trochanter, and the groove visible at the opposite end, which may represent the epicondylar groove at the distal end of the femur (Fig. 2b, c). The femur of Early Jurassic sauropod taxon Barapasaurus tagorei has closer proportions to NMS G.2020.10.1 (Table 1; Fig. 5b, c), suggesting that if NMS G.2020.10.1 is a sauropod femur, it belonged to a gracile taxon, and possibly not a cetiosaurid – Barapasaurus is currently placed outside of the cetiosaurid clade (Holwerda & Pol Reference Holwerda and Pol2018).

Figure 5 Postcranial elements of sauropod dinosaurs. Cetiosaurus oxoniensis femur OUMNH J.13615 in posterior view (a); Barapasaurus tagorei ISIR741 femur in anterior view (B) and posterior view (C); Cetiosaurus oxoniensis OUMNH J.29807 fibula in anterior view (D); Rhoetosaurus brownei QMF 1659 fibula in anterior (E) and posterior (F) view; Spinophrosaurus nigerensis GCP-CV-4429 fibula in anterior view (G); Tazoudasaurus naimi Pt-1 humerus in anterior (H) and posterior (I) view; Cetiosaurus oxoniensis OUMNH J.13611 ulna (J) and radius (K) in anterolateral view. Scale bar = 100 mm.
If NMS G.2020.10.1 belongs to a sauropod, we consider it most likely to be a fibula, as they are similar in length-to-width ratio to NMS G.2020.10.1 (Table 1). Sauropod fibulae bear a posterior projection on the distal end of the bone above the astragalar articular surface, as seen most clearly in Spinophorosaurus nigeriensis (Remes et al. Reference Remes, Ortega, Fierro, Joger, Kosma, Ferrer, Ide and Maga2009) (Fig. 5g), but also, to a lesser extent, in C. oxoniensis (Fig. 5d). This feature may correspond to the lateral projection at one end of NMS G.2020.10.1 (Fig. 3). Moreover, NMS G.2020.10.1 possesses a ridge which may correspond to the ridge for the accommodation of the tibia, similar to those in fibulae of the contemporaneous Cetiosaurus (Fig. 5d), and of the possibly Oxfordian Rhoetosaurus brownei (Fig. 5e, f). If that interpretation is correct, it would mean this end of NMS G.2020.10.1 corresponds to the proximal half. The somewhat triangular shape of this ‘proximal’ end of NMS G.2020.10.1 is similar to that in the juvenile Cetiosaurus OUMNH J.29807 and Rhoetosaurus QMF 1659.
The extreme mid-shaft compression and proximal and distal flaring of the humerus in sauropods is not present in NMS G.2020.10.1. All sauropods show this morphology, even among juvenile individuals such as Tazoudasaurus naimi Pt-1 (Allain & Aquesbi Reference Allain and Aquesbi2008) (Fig. 5h, i). Therefore, identification of NMS G.2020.10.1 as a sauropod humerus can be ruled out. There is no proximal flaring of the bone, as seen in the cnemial crest of sauropod tibiae. NMS G.2020.10.1 does not possess the slight sinusoidal curvature or proximal mediolateral widening and distal posterolateral widening seen in the radius of C. oxoniensis OUMNH J.13611 (Fig. 5k). Sauropod radii are oval in cross-section proximally (Upchurch et al. Reference Upchurch, Barrett, Dodson, Weishampel, Dodson and Osmólska2004), whereas NMS G.2020.10.1 is more triangular.
Although the length-to-width ratio is similar between NMS G.2020.10.1 and sauropod ulnae (Table 1), and there is a similar triangular cross-section near the proximal end of the bone, NMS G.2020.10.1 lacks the narrow distal end, as well as the triradiate anteromedial and anterolateral proximal expansions seen in sauropod ulnae – for example, C. oxoniensis OUMNH J.13611 (Fig. 5j).
4.4. Ornithischia
The Middle Jurassic body fossil record of Ornithischia is restricted to small, bipedal forms (e.g., Ruiz-Omenaca et al. Reference Ruiz-Omenaca, Pereda-Suberbiola, Galton and Carpenter2006), with the exception of the armoured dinosaurs, Thyreophora, which were the first ornithischians to attain large body mass and quadrupedality (Galton & Upchurch Reference Galton, Upchurch, Weishampel, Dodson and Osmólska2004; Barrett & Maidment Reference Barrett and Maidment2017). Thyreophoran remains are known from Middle Jurassic deposits in the UK, such as Loricatosaurus priscus and Sarcolestes leedsi from the Callovian Oxford Clay Formation (Galton Reference Galton1983, Reference Galton1985; Maidment et al. Reference Maidment, Norman, Barrett and Upchurch2008), indeterminate stegosaur remains from the Sharp's Hill Formation of Oxfordshire (Boneham & Forsey Reference Boneham and Forsey1992), and body fossils of thyreophorans from the Great Estuarine Group of the Isle of Skye (Clark Reference Clark2001). There are also trackways attributed to the ichnogenus Deltapodus from Skye (dePolo et al. Reference dePolo, Brusatte, Challands, Foffa, Wilkinson, Clark, Hoad, da Pereira, Ross and Wade2020) and the Middle Jurassic of Yorkshire (Whyte et al. Reference Whyte, Romano and Elvidge2007), attributed to a stegosaur trackmaker. Possible larger-bodied ornithopod footprints have recently been suggested for some of the trackways on Skye (Delair and Sarjeant, Reference Delair and Sarjeant1985, dePolo et al. Reference dePolo, Brusatte, Challands, Foffa, Wilkinson, Clark, Hoad, da Pereira, Ross and Wade2020), but no conclusive evidence for their presence is currently known.
The femora of thyreophorans are proportionally short and robust, with rounded shaft cross-sections (Fig. 6a, b). In contrast, the shaft of NMS G.2020.10.1 is slender and elongate, and flattened on one side (although this may have been accentuated by crushing). Humeri in thyreophorans are characterised by prominent deltopectoral crests that occupy much of the length of the bone, and flared distal ends (Fig. 6c, d), unlike the shape in NMS G.2020.10.1. The ulnae of thyreophorans are short, proportionally short and robust, and proximally triradiate (Fig. 6e, f), unlike NMS G.2020.10.1, and the radii are shorter and much less slender than NMS G.2020.10.1 (Fig. 6g, h). The cross-sectional geometry of NMS G.2020.10.1 is similar to the tibiae of thyreophorans (Fig. 6i, j), although the proximal and distal ends are much more flared than in NMS G.2020.10.1.

Figure 6 Postcranial elements of thyreophoran dinosaurs. Anterior views of Stegosaurus stenops NHMUK PV R36730 femora (A), humerus (C), ulna (E), radius (G), fused tibia and fibula (I); anterior view of Edmontonia sp. CMN 8531 femur (B); anterior view of Euoplocephalus tutus AMNH 5337 humerus (D) and radius (H); anterior view of Euoplocephalus tutus AMNH 5403 ulna (F); anterior view of Polacanthus foxii NHMUK PV R175 tibia with partial fibula fused to distal end (J); posterior view of Ankylosaurus magniventris AMNH 5214 fibula (K). Scale bar = 100 mm.
NMS G.2020.10.1 is similar in overall proportions and cross-sectional geometry to the fibulae of thyreophorans (Fig. 6i, k). It is possible, therefore, that NMS G.2020.10.1 is a fibula of a thyreophoran dinosaur. However, there are no thyreophoran synapomorphies of the fibula present (Raven & Maidment Reference Raven and Maidment2017) and so NMS G.2020.10.1 cannot be unequivocally referred to Thyreophora by comparative anatomy alone (but see 5. Osteohistology).
5. Osteohistology
A transverse thin section of NMS G.2020.10.1 shows it is extensively fractured and moderately crushed, which has collapsed some of the internal trabeculae (Fig. 7). Despite this damage, it is clear that the medullary cavity was not open, and that trabeculae extended throughout the medullary region. Cortical thickness is relatively high (~50%) in some regions, but varies around the cortex.

Figure 7 Overview of the osteohistology of NMS G.2020.10.1. (A) Column through the cortex, showing medullary spaces endosteally, dense Haversian bone throughout most of the cortex, and primary fibrolamellar bone in the outer cortex; (B) overview of entire slide, showing the arrangement of the medullary cavity and the cortex, and position of the LAG (arrow) in the middle cortex; (C) outer cortex, showing primary fibrolamellar bone with longitudinal–reticular vascularity and consistent vascularity to the periosteal surface; (D) outer cortex, showing zone of dense Haversian bone grading into primary fibrolamellar bone with a LAG (arrow), and a second, isolated zone of secondary remodelling. All images under normal light. Abbreviations: FLB = fibrolamellar bone; HB = Haversian bone; LAG = line of arrested growth; longvasc = longitudinal vascularity; Retvasc = reticular vascularity; SOs = secondary osteons; SR = secondary remodelling.
Most of the cortex is heavily remodelled, resulting in dense Haversian bone (Francillon-Vieillot et al. Reference Francillon-Vieillot, de Buffrenil, Castanet, Gkraudie, Meunier, Sire, Zylberberg, de Ricqlès and Carter1990), and, combined with expansion of the medullary cavity, this feature has obscured all primary bone in the inner cortex. The trabeculae of the medullary cavity are formed of lamellar bone (Francillon-Vieillot et al. Reference Francillon-Vieillot, de Buffrenil, Castanet, Gkraudie, Meunier, Sire, Zylberberg, de Ricqlès and Carter1990) with flattened osteocyte lacunae (Fig. 8d). Within the medullary spaces, linings of endosteally derived lamellar bone (Bromage et al. Reference Bromage, Lacruz, Hogg, Goldman, McFarlin, Warshaw, Dirks, Perez-Ochoa, Smolyar, Enlow and Boyde2009) are apparent. The size of the medullary spaces decreases periosteally (Fig. 7b), and close to the cortex, some of the medullary spaces resemble large secondary osteons (Fig. 7a). There is a stark transition between the zone of dense Haversian bone and the trabeculae of the medullary cavity. At this transition, the diameter of vascular spaces decreases significantly and no endosteal lamellar bone is visible between the secondary osteons. The zone of dense Haversian bone is defined here as the region where secondary remodelling completely obscures any intervening primary tissue. Secondary osteons within the zone of dense Haversian bone are longitudinally oriented and decrease in size periosteally (Fig. 7a). Endosteally, several overlapping generations of secondary osteons can be discerned, and in some areas, there are at least three and maybe four generations of secondary osteons (Fig. 8c). The density of secondary remodelling decreases periosteally, so that there are fewer overlapping secondary osteons, and more primary bone is visible between them (Fig. 7d). We interpret this zone as more representative of abundant secondary remodelling rather than true dense Haversian bone, because primary tissue is visible between the secondary osteons.
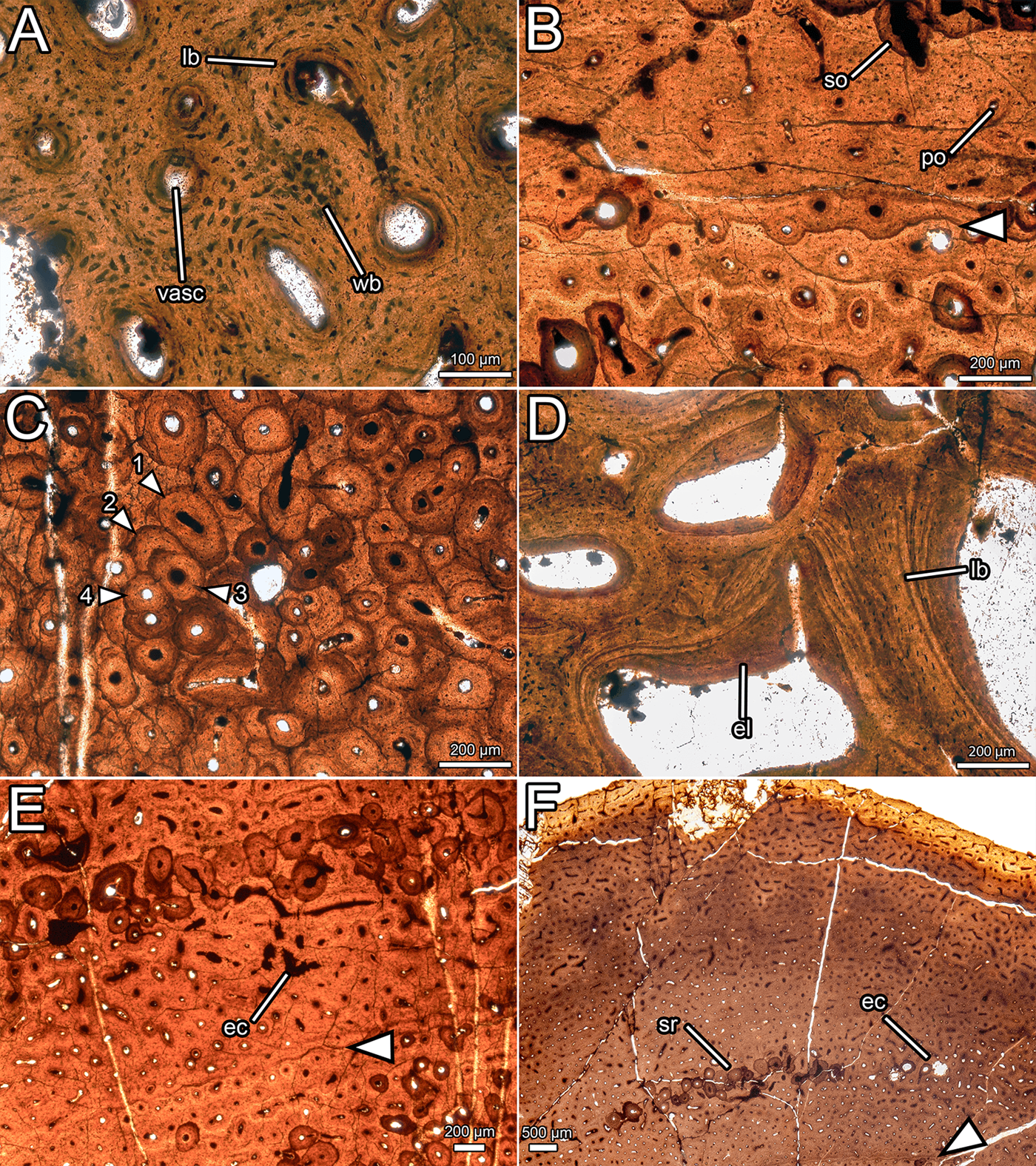
Figure 8 Histological details of NMS G.2020.10.1. (A) Primary osteons in the outer cortex, showing fibrolamellar bone matrix and variation in osteocyte shape and density; (B) primary and secondary osteons with a LAG (arrow) in the outer cortex; (C) overlapping generations of secondary osteons within the dense Haversian bone of the inner cortex (numbers indicate order of deposition); (D) trabeculae composed of lamellar bone and infilled with endosteal lamellae in the medullary cavity; (E) erosive cavities in the fibrolamellar bone separating the two zones of secondary remodelling; (F) outer cortex, showing consistent vascular orientation and density, localised secondary remodelling, and erosional cavities. All images under normal light. Abbreviations: ec = erosional cavity; el = endosteal lamellae; lb = lamellar bone; po = primary osteon; so = secondary osteon; sr = secondary remodelling; vasc = vascular canal; wb = woven bone.
In the thickest part of the cortex, an extensive area of primary bone is preserved towards the periosteal surface (Fig. 7c). This primary bone is fibrolamellar, with relatively sparse osteocyte lacunae (~14,500 mm−3). Osteocyte lacunae are lenticular where they surround primary osteons, but are denser and more globose in the intermittent areas of woven bone (Fig. 8a). The primary bone is relatively poorly vascularised (Fig. 7c) compared to most dinosaurs (Horner et al. Reference Horner, de Ricqlès and Padian1999; Horner & Padian Reference Horner and Padian2004; Padian et al. Reference Padian, Horner and De Ricqlès2004; Sander et al. Reference Sander, Klein, Stein, Wings, Klein, Remes, Gee and Sander2011a, Reference Sander, Christian, Clauss, Fechner, Gee, Griebeler, Gunga, Hummel, Mallison, Perry and Preuschoftb; Woodward et al. Reference Woodward, Freedman Fowler, Farlow and Horner2015), and the density, orientation, and size of vascular canals varies throughout the cortex. In most areas, vasculature is longitudinal in orientation, and these canals are randomly dispersed rather than arranged into circumferential rings (Fig. 7c). Several areas have a higher proportion of anastomosing canals and thus exhibit reticular vascularity, but these are confined to small, randomly distributed patches rather than continuous layers or zones (Fig. 7c). The diameter of the longitudinal vascular canals varies randomly from ~20 μm to ~100 μm, and even adjacent canals can be considerably different in size. Vascularity is consistent in density and orientation approaching the periosteal surface, and numerous vascular canals are open to the periosteal surface (Figs 7c, d, 8f). One line of arrested growth (LAG) is visible in the primary bone of the cortex, just periosteal to the zone of Haversian bone (Fig. 7d). There are no LAGs near the periosteal surface, nor is there development of an external fundamental system (Horner et al. Reference Horner, de Ricqlès and Padian1999; Woodward et al. Reference Woodward, Horner and Farlow2011, Reference Woodward, Freedman Fowler, Farlow and Horner2015).
A region of secondary remodelling is present within the primary cortical bone, about one third of the distance from the zone of dense Haversian bone to the periosteal surface, approximately 0.5–1 mm external to the LAG (Fig. 7d). This region is separated from the Haversian bone by a band of primary fibrolamellar bone (Fig. 7d) with small, longitudinally oriented canals, where the LAG is situated (Fig. 8b). The secondary osteons in the zone of remodelling are generally larger than those in the periosteal portion of the Haversian zone, and are more comparable in size to those nearer the medullary cavity. The secondary osteons in the zone of remodelling frequently interconnect, which creates a reticular pattern of vascularity overall (Fig. 7d). No cross-cutting of the secondary osteons can be detected, in contrast with the zone of Haversian bone. Where the primary bone is visible between the secondary osteons in this region of secondary remodelling, some erosive cavities can be discerned (Fig. 8e). These can be differentiated from primary osteons by their scalloped edges, created by Howship's lacunae, which are evidence of resorption by osteoclasts (Francillon-Vieillot et al. Reference Francillon-Vieillot, de Buffrenil, Castanet, Gkraudie, Meunier, Sire, Zylberberg, de Ricqlès and Carter1990).
Assuming NMS G.2020.10.1 is a hind limb bone, the bone matrix and internal structure exclude certain identifications. Fibrolamellar bone is known only in amniotes (Francillon-Vieillot et al. Reference Francillon-Vieillot, de Buffrenil, Castanet, Gkraudie, Meunier, Sire, Zylberberg, de Ricqlès and Carter1990), specifically in synapsids some marine reptiles (de Buffrénil & Mazin Reference de Buffrénil and Mazin1990; Klein Reference Klein2010; Kolb et al. Reference Kolb, Sánchez-Villagra and Scheyer2011; Houssaye et al. Reference Houssaye, Scheyer, Kolb, Fischer and Sander2014; Klein et al. Reference Klein, Houssaye, Neenan and Scheyer2015; Wintrich et al. Reference Wintrich, Hayashi, Houssaye, Nakajima and Sander2017), and archosaurs (Horner et al. Reference Horner, Padian and de Ricqlès2001; de Ricqlès et al. Reference de Ricqlès, Padian and Horner2003; Padian et al. Reference Padian, Horner and De Ricqlès2004). The large size of the bone precludes a synapsid identification, while the lack of highly porous (osteoporotic), or compacted (pachyostotic) bone rules out marine reptiles, which have these features as adaptations for a marine lifestyle (Houssaye Reference Houssaye2009; Houssaye et al. Reference Houssaye, Scheyer, Kolb, Fischer and Sander2014). Additionally, the cortical thickness of NMS G.2020.10.1 is greater than would be expected of pelagic animals like ichthyosaurs.
The histology of NMS G.2020.10.1 is most similar to that of dinosaurs, which typically have highly vascularised fibrolamellar zonal bone with LAGs (Horner et al. Reference Horner, de Ricqlès and Padian1999; Padian et al. Reference Padian, Horner and De Ricqlès2004; Padian & Lamm Reference Padian and Lamm2013). In contrast, crocodylomorphs and pseudosuchians more commonly show lamellar-zonal bone with lower vascularity and less fibrolamellar tissue (de Ricqlès et al. Reference de Ricqlès, Padian and Horner2003; de Andrade & Sayão Reference de Andrade and Sayão2014; Sayão et al. Reference Sayão, Bantim, Andrade, Lima, Saraiva, Figueiredo and Kellner2016; Cubo et al. Reference Cubo, Köhler and de Buffrénil2017), whereas pterosaurs have extensive medullary cavities with extremely thin cortical walls (de Ricqlès et al. Reference de Ricqlès, Padian, Horner and Francillon-Vieillot2000; Padian et al. Reference Padian, Horner and De Ricqlès2004).
Detailed comparative anatomy suggests that NMS G.2020.10.1 s most likely to be either a thyreophoran or sauropod fibula. The distinctive combination of osteohistological features in NMS G.2020.10.1 provides further clues, but uncertainty over the identity of the element makes the significance of certain features – like vascular orientation – unclear. Different bones of the same individual, and even different regions within the same bone, can produce markedly different histological signatures (Horner et al. Reference Horner, de Ricqlès and Padian1999; Cullen et al. Reference Cullen, Evans, Ryan, Currie and Kobayashi2014; Woodward et al. Reference Woodward, Horner and Farlow2014; Nacarino-Meneses et al. Reference Nacarino-Meneses, Jordana and Köhler2016). Smaller bones tend to grow at slower rates and may experience more rapid secondary remodelling (Horner et al. Reference Horner, de Ricqlès and Padian1999), and fibulae especially tend to show more remodelling. The pervasive remodelling in NMS G.2020.10.1 may, therefore, be the result of the element rather than taxonomic identity. However, as NMS G.2020.10.1 likely represents a large hind limb bone, its histology probably generally reflects the overall growth of the individual rather than solely exhibiting a biomechanical signal.
The microstructure of NMS G.2020.10.1 differs from theropod limb bones in that the medullary cavity is not hollow (Chinsamy Reference Chinsamy1990; Horner & Padian Reference Horner and Padian2004; Bybee et al. Reference Bybee, Lee and Lamm2006; Lee & O'Connor Reference Lee and O'Connor2013; Cullen et al. Reference Cullen, Evans, Ryan, Currie and Kobayashi2014). In theropods, some sparse trabeculae can be present in the medullary cavity where the diaphysis grades into the metaphysis. However, it is unlikely that the closed medullary cavity in NMS G.2020.10.1 is attributable to this phenomenon, because trabeculae completely fill the medullary cavity, and because the section was taken relatively close to the mid-shaft (Fig. 2). Sauropod osteohistology is well studied, and their limb bone cortices are characterised by a laminar vascular arrangement indicative of rapid growth (Sander Reference Sander2000, Reference Sander2004; Klein & Sander Reference Klein and Sander2008; Woodward & Lehman Reference Woodward and Lehman2009; Sander et al. Reference Sander, Klein, Stein, Wings, Klein, Remes, Gee and Sander2011a, Reference Sander, Christian, Clauss, Fechner, Gee, Griebeler, Gunga, Hummel, Mallison, Perry and Preuschoftb), even in smaller forms (Sander et al. Reference Sander, Mateus, Laven and Knötschke2006; Stein et al. Reference Stein, Csiki, Rogers, Weishampel, Redelstorff, Carballido and Sander2010). This arrangement is not the case in NMS G.2020.10.1, where vasculature is arranged randomly rather than into circumferential rows (Fig. 7c). Neosauropods tend to lack distinct LAGs (Sander et al. Reference Sander, Klein, Stein, Wings, Klein, Remes, Gee and Sander2011a, Reference Sander, Christian, Clauss, Fechner, Gee, Griebeler, Gunga, Hummel, Mallison, Perry and Preuschoftb), and in many cases growth marks are preserved instead as polish lines visible in reflected light (de Ricqlès Reference de Ricqlès1983). The presence of a LAG in NMS G.2020.10.1, therefore, argues against a neosauropod affinity for the specimen. The low osteocyte lacunar density of NMS G.2020.10.1 is further evidence against a sauropod affinity, as sauropods typically have much denser osteocyte lacunae than other comparably sized animals (Stein & Werner Reference Stein and Werner2013).
Of the possible dinosaur groups, the histology of NMS G.2020.10.1 is most similar to that of thyreophoran dinosaurs. The combination of predominantly longitudinal vascularity indicative of a relatively low growth rate and abundant secondary remodelling is seen in this group (Hayashi et al. Reference Hayashi, Carpenter and Suzuki2009; Redelstorff & Sander Reference Redelstorff and Sander2009; Redelstorff et al. Reference Redelstorff, Hübner, Chinsamy and Sander2013; Stein et al. Reference Stein, Hayashi and Sander2013; Maidment et al. Reference Maidment, Woodruff and Horner2018). Most osteohistological work on thyreophorans has focused on their osteoderms (e.g., Hayashi et al. Reference Hayashi, Carpenter and Suzuki2009; Burns & Currie Reference Burns and Currie2014; Horner et al. Reference Horner, Woodward and Bailleul2016), but a few studies have sampled long bones. In a review of ankylosaur osteohistology, Stein et al. (Reference Stein, Hayashi and Sander2013) noted abundant structural fibres within the primary and secondary bone of the limb elements of derived North American ankylosaurs. In contrast, stegosaurs lack structural fibres and have slightly less – but still abundant – secondary remodelling at equivalent ontogenetic stages (Hayashi et al. Reference Hayashi, Carpenter and Suzuki2009; Redelstorff & Sander Reference Redelstorff and Sander2009; Redelstorff et al. Reference Redelstorff, Hübner, Chinsamy and Sander2013; Stein et al. Reference Stein, Hayashi and Sander2013). Hayashi et al. (Reference Hayashi, Carpenter and Suzuki2009) sampled fibulae from an ontogenetic sequence of Stegosaurus, and Maidment et al. (Reference Maidment, Woodruff and Horner2018) sampled a fibula of the stegosaur Hesperosaurus. Both showed that vasculature in medium- to large-sized individuals was predominantly longitudinal with extensive secondary remodelling and the development of LAGs.
The histology of NMS G.2020.10.1 is remarkably similar to the medium- to large-sized Stegosaurus fibulae described by Hayashi et al. (Reference Hayashi, Carpenter and Suzuki2009), except that an external fundamental system is not developed. This difference could be explained by a slightly younger ontogenetic stage in NMS G.2020.10.1, as the external fundamental system is only developed late in life (Horner et al. Reference Horner, de Ricqlès and Padian1999; Woodward et al. Reference Woodward, Horner and Farlow2011, Reference Woodward, Freedman Fowler, Farlow and Horner2015). In this aspect, NMS G.2020.10.1 is more like the fibula of Hesperosaurus MOR 9728 described by Maidment et al. (Reference Maidment, Woodruff and Horner2018), which also lacks an external fundamental system. The two specimens are virtually identical in cross-sectional shape, and although the cortical thickness of MOR 9728 is greater than NMS G.2020.10.1, this could be because the samples were taken at different locations of the mid-shaft. MOR 9728 is more extensively remodelled than NMS G.2020.10.1, but where primary bone remains near the periosteal surface, the vasculature is sparse and longitudinally oriented, as in NMS G.2020.10.1.
The osteohistological signal of slow growth with extensive remodelling is evident in Kentrosaurus. Based on the femora, Kentrosaurus had a slightly faster growth rate than Stegosaurus or NMS G.2020.10.1, but still lower than other comparably sized ornithischians (Redelstorff & Sander Reference Redelstorff and Sander2009; Redelstorff et al. Reference Redelstorff, Hübner, Chinsamy and Sander2013). NMS G.2020.10.1 shares with stegosaurs the abundant secondary remodelling (Figs 2c, 7a), randomly arranged longitudinal–reticular vasculature (Fig. 7c), and the absence of the structural fibres, as present in ankylosaurs. Of the dinosaurian candidates, the histology of NMS G.2020.10.1 is, therefore, most consistent with stegosaurs.
The fibrolamellar bone matrix of NMS G.2020.10.1 is indicative of relatively high growth rates compared to more basal tetrapods (Francillon-Vieillot et al. Reference Francillon-Vieillot, de Buffrenil, Castanet, Gkraudie, Meunier, Sire, Zylberberg, de Ricqlès and Carter1990; Castanet et al. Reference Castanet, Rogers, Cubo and Jacques-Boisard2000; Padian & Lamm Reference Padian and Lamm2013). Based on the vascular canals within the primary cortical bone, the predominantly longitudinal vascularity with small regions of reticular vascularity suggests, however, that growth in this element was on the low end of the spectrum of fibrolamellar growth rates (Castanet et al. Reference Castanet, Rogers, Cubo and Jacques-Boisard2000; de Margerie Reference de Margerie2004). Vascularity at the periosteal surface and the absence of an external fundamental system also suggest this animal was actively growing at the time of death (Horner et al. Reference Horner, de Ricqlès and Padian1999; Woodward et al. Reference Woodward, Horner and Farlow2011, Reference Woodward, Freedman Fowler, Farlow and Horner2015).The position of the single LAG towards the middle of the cortex indicates considerable growth in the last year of life. The consistent density and orientation of vascularity in the periosteal portion of the cortex suggests that growth had not slowed, and that NMS G.2020.10.1 was in the maximum growth phase of its life when it died (Lee et al. Reference Lee, Huttenlocker, Padian, Woodward, Padian and Lamm2013).
Establishing the chronological age of NMS G.2020.10.1 is difficult because of the extensive secondary remodelling of the cortex and expansion of the medullary cavity. The combination of active growth and extensive secondary remodelling is unusual, as these typically characterise different phases of growth (Klein & Sander Reference Klein and Sander2008; Padian & Lamm Reference Padian and Lamm2013). Secondary remodelling usually progresses from the inner cortex outwards (Mitchell & Sander Reference Mitchell and Sander2014), and, therefore, Haversian bone in the outer cortex only occurs later in life (Kerley Reference Kerley1965; Klein & Sander Reference Klein and Sander2008). However, it can be induced by biomechanical stress or other environmental factors (Padian & Lamm Reference Padian and Lamm2013), which may explain the abundance of secondary osteons in conjunction with high growth rates. It is clear from the single LAG that this individual was at least one year old at the time of death, but it was almost certainly considerably older. The abundance of secondary remodelling and overlapping generations of secondary osteons are typically associated with advanced age (Kerley Reference Kerley1965; Uytterschaut Reference Uytterschaut, Grupe and Garland1993; Horner et al. Reference Horner, de Ricqlès and Padian1999; Klein & Sander Reference Klein and Sander2008; Sander et al. Reference Sander, Klein, Stein, Wings, Klein, Remes, Gee and Sander2011a, Reference Sander, Christian, Clauss, Fechner, Gee, Griebeler, Gunga, Hummel, Mallison, Perry and Preuschoftb). Unfortunately, retrocalculation of growth marks is not possible with only a single LAG (Cooper et al. Reference Cooper, Lee, Taper and Horner2008; Lee et al. Reference Lee, Huttenlocker, Padian, Woodward, Padian and Lamm2013), so the exact age of NMS G.2020.10.1 at death cannot be determined.
6. Conclusion
This specimen, NMS G.2020.10.1, is the first unequivocal dinosaur fossil found in Scotland outside of Skye. Identification of damaged isolated bones can be challenging, but finding ways to approach such identification is especially relevant for the dinosaur fossil record in Scotland, which comprises relatively incomplete material compared to contemporaneous sites in England.
Through detailed anatomical comparison, we find the overall proportions and cross-sectional geometry similar to the fibulae of thyreophorans. The length-to-width ratio and certain features such as a longitudinal ridge are similar to features present in a sauropod fibula, and NMS G.2020.10.1 bears resemblance to the fibula of juvenile Cetiosaurus. However, examination of the microstructure of the bone through histological analysis reveals a combination of predominantly longitudinal vascularity indicative of a relatively low growth rate, with abundant secondary remodelling – both strongly indicative of thyreophoran (particularly stegosaur) limb bone microstructure. The vascularity at the periosteal surface and absence of an external fundamental system indicate it belonged to a juvenile animal still rapidly growing at the time of death. We therefore consider NMS G.2020.10.1 most likely to represent a juvenile stegosaur fibula.
The presence of a thyreophoran bone on the Isle of Eigg adds a significant new datapoint for dinosaur distribution in the Middle Jurassic. The dinosaur body fossil record is sparse in Scotland, and this specimen provides evidence for a large-bodied animal in a locality previously not known for dinosaur fossils. Weathering, tooth marks, and a layer of small molluscs on the underside of the femur suggest transport and scavenging of the carcass prior to deposition, which is consistent with its entombment in the fluvio-deltaic Valtos Sandstone Formation.
This specimen increases the palaeontological significance of the Isle of Eigg. The island is already well known for the fossiliferous ‘Hugh Miller Reptile Bed’ (Miller Reference Miller1858; Hudson Reference Hudson1966; Benton Reference Benton, Spencer, Benton and Spencer1995), and for the distinctive features of its geological landscape, such as the Sgurr of Eigg. Although a theropod tooth fragment from Eigg was mentioned by Rees & Underwood (Reference Rees and Underwood2005), it has subsequently been lost and this observation cannot be confirmed. This makes NMS G.2020.10.1 the first unequivocal dinosaur specimen from the island.
This discovery hints that continued exploration of the Valtos Sandstone Formation – and other parts of the Great Estuarine Group – could yield further vital fossil material. These finds would undoubtedly continue to enrich our picture of ecosystem diversity in Middle Jurassic Scotland.
7. Acknowledgements
We would like to thank the Eigg Community Trust, Maggie Fyffe, Craig Lovatt, and people of Eigg for permitting us access and for supporting our work on their beautiful island, and Scottish Natural Heritage (including Sarah McGrory and Colin MacFadyen) for permits and logistical support. We thank Western Isles Ferries and Alastair Kirk for helping to collect and transport the bone. Thanks go to Roger Benson for providing images of specimens. Our research was funded by a National Geographic Grant GEFNE185-16 awarded to SLB. Histological work was funded primarily by a Newton International Fellowship to GFF and partially by a Philip Leverhulme Prize to SLB and the School of Geosciences, University of Edinburgh. Authors TC to MW are listed alphabetically for their equal contribution to fieldwork and to this manuscript. We also thank our two anonymous reviewers for their helpful and constructive comments, which improved this manuscript.












