Lack of insight (i.e. poor awareness of illness) is a common feature of schizophrenia, and indeed of psychosis in general (Reference Amador and DavidAmador & David, 2004). Impaired insight is of clinical importance, as it has been linked to poor treatment compliance (Reference Kemp, David and BlackwellKemp & David, 1997), poorer global functioning (Reference Pyne, Bean and SullivanPyne et al, 2001), severity of psychopathology (Reference Mintz, Dobson and RomneyMintz et al, 2003), recurrence, and poorer outcome (Reference David, van Os and JonesDavid et al, 1995). As early as 1934 impaired insight was argued to arise from a neuropsychological deficit (Reference LewisLewis, 1934; Reference DavidDavid, 1999), although others maintain that ‘poor insight’ should not be conceived of solely as a defect within the individual but more as a sociocultural response (see Reference Saravanan, Jacob and PrinceSaravanan et al, 2004). Empirical testing of the neuropsychological hypothesis has only occurred over the past decade. In particular, it has been proposed that impaired functioning of the prefrontal cortex, which subserves mental flexibility, abstract reasoning, concept formation and self-reflection, may lead to impaired insight (Reference DavidDavid, 1990). Although numerous studies report unawareness of illness to be associated with dysfunction on several neuropsychological tests (Reference Morgan, David, Amador and DavidMorgan & David, 2004), the findings are inconsistent. For example, although an association of insight with performance on the Wisconsin Card Sorting Test (WCST) has repeatedly been reported (e.g. Reference Young, Davila and ScherYoung et al, 1993; Reference Rossell, Coakes and ShapleskeRossell et al, 2003), a number of studies have failed to replicate such a relationship (e.g. Reference Cuesta, Peralta and CaroCuesta et al, 1995; Reference Dickerson, Boronow and RingelDickerson et al, 1997).
In this paper we present meta-analyses of insight and cognition studies, in order to synthesise and integrate the published findings, and to estimate the magnitude of any association. Furthermore, we test the hypothesis whether insight is related to general intellectual dysfunction (e.g. IQ score) or more specifically to prefrontal cognitive dysfunction (e.g. WCST performance). Separate analyses are conducted for samples with a diagnosis of schizophrenia (as opposed to other psychoses) because schizophrenia has been associated with substantial cognitive impairment (Reference Heinrichs and ZakzanisHeinrichs & Zakzaknis, 1998; Reference Aleman, Hijman and De HaanAleman et al, 1999). Finally, insight is a multidimensional construct, and measures of insight vary considerably, ranging from 1 to 74 items (Reference Amador, Kronengold, Amador and DavidAmador & Kronengold, 2004). Therefore, we address the issue whether different components of the insight construct and different measures thereof moderate the insight-cognition relationship.
METHOD
Study selection
Articles for consideration were identified using a three-step procedure. First, a literature search was conducted in the electronic databases PubMed and Web of Science in the period between 1980 and April 2004, using the keywords INSIGHT or UNAWARENESS combined with PSYCHOSIS or SCHIZOPHRENIA combined with COGNITI* or NEUROPSYCHOLOG* or INTELLIGENCE, MEMORY or WCST. This yielded 215 papers, of which 33 fulfilled our criteria (see below). Studies were included after consensus was reached between two authors (A.A. and N.A.) that the paper met all inclusion criteria. Second, we screened the reference list of a recent review by Morgan & David (Reference Morgan, David, Amador and David2004), which yielded two additional papers for inclusion in the meta-analysis (Reference Takai, Uermatsu and UekiTakai et al, 1992; Reference Goldberg, Green-Paden and LehmanGoldberg et al, 2001). Finally, in order not to miss new articles that might not yet have been electronically indexed, a hand search of key journals was conducted for the period April 2003 to April 2004; this included the American Journal of Psychiatry, Archives of General Psychiatry, British Journal of Psychiatry, Journal of Nervous and Mental Disease, Psychiatry Research, Psychological Medicine, Schizophrenia Bulletin and Schizophrenia Research. This did not yield any additional paper suitable for inclusion.
To be included in the meta-analysis, studies had to report correlations between insight scales and cognitive performance measures. In addition, they had to satisfy the following criteria:
-
(a) the sample comprised patient groups with a psychotic disorder, whether affective or non-affective;
-
(b) sufficient information was reported to compute effect sizes, or to determine the direction and significance of the effect;
-
(c) the article had been published in a peer-reviewed English-language journal.
Studies had to include a valid measure of insight, such as the insight item from the Positive and Negative Syndrome Scale (PANSS; Reference Kay, Fiszbein and OplerKay et al, 1987) or Present State Examination (PSE; Reference Wing, Cooper and SartoriusWing et al, 1974); the 11-item Schedule for the Assessment of Insight, original and Expanded version (SAI-E; Reference Kemp, David and BlackwellKemp & David, 1997; Reference Sanz, Constable and Lopez-IborSanz et al, 1998); the 74-item Scale to Assess Unawareness of Mental Disorder (SUMD; Reference Amador, Strauss and YaleAmador et al, 1993); or the 11-item Insight and Treatment Attitudes Questionnaire (ITAQ; Reference McEvoy, Apperson and AppelbaumMcEvoy et al, 1989).
With regard to the cognitive domains, we defined five domains. The first was total cognition, in which all cognitive test results published in the same paper were pooled. Examples of such tests are the Wechsler Adult Intelligence Scale (WAIS), or subtests of the WAIS such as Digit Span; the Mini-Mental State Examination (MMSE); the National Adult Reading Test (NART); the Trail Making Test; the Wisconsin Card Sorting Test (WCST); and measures of attention, memory tests and verbal fluency tests (see Reference LezakLezak, 1995 for a description of these measures). The second domain was IQ only, which in most cases pertained to the WAIS. Other estimates of intelligence with an established validity were also included. In cases where only a number of WAIS sub-tests were included, these subtests were pooled. The third domain, memory, included established measures of verbal and visual memory performance (see Reference Aleman, Hijman and De HaanAleman et al, 1999). Fourth was frontal executive function, which included the Trail Making Test B, verbal fluency and the WCST. Finally, a separate analysis limited to the WCST was included, because several authors have postulated a specific relationship between perseveration as assessed by the WCST and poor insight (cf. Reference Morgan, David, Amador and DavidMorgan & David, 2004). Furthermore, in a construct validation factor analysis (involving data from 473 clinical cases) WCST scores loaded independently of other neuropsychological variables, indicating that the WCST contributes uniquely to neuropsychological evaluation (Reference Greve, Ingram and BianchiniGreve et al, 1998). For the WCST analysis we included categories completed as well as perseverative responses, which were pooled when both were reported in a single paper (cf. Reference Nieuwenstein, Aleman and De HaanNieuwenstein et al, 2001). These parameters of the WCST have been shown to load on the same factor, termed ‘perseveration’ (Reference Cuesta, Peralta and CaroCuesta et al, 1995).
Data analysis
For each study an effect size was calculated, which was the mean r weighted for sample size (Reference Hunter and SchmidtHunter & Schmidt, 1990). When the precise r values were not given, an estimate was computed from exact P, t or F values (Reference Lipsey and WilsonLipsey & Wilson, 2001). A problem in meta-analysis concerns the handling of missing values owing to studies reporting that a result was non-significant without providing the exact statistics. The most common method is to exclude such studies completely; this is unfortunate, and will bias the results considerably when there is a substantial number of studies with this problem (as in the present case). Another method is to give such studies values of r=0, which is a highly conservative approach that may obscure the existence of small effects. A third method is to assign such studies the mean effect size of the studies that report an insignificant effect size. A problem of this method is that it may yield an overestimation of the non-significant effect sizes (as authors will more readily report non-significant effect sizes of some magnitude than very small ones). A reasonable compromise, in our view, is to include the lowest value of the 95% confidence interval of the mean effect size for non-significant studies that do report precise statistics. This is a conservative approach, but not as extremely conservative as the zero substitution approach. We adopted this strategy for the overall analysis, to be able to include as many informative papers as possible. After computing effect sizes for each study we applied meta-analytic methods in order to obtain a combined effect size, which indicated the magnitude of the association across all studies (cf. Reference Nieuwenstein, Aleman and De HaanNieuwenstein et al, 2001). Effect sizes were weighted for sample size, in order to correct for upwardly biased estimation of the effect in small sample sizes (Reference RosenthalRosenthal, 1991). The corresponding z-value and significance level provide an indication of the statistical significance of the association. We also calculated a homogeneity statistic, Q, to test whether the studies can be taken to share a common population effect size. A significant Q statistic indicates heterogeneity of the individual study effect sizes which poses a threat to a reliable interpretation of the results. However, when a small number of studies are included in the meta-analysis, the Q statistic can underreport heterogeneity, and an exploration of heterogeneity is warranted through subgroup analyses. All analyses were carried out in the random-effects model, using the Comprehensive Meta-Analysis package (see http://www.meta-analysis.com). In contrast to the fixed-effects model, the random-effects model permits generalisation to studies not yet in the sample, and is to be preferred when studying psychiatric phenomena which may be not only empirically but also conceptually heterogeneous (Reference Rosenthal and DiMatteoRosenthal & DiMatteo, 2001).
RESULTS
Characteristics of studies included in the meta-analyses are listed in Table 1. The first analyses concerned studies reporting on psychosis in general. Tables 2 and 3 show all results for these analyses. The mean weighted effect size for the relationship between insight and all cognitive measures pooled together was r=0.17 (95% CI 0.13-0.21, z=8.3, P<0.0001). This concerned the results of 35 studies with a total of 2354 individuals. The effect sizes were not heterogeneous between studies. For IQ measures, the mean weighted effect size was r=0.14 (20 studies). The effect size for memory measures was small and non-significant: r=0.08, z=1.3, P<0.19 (10 studies). For measures of frontal executive function, the mean weighted effect size was r=0.19 (21 studies), whereas an analysis limited to the WCST revealed an effect size of r=0.23 (13 studies). The difference between the effect sizes for IQ and WCST was significant (Q B=6.4; P=0.01), as was the difference between frontal executive function and memory (Q B=4.1; P=0.04). Other comparisons were not significant. Figures 1, 2, 3 show forest plots of the analyses for total cognition, IQ and WCST respectively.

Fig. 1 Forest plot of studies included in the meta-analysis of the relationship between insight and ‘total cognition’ (all cognitive tests pooled together).
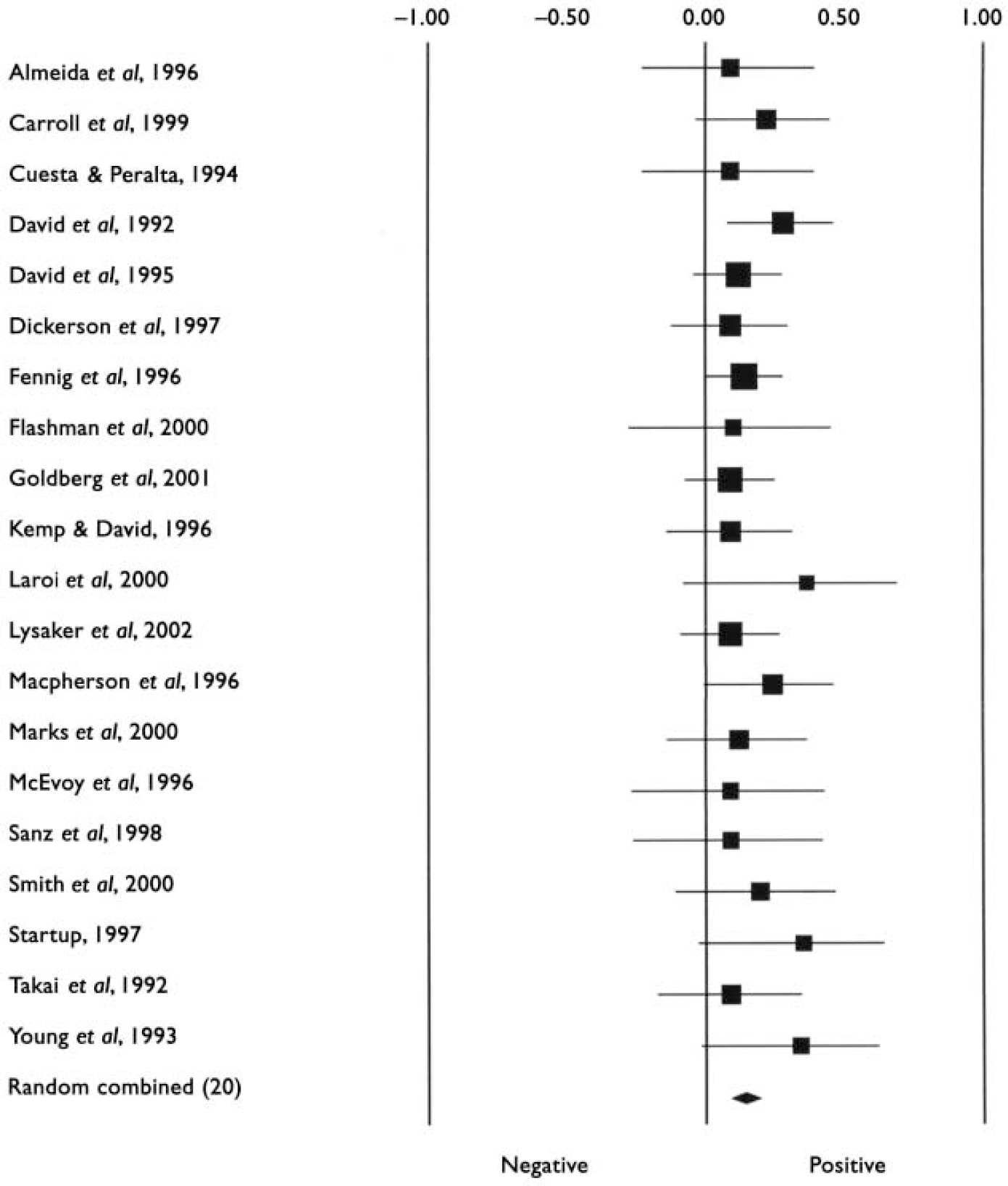
Fig. 2 Forest plot of studies included in the meta-analysis of the relationship between insight and IQ test performance.
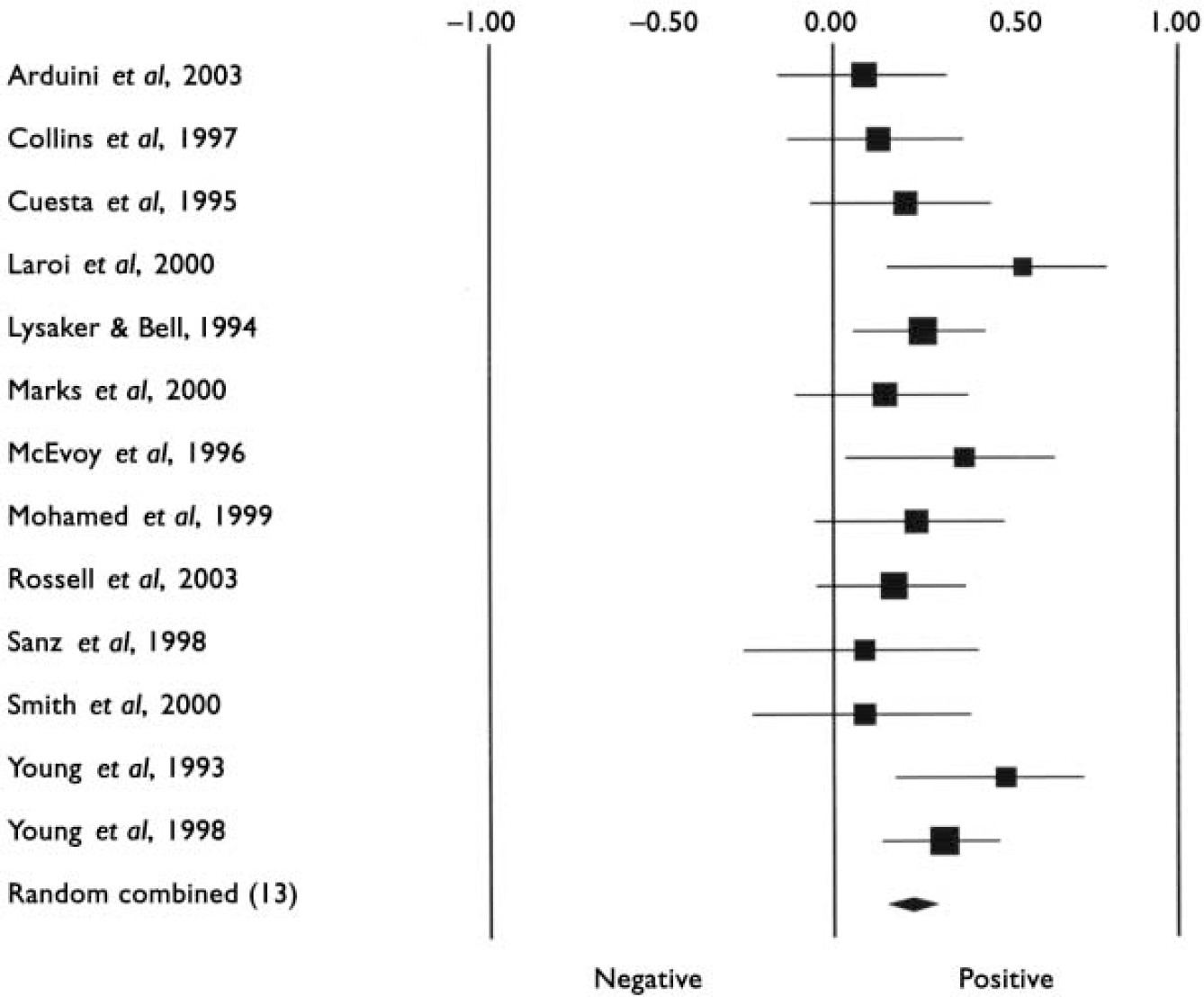
Fig. 3 Forest plot of studies included in the meta-analysis of the relationship between insight and Winsconsin Card Sorting Test performance.
Table 1 Characteristics of studies included in the meta-analyses
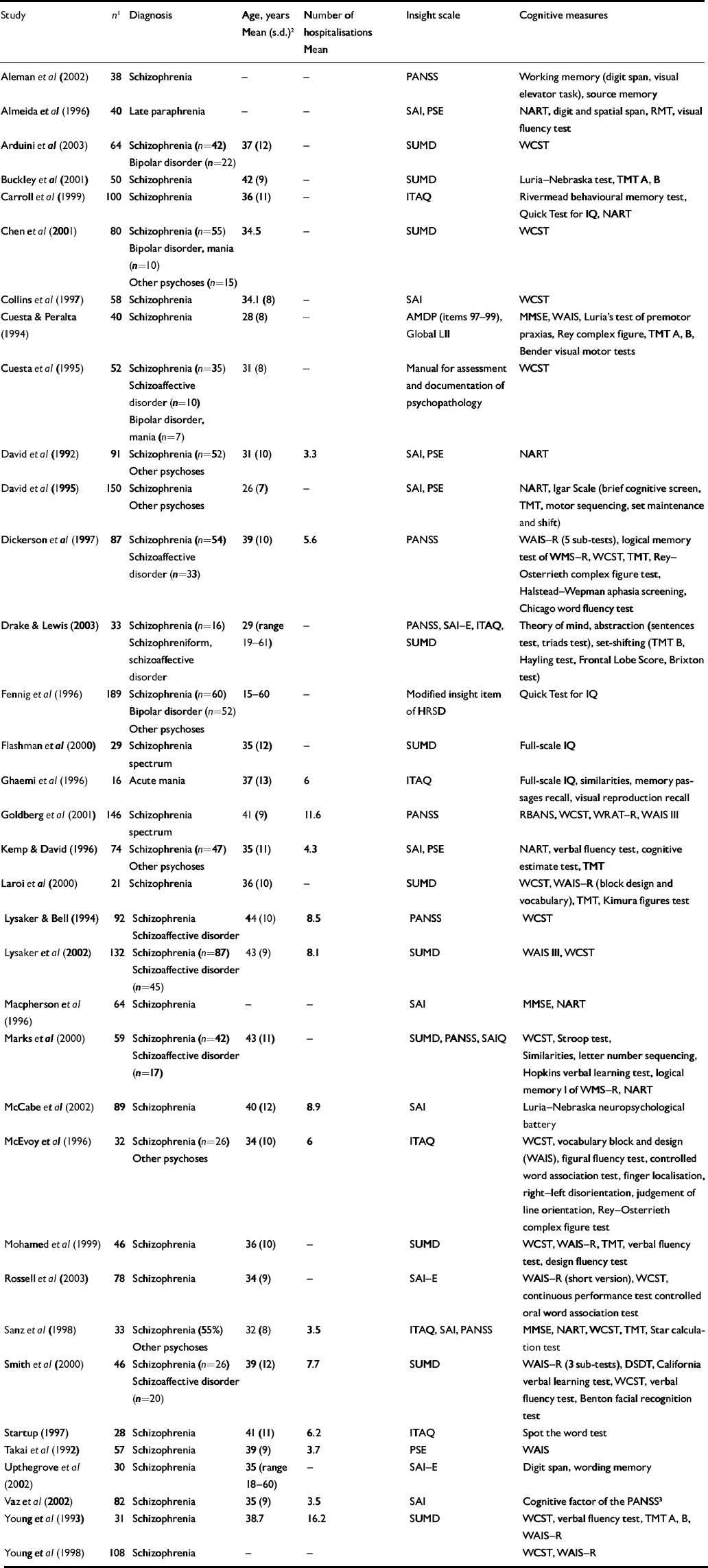
| Study | n 1 | Diagnosis | Age, years Mean (s.d.) 2 | Number of hospitalisations Mean | Insight scale | Cognitive measures |
|---|---|---|---|---|---|---|
| Aleman et al (Reference Aleman, De Haan and Kahn2002) | 38 | Schizophrenia | – | – | PANSS | Working memory (digit span, visual elevator task), source memory |
| Almeida et al (Reference Almeida, Levy and Howard1996) | 40 | Late paraphrenia | – | – | SAI, PSE | NART, digit and spatial span, RMT, visual fluency test |
| Arduini et al (Reference Arduini, Kalyvoka and Stratta2003) | 64 | Schizophrenia (n=42) Bipolar disorder (n=22) | 37 (12) | – | SUMD | WCST |
| Buckley et al (Reference Buckley, Hasan and Friedman2001) | 50 | Schizophrenia | 42 (9) | – | SUMD | Luria–Nebraska test, TMT A, B |
| Carroll et al (Reference Carroll, Fattah and Clyde1999) | 100 | Schizophrenia | 36 (11) | – | ITAQ | Rivermead behavioural memory test, Quick Test for IQ, NART |
| Chen et al (Reference Chen, Kwok and Chen2001) | 80 | Schizophrenia (n=55) Bipolar disorder, mania (n=10) Other psychoses (n=15) | 34.5 | – | SUMD | WCST |
| Collins et al (Reference Collins, Remington and Coulter1997) | 58 | Schizophrenia | 34.1 (8) | – | SAI | WCST |
| Cuesta & Peralta (Reference Cuesta and Peralta1994) | 40 | Schizophrenia | 28 (8) | – | AMDP (items 97–99), Global LII | MMSE, WAIS, Luria's test of premotor praxias, Rey complex figure, TMT A, B, Bender visual motor tests |
| Cuesta et al (Reference Cuesta, Peralta and Caro1995) | 52 | Schizophrenia (n=35) Schizoaffective disorder (n=10) Bipolar disorder, mania (n=7) | 31 (8) | – | Manual for assessment and documentation of psychopathology | WCST |
| David et al (Reference David, Buchanan and Reed1992) | 91 | Schizophrenia (n=52) Other psychoses | 31 (10) | 3.3 | SAI, PSE | NART |
| David et al (Reference David, van Os and Jones1995) | 150 | Schizophrenia Other psychoses | 26 (7) | – | SAI, PSE | NART, Igar Scale (brief cognitive screen, TMT, motor sequencing, set maintenance and shift) |
| Dickerson et al (Reference Dickerson, Boronow and Ringel1997) | 87 | Schizophrenia (n=54) Schizoaffective disorder (n=33) | 39 (10) | 5.6 | PANSS | WAIS–R (5 sub-tests), logical memory test of WMS–R, WCST, TMT, Rey–Osterrieth complex figure test, Halstead–Wepman aphasia screening, Chicago word fluency test |
| Drake & Lewis (Reference Drake and Lewis2003) | 33 | Schizophrenia (n=16) Schizophreniform, schizoaffective disorder | 29 (range 19–61) | – | PANSS, SAI–E, ITAQ, SUMD | Theory of mind, abstraction (sentences test, triads test), set-shifting (TMT B, Hayling test, Frontal Lobe Score, Brixton test) |
| Fennig et al (Reference Fennig, Everett and Bromet1996) | 189 | Schizophrenia (n=60) Bipolar disorder (n=52) Other psychoses | 15–60 | – | Modified insight item of HRSD | Quick Test for IQ |
| Flashman et al (Reference Flashman, McAllister and Andreasen2000) | 29 | Schizophrenia spectrum | 35 (12) | – | SUMD | Full-scale IQ |
| Ghaemi et al (Reference Ghaemi, Hebben and Stoll1996) | 16 | Acute mania | 37 (13) | 6 | ITAQ | Full-scale IQ, similarities, memory passages recall, visual reproduction recall |
| Goldberg et al (Reference Goldberg, Green-Paden and Lehman2001) | 146 | Schizophrenia spectrum | 41 (9) | 11.6 | PANSS | RBANS, WCST, WRAT–R, WAIS III |
| Kemp & David (Reference Kemp and David1996) | 74 | Schizophrenia (n=47) Other psychoses | 35 (11) | 4.3 | SAI, PSE | NART, verbal fluency test, cognitive estimate test, TMT |
| Laroi et al (Reference Laroi, Fannemel and Ronneberg2000) | 21 | Schizophrenia | 36 (10) | – | SUMD | WCST, WAIS–R (block design and vocabulary), TMT, Kimura figures test |
| Lysaker & Bell (Reference Lysaker and Bell1994) | 92 | Schizophrenia Schizoaffective disorder | 44 (10) | 8.5 | PANSS | WCST |
| Lysaker et al (Reference Lysaker, Bryson and Bell2002) | 132 | Schizophrenia (n=87) Schizoaffective disorder (n=45) | 43 (9) | 8.1 | SUMD | WAIS III, WCST |
| Macpherson et al (Reference MacPherson, Jerrom and Hughes1996) | 64 | Schizophrenia | – | – | SAI | MMSE, NART |
| Marks et al (Reference Marks, Fastenau and Lysaker2000) | 59 | Schizophrenia (n=42) Schizoaffective disorder (n=17) | 43 (11) | – | SUMD, PANSS, SAIQ | WCST, Stroop test, Similarities, letter number sequencing, Hopkins verbal learning test, logical memory I of WMS–R, NART |
| McCabe et al (Reference McCabe, Quayle and Beirne2002) | 89 | Schizophrenia | 40 (12) | 8.9 | SAI | Luria–Nebraska neuropsychological battery |
| McEvoy et al (Reference McEvoy, Hartman and Gottlieb1996) | 32 | Schizophrenia (n=26) Other psychoses | 34 (10) | 6 | ITAQ | WCST, vocabulary block and design (WAIS), figural fluency test, controlled word association test, finger localisation, right–left disorientation, judgement of line orientation, Rey–Osterrieth complex figure test |
| Mohamed et al (Reference Mohamed, Fleming and Penn1999) | 46 | Schizophrenia | 36 (10) | – | SUMD | WCST, WAIS–R, TMT, verbal fluency test, design fluency test |
| Rossell et al (Reference Rossell, Coakes and Shapleske2003) | 78 | Schizophrenia | 34 (9) | – | SAI–E | WAIS–R (short version), WCST, continuous performance test controlled oral word association test |
| Sanz et al (Reference Sanz, Constable and Lopez-Ibor1998) | 33 | Schizophrenia (55%) Other psychoses | 32 (8) | 3.5 | ITAQ, SAI, PANSS | MMSE, NART, WCST, TMT, Star calculation test |
| Smith et al (Reference Smith, Hull and Israel2000) | 46 | Schizophrenia (n=26) Schizoaffective disorder (n=20) | 39 (12) | 7.7 | SUMD | WAIS–R (3 sub-tests), DSDT, California verbal learning test, WCST, verbal fluency test, Benton facial recognition test |
| Startup (Reference Startup1997) | 28 | Schizophrenia | 41 (11) | 6.2 | ITAQ | Spot the word test |
| Takai et al (Reference Takai, Uermatsu and Ueki1992) | 57 | Schizophrenia | 39 (9) | 3.7 | PSE | WAIS |
| Upthegrove et al (Reference Upthegrove, Oyebode and George2002) | 30 | Schizophrenia | 35 (range 18–60) | – | SAI–E | Digit span, wording memory |
| Vaz et al (Reference Vaz, Bejar and Casado2002) | 82 | Schizophrenia | 35 (9) | 3.5 | SAI | Cognitive factor of the PANSS 3 |
| Young et al (Reference Young, Davila and Scher1993) | 31 | Schizophrenia | 38.7 | 16.2 | SUMD | WCST, verbal fluency test, TMT A, B, WAIS–R |
| Young et al (Reference Young, Zakzanis and Bailey1998) | 108 | Schizophrenia | – | – | WCST, WAIS–R |
AMDP, Manual for the Assessment and Documentation of Psychopathology; DSDT, Digit Span Distractibility Test; HRSD, Hamilton Rating Scale for Depression; ITAQ, Insight and Treatment Attitude Questionnaire; LII, Lack of Insight Index; MMSE, Mini-Mental State Examination; NART, National Adult Reading Test; PANSS, Positive and Negative Syndrome Scale; PSE, Present State Examination; RBANS, Repeatable Battery for Assessment of Neuropsychological Status; RMT, Recognition Memory Test; SAI(–E), Schedule for the Assessment of Insight (Extended version); SUMD, Scale to Assess Unawareness of Mental Disorder; TMT, Trail Making Test; WAIS, Wechsler Adult Intelligence Scale; WCST, Wisconsin Card Sorting Test; WMS–R, Wechsler Memory Scale – Revised; WRAT–R, Wide Range Achievement Test – Reading
Table 2 Results of meta-analyses on insight–cognition relationships in samples of patients with (a) psychosis and (b) with a diagnosis of schizophrenia
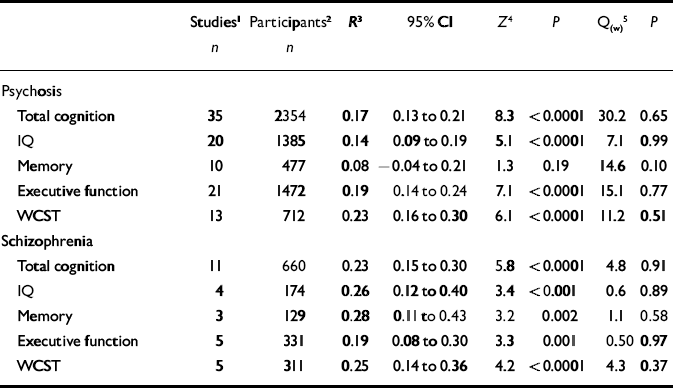
| Studies 1 | Participants 2 | R 3 | 95% CI | Z 4 | P | Q(w) 5 | P | |
|---|---|---|---|---|---|---|---|---|
| n | n | |||||||
| Psychosis | ||||||||
| Total cognition | 35 | 2354 | 0.17 | 0.13 to 0.21 | 8.3 | <0.0001 | 30.2 | 0.65 |
| IQ | 20 | 1385 | 0.14 | 0.09 to 0.19 | 5.1 | <0.0001 | 7.1 | 0.99 |
| Memory | 10 | 477 | 0.08 | –0.04 to 0.21 | 1.3 | 0.19 | 14.6 | 0.10 |
| Executive function | 21 | 1472 | 0.19 | 0.14 to 0.24 | 7.1 | <0.0001 | 15.1 | 0.77 |
| WCST | 13 | 712 | 0.23 | 0.16 to 0.30 | 6.1 | <0.0001 | 11.2 | 0.51 |
| Schizophrenia | ||||||||
| Total cognition | 11 | 660 | 0.23 | 0.15 to 0.30 | 5.8 | <0.0001 | 4.8 | 0.91 |
| IQ | 4 | 174 | 0.26 | 0.12 to 0.40 | 3.4 | <0.001 | 0.6 | 0.89 |
| Memory | 3 | 129 | 0.28 | 0.11 to 0.43 | 3.2 | 0.002 | 1.1 | 0.58 |
| Executive function | 5 | 331 | 0.19 | 0.08 to 0.30 | 3.3 | 0.001 | 0.50 | 0.97 |
| WCST | 5 | 311 | 0.25 | 0.14 to 0.36 | 4.2 | <0.0001 | 4.3 | 0.37 |
WCST, Wisconsin Card Sorting Test
Table 3 Different insight scales and mean correlations with neuropsychological function across studies
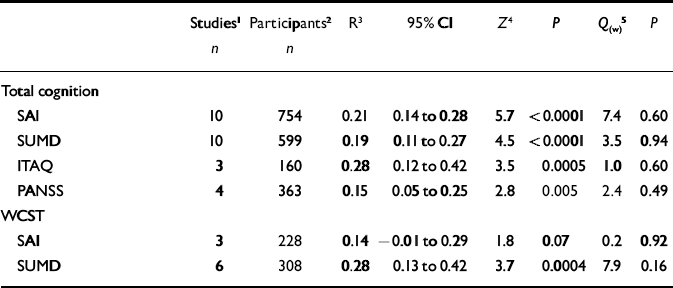
| Studies 1 | Participants 2 | R 3 | 95% CI | Z 4 | P | Q (w) 5 | P | |
|---|---|---|---|---|---|---|---|---|
| n | n | |||||||
| Total cognition | ||||||||
| SAI | 10 | 754 | 0.21 | 0.14 to 0.28 | 5.7 | <0.0001 | 7.4 | 0.60 |
| SUMD | 10 | 599 | 0.19 | 0.11 to 0.27 | 4.5 | <0.0001 | 3.5 | 0.94 |
| ITAQ | 3 | 160 | 0.28 | 0.12 to 0.42 | 3.5 | 0.0005 | 1.0 | 0.60 |
| PANSS | 4 | 363 | 0.15 | 0.05 to 0.25 | 2.8 | 0.005 | 2.4 | 0.49 |
| WCST | ||||||||
| SAI | 3 | 228 | 0.14 | –0.01 to 0.29 | 1.8 | 0.07 | 0.2 | 0.92 |
| SUMD | 6 | 308 | 0.28 | 0.13 to 0.42 | 3.7 | 0.0004 | 7.9 | 0.16 |
ITAQ, Insight and Treatment Attitude Questionnaire; PANSS, Positive and Negative Syndrome Scale; SAI, Schedule for the Assessment of Insight; SUMD, Scale to Assess Unawareness of Mental Disorder; WCST, Wisconsin Card Sorting Test
1. Number of studies included in the analysis
2. Total number of participants across studies
3. Mean weighted correlation coefficient
4. Fisher's Z, and significance of effect size (P)
5. Within-category heterogeneity between studies
1. Number of studies included in the analysis
2. Total number of participants across studies
3. Mean weighted correlation coefficient; 95% CI=95% confidence interval
4. Fisher's Z, and significance of effect size (P)
5. Within-category heterogeneity between studies
1. Numbers in this column are those reported at the start of the Method section of the papers concerned; the numbers included in our statistical analysis may be smaller owing to drop-outs
2. Dashes indicate data are not available
3. The cognitive factor of the PANSS correlates well with neuropsychological performance (Reference Bell, Lysaker and MilsteinBell et al, 1994) and was therefore included in the overall analysis
The next analyses were confined to studies reporting on samples of patients with a diagnosis of schizophrenia made using explicit diagnostic criteria. Mean effect sizes for insight-cognition relationships were in the small to medium range, and varied from 0.19 (executive function) to 0.28 (memory). The effect sizes did not differ significantly from each other. Thus, in contrast to the first analysis for psychosis in general, the WCST-insight relationship was not stronger than the IQ-insight relationship. In addition, the correlation between memory performance and insight was significant in this analysis limited to schizophrenia, but not in the first analysis including psychosis in general. Table 2 lists all results for these analyses.
Finally, associations of different insight scales with cognition were examined (including studies that reported on psychosis in general). These analyses focused on the four scales that have most been used (Reference Morgan, David, Amador and DavidMorgan & David, 2004), all of which showed significant correlations with total cognition (Table 3). With regard to associations with general cognition, mean correlations varied from 0.15 (PANSS) to 0.28 (ITAQ). The differences in magnitude of effect size did not attain significance, however. For the WCST the mean correlation with the SUMD was 0.28, whereas the correlation with the SAI was 0.14. Again, however, this difference was not significant: Q B=1.8, P=0.18.
Publication bias
A concern in the interpretation of meta-analytic findings is the possibility of an upward bias of the mean effect due to the omission of unpublished studies with null effects. Small studies with significant results tend to be published, whereas small studies without statistically significant findings tend to remain in the file drawer. Figure 4 is a funnel plot of the studies included in the primary analysis: this is a scatterplot of the effect size by sample size of each study, which should take the shape of a funnel, as there should be greater variability among the effect sizes based on small samples than those based on large samples (Reference Wang and BushmanWang & Bushman, 1998). The underrepresentation of small studies with small effects could be indicative of publication bias. Figure 4 shows that such studies are indeed somewhat underrepresented, but the asymmetry is not strong. To assess whether sampling bias would be large enough to render the mean effect size insignificant, we computed an additional statistic, the fail-safe n (Reference OrwinOrwin, 1983; Reference RosenthalRosenthal, 1991). Specifically, we determined the number of studies with an effect size of zero needed to reduce the mean effect size to a negligible effect (r=0.05). The fail-safe n was 84 for the overall analysis (35 studies) and 40 for the analysis confined to the diagnosis of schizophrenia (11 studies). It is unlikely that such a large number of unpublished studies with null effects reside in file drawers.

Fig. 4 Funnel plot of the studies included in the primary analysis: the effect size r (x-axis) is plotted against the sample size (y-axis) of each study (the vertical line indicates the mean weighted effect size).
DISCUSSION
Several findings emerge from our meta-analysis of published neuropsychological studies of insight in patients with psychotic disorders. First, insight showed a small, albeit statistically significant, positive relationship with general cognitive functioning. Second, the relationship between WCST performance and insight was significantly stronger than the association with IQ, with a magnitude corresponding to a medium effect size (Reference CohenCohen, 1988). Third, whereas the WCST-insight association was stronger than the IQ-insight association in samples of patients with psychotic disorders in general, there was no difference between these associations when analyses were limited to samples of patients with a diagnosis of schizophrenia. Overall, however, the insight-cognition assessment was slightly stronger in patients with schizophrenia v. patients with psychosis in general. Finally, analyses of different insight scales did not reveal significant differences in their association with cognitive performance.
Neuropsychological dysfunction
It has been a matter of debate in recent years whether poor insight in psychotic disorders can, in part, be explained by neuropsychological dysfunction. The association of insight with general cognitive function observed in our meta-analysis may imply that the interpretation and attribution of anomalous mental experiences is indeed hampered by poor general cognition. Notably, although the effect sizes were in the small to medium range, a recent meta-analysis meta-analysis of insight studies reported comparable mean correlations for the association with positive and negative symptoms; Mintz et al (Reference Mintz, Dobson and Romney2003) observed a correlation of 0.25 for positive symptoms (based on 22 studies) and a correlation of 0.23 for negative symptoms (based on 20 studies). Hence, it could be argued that poor insight in psychosis is part and parcel of psychopathology and cognitive dysfunction in roughly equal measure. However, although psychotic symptoms have been suggested to be largely independent of cognitive functioning (Reference Nieuwenstein, Aleman and De HaanNieuwenstein et al, 2001), any conclusion regarding independent contributions of psychopathological disorder and cognitive deficits to impaired insight should be confirmed by a regression model in the same sample. Furthermore, interpretations of association should not assume causality. It is possible that the assessment of insight is confounded by cognition, since a clinician's tendency to infer insight may be coloured by the patient's ability to express complex attitudes and experiences. Independent rating of insight and cognition would minimise this effect.
The finding that in patients with a psychotic disorder the relationship between WCST performance and insight was significantly stronger than the association with IQ may imply a specific role of perseveration. With regard to the cognitive mechanisms of insight, WCST performance has been suggested to be of particular relevance (Reference Drake and LewisDrake & Lewis, 2003). Cognitive flexibility is important, as it refers to the capacity ‘to hold an abstract representation related to an actual situation, but different from it, at the same time as the more obvious immediate representation’ (Reference Drake and LewisDrake & Lewis, 2003). This capacity would enable people to evaluate their own perceptions, thoughts and behaviour in relation to knowledge of symptoms of mental illness (shaped by social and cultural influences). In a sample of 33 people with acute psychosis, Drake & Lewis (Reference Drake and Lewis2003) reported a specific strong correlation (r=0.59) between insight and perseverative errors, rather than more general measures of abstraction. This is consistent with the notion that a failure to change cognitive set and to monitor error responses may lead to impaired insight. It should be kept in mind, none the less, that although ‘perseveration’ is widely recognised as a key cognitive process called upon during WCST performance, the cognitive processes underlying WCST performance remain poorly understood.
Diagnosis of schizophrenia
Whereas the WCST-insight association was stronger than the IQ-insight association in samples of patients with psychotic disorders in general, there was no difference between these associations when analyses were limited to samples of patients with a diagnosis of schizophrenia. This was not owing to a smaller effect size for WCST, but could be attributed to a larger effect size for IQ in patients with schizophrenia. Schizophrenia involves more negative symptoms and more pronounced intellectual impairment in comparison with other psychotic disorders, and this could lead to a larger correlation between IQ and insight; that is, a certain IQ level might be required to enable the person to make the complex judgements involved in self-reflection and monitoring of unusual or ambiguous experiences. Another difference between the analysis confined to schizophrenia proper concerned the relationship between memory performance and insight. This association was significant in schizophrenia, but was absent when all psychoses were included in the analysis. In schizophrenia, large effect sizes of memory impairment have been documented (Reference Aleman, Hijman and De HaanAleman et al, 1999) and a certain level of memory function may be necessary for intact insight.
An alternative interpretation of the different results in the analyses including schizophrenia v. ‘all psychoses’ would be that this does not imply a specific role of perseveration in impaired insight, but rather reflects an effect of clinical diagnosis. That is, in patients with less severe psychotic disorders only executive and attentional functioning might be impaired, whereas schizophrenia is characterised by generalised cognitive impairment. Thus, a relationship with insight is found only for cognitive functions that are impaired. Direct comparison between different patient groups is needed to test this possibility. The results of a study published after our meta-analysis was completed may be consistent with this notion (Reference Keshavan, Rabinowitz and DeSmedtKeshavan et al, 2004): in a sample of 535 patients with first-episode psychosis, the authors observed significant associations of impaired insight with multiple cognitive domains, including memory, learning and executive functions. However, another recent study of a sample comprising 122 out-patients with schizophrenia failed to find significant associations between insight and neuropsychological tests sensitive to frontal lobe function (Reference Freudenreich, Deckersbach and GoffFreudenreich et al, 2004).
Different scales
With regard to the use of different insight scales, the PANSS, SAI, SUMD and ITAQ apparently yielded effect sizes in the same range. This is consistent with studies that observed high correlations between these different insight measures (Reference Sanz, Constable and Lopez-IborSanz et al, 1998; Reference Cuesta, Peralta and ZarzuelaCuesta et al, 2000; Reference Drake and LewisDrake & Lewis, 2003). Unfortunately, owing to the small number of studies reporting separate data for different components of insight scales, it was not possible to analyse differential relations in this regard. It has been suggested that reduced symptom relabelling abilities might be more closely related to deficits in cognitive functioning than other domains of insight such as illness awareness and treatment compliance (Reference Morgan, David, Amador and DavidMorgan & David, 2004). The latter components would be less independent of external factors such as social and cultural variations (Reference Saravanan, Jacob and PrinceSaravanan et al, 2004).
A possible explanation for the small and inconsistent correlations observed in a number of studies might be that the relationship between insight and cognitive deficits is non-linear. According to Startup (Reference Startup1996), both motivational and cognitive deficits affect insight, with a trade-off between the two processes, giving rise to quadratic relationships between insight and cognitive test performance. Unfortunately, the other studies included in our meta-analysis did not examine curvilinear associations.
It should be noted that the effect sizes were rather small, implying that cognitive function may explain only a limited portion of the observed variance in insight ratings. The method of meta-analysis has been criticised for mixing dissimilar studies, publication bias and inclusion of poor-quality studies. We addressed these issues by imposing strict inclusion criteria and exploring publication bias. Indeed, as Rosenthal & DiMatteo (Reference Rosenthal and DiMatteo2001) have noted; ‘criticisms of meta-analysis that are applicable are equally applicable to traditional, non-quantitative, narrative reviews of the literature’.
Future research should focus more in detail on the role of metacognitive processes, and examine separate components of the insight construct. Koren et al (Reference Koren, Seidman and Poyurovsky2004) reported in a sample of 30 patients with first-episode schizophrenia that prediction of poor insight was significantly improved by adding the new, free-choice metacognitive measures to the conventional WCST measures. These preliminary results suggest that metacognition might be an important mediator between basic cognitive deficits and poor insight, and might be even more relevant to poor insight than cognitive deficits per se.
Acknowledgement
A.A. was supported by an Innovational Research Grant (016.026.027) from the Netherlands Organisation for Scientific Research.










eLetters
No eLetters have been published for this article.