INTRODUCTION
Salmonella enterica serotype Typhi (S. Typhi), the organism that causes typhoid fever is common in areas with compromised sanitary and water conditions. Typhoid fever was estimated to have caused about 22 million cases and 216 000 deaths in 2000 [Reference Crump, Luby and Mintz1]. Most studies that were used for burden-of-disease estimation are from the South and South East Asian region. Only one study representing Africa was included. Therefore these estimates are considered conservative for the global burden of typhoid fever. Population-based incidence estimates for typhoid fever are not available globally, but typhoid fever complications are frequently reported in the literature from almost all regions of the world [Reference Crump2, Reference Parry3].
Typhoid fever is mainly spread by the faecal–oral route; therefore transmission is high in areas where the risk of food and especially water contamination is high. Factors that influence contamination of drinking water are the non-availability of piped water, and improper drainage of sewage [Reference Farooqui, Khan and Kazmi4]. Individual risky behaviours such as not washing hands after defecation and before meals are another contributing factor for typhoid spread. In South East Asia, the incidence of typhoid fever in children is high in urban low socioeconomic squatter settlements where population density is high and living conditions are compromised [Reference Brooks5].
Humans are the exclusive host for S. Typhi. The most common source of infection is the ingestion of food contaminated with S. Typhi [Reference Swaddiwudhipong and Kanlayanaphotporn6]. Occasionally contaminated eggs and frozen oysters have been found to be associated with typhoid infection [Reference Elsarnagawy7]. The documented risk factors based on studies in high-risk areas such as Pakistan are consumption of ice cream and flavoured iced drinks from street vendors, raw fruit and vegetables, and a history of contact with a typhoid patient before illness [Reference Ram8]. Hand washing and past evidence of infection with Helicobacter pylori are among other risk factors [Reference Bhan9]. Individual host factors such as HLA-linked genes may also increase susceptibility to infection; however, the underlying mechanism of action is not yet completely understood [Reference Dunstan10].
Typhoid fever remains a major health problem in the rapidly growing urban settlements of Asia and Africa. Migrating populations frequently have to settle on untitled land which usually has no electricity, water supply, or sanitation infrastructure. The identification of risk factors for an enteric disease such as typhoid fever within such communities will be beneficial for control of typhoid fever and help in devising cost-effective interventions for its prevention.
METHODS
We analysed the data from the recipients of the control vaccine in a cluster randomized trial conducted in low socioeconomic settlements of Karachi. The data were collected prospectively using a surveillance network to capture typhoid fever cases in children aged 2–16 years. We assessed the risk factors for typhoid fever in these informal settlements. As part of the study we collected information at three levels: individual, household and geographical clusters.
Study setting and population
The study population represented different ethnic groups from throughout Pakistan as described in a previous report [Reference Ochiai11]. The study site comprised of three different colonies as divided by the city district administration. Two colonies are adjacent (Sultanabad and Hijrat) but separated by a sewerage line and the third colony (Bilal) is located at a distance of ~20 km from the centre of the two other colonies in an industrial area of Karachi. The study site was divided arbitrarily into 120 geographical clusters so that the size of population in the clusters within a colony would not vary significantly from one another. The results of the analysis presented here use the data from 60 control clusters that were randomized to receive hepatitis A vaccine.
The target age group for the vaccine trial was children aged 2–16 years. Prior to vaccination, a household census was conducted to enumerate the population of the study area and therefore allocate unique identification numbers to the residents. These unique identification numbers provided the key to link to different datasets used in this study. The total population in the study area was 122 894 of which 51 965 (42%) were children aged 2–16 years.
Eligibility criteria
All children aged 2–16 years were under surveillance for fever during the study period from August 2003 to July 2006. The project health clinics provided outpatient care for fever and other common illnesses.
A typhoid fever case was an eligible child who (i) visited the project health clinics with a history of fever for ⩾3 days, (ii) registered and had venous blood taken for microbiological confirmation, (iii) had S. Typhi isolated and his/her identity confirmed through a home visit. Enrolment in the surveillance was irrespective of the randomization clusters. The assessment of factors that determined the outcome of typhoid fever was based on individual, household and geographical clusters. These included basic demographic variables such as age, sex, household income, number of individuals living in the house, population density and distance to the health centre. Exclusion criteria were: children who enrolled in the study but did not have a blood sample taken, or the blood culture results were missing, or residential confirmation was not possible on a follow-up visit. Typhoid cases were compared with culture-negative fever episodes for the evaluation of risk factors for typhoid fever in children who were residing in the hepatitis A group clusters.
Data collection
Surveillance of febrile episodes was conducted through household visits and project health clinics. A team of community health workers (CHWs) visited the households on a weekly basis to collect information on febrile children. Additional information of common childhood illnesses was also recorded during the household visit. To identify a febrile episode, parents were encouraged to seek healthcare either at the project health clinics or from their family physician. Three project health clinics, one in each site, were established to provided care to febrile children for 10 hours, 6 days a week (Monday–Saturday) working in two shifts. Clinical- and household-level information was collected by study physicians using a structured questionnaire. Blood samples were taken following standard phlebotomy techniques in culture bottles (BACTEC) and were transported to the clinical laboratory of the Aga Khan University Hospital (AKUH) clinical laboratory for microbiological confirmation of typhoid fever. Patients were provided empirical therapy following a standard algorithm and were requested for a follow-up visit. The treatment regimen was either changed or continued upon the receipt of the laboratory results. The study established a referral mechanism with the privately practising physicians in the area. The doctor contacted the study phlebotomy team for information collection and blood sampling [Reference Khan12].
Data management and statistical analysis
All data checked by the field supervisor of data collection teams were signed and then handed over to the data management unit, established at each site. Logbooks were maintained to ensure a proper flow of the study data forms. The data were doubly entered in Visual FoxPro version 7.0 data management system (Microsoft Corp., USA). Key punching errors were rectified by the data entry supervisor and the logical errors were corrected with help from the study physicians.
Descriptive analysis was performed to understand distribution of individuals, households and cluster of residential characteristics. Means and proportions were calculated and statistical tests (t test for continuous variables and χ2 for categorical variables) were applied to assess statistical significance of variables in determining the outcome of typhoid fever in children. Adjustment for cluster-level effect was performed using generalized estimating equations (GEE) for log-binomial regression analysis [Reference Zeger and Liang13]. The final model building was based on checking estimates of independent effect on a variable and estimated after adjustment with other variables. Data were analysed using statistical analysis software Stata version 10.0 (StataCorp LP, USA).
Ethical approvals
The study was approved by the Ethical Review Committee of the Aga Khan University Karachi, Pakistan, and the Institutional Review Board of International Vaccine Institute Seoul, Korea.
RESULTS
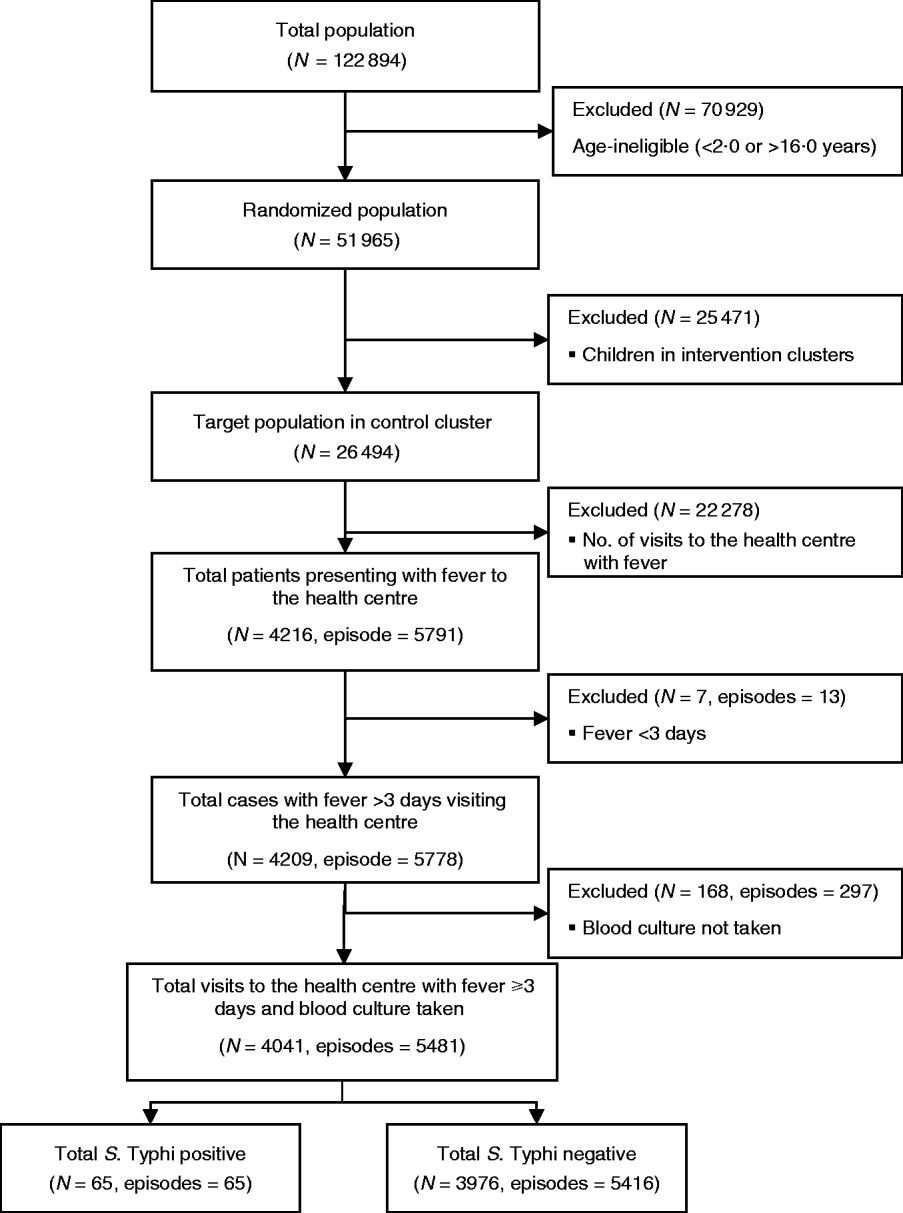
A total of 26 494 children aged 2–16 years living in 60 geographical clusters from Sultanabad [5297 (20%)], Hijrat [6486 (24%)] and Bilal [14 711 (56%)] colonies were randomized to receive hepatitis A vaccine (Fig. 1). The mean age of the children was 9 (s.d.=4) years and 6482 (24%) were aged 2–5 years, 10 090 (38%) were aged 5–10 years and 9922 (37%) were aged between 10 and 16 years. Male children comprised 52% (13 883) of the target study population (Table 1).
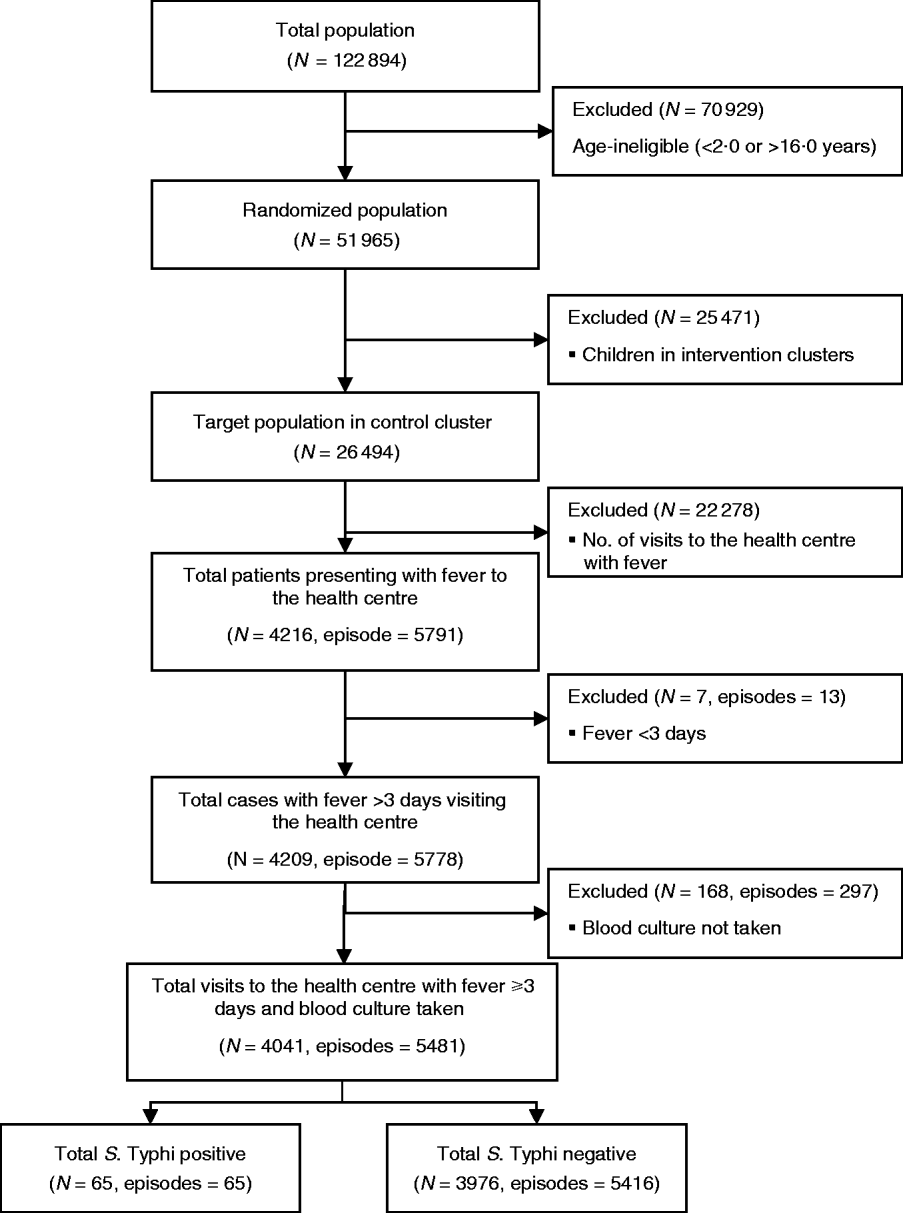
Fig. 1. Flowchart of the study population and sample size for the analysis of risk factor for typhoid fever in children in Karachi, Pakistan.
Table 1. Distribution and estimate of risk for factors associated with typhoid fever in children of urban squatter settlements in Karachi, Pakistan
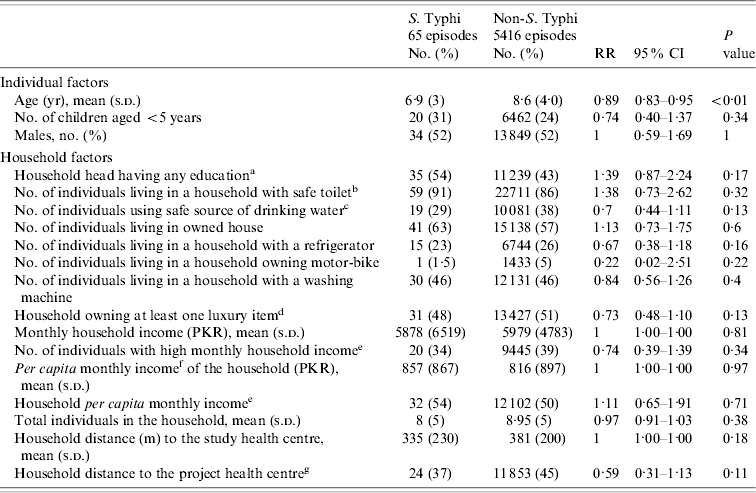
RR, Risk ratio; CI, confidence interval; PKR, Pakistani rupees.
a Any education: any type of education is counted; compared to ‘no education’.
b Safe toilet: use of own (non-shared) flush toilet.
c Safe water source: consumption of tap (communal/personal) water or bottled water.
d Luxury items: refrigerator, motor-bike, and/or washing machine.
e High (household/per capita household) income: more than the median household/per capita household income; median derived using the vaccinated population (0, low income; 1, high income).
f Per capita income: calculated from total household income divided by the number of active members in the household prior to vaccination (US$1=PKR 60).
g Longer distance to the project health centre: more than the median distance to the project health centre; median derived using the vaccinated population (0, close to the project health centre; 1, long distance to the project health centre).
During the 2 years of follow-up, 5791 episodes of fever were captured in the surveillance from 4216 individuals. Thirteen episodes were of <3 days fever, and a blood sample was not collected for microbiological confirmation in 297 episodes which were excluded from the analysis. In total, 65 fever cases were blood culture-positive for S. Typhi during 2 years of follow-up. Annual incidence of typhoid in children aged 2–16 years in the hepatitis A arm was 151/100 000 population. Mean number of household members in the study setting was nine (s.d.=5, median=8). In 57% households the head of household had no formal education. Most (86%) reported use of a flush toilet. Only 38% had a safe drinking facility at home. Sixty percent of the respondents reported ownership of current residence. Mean monthly household income for the study population was 5978 Pakistani rupees (PKR) (US$104.0) [s.d.=PKR 4788 (US$83.0)]; median=PKR 5000 (US$87.0) and 4532 households reported living below poverty line. The mean cluster population density as defined by number of individuals/100 m2 was 9·2. Mean population density was higher in Sultanabad (15 individuals/100 m2) than in Hijrat (11 individuals/100 m2), and was lowest in Bilal (6 individuals/100 m2) (Table 2).
Table 2. Distribution and estimate of risk for cluster-level factors associated with typhoid fever in children of urban squatter settlements in Karachi, Pakistan
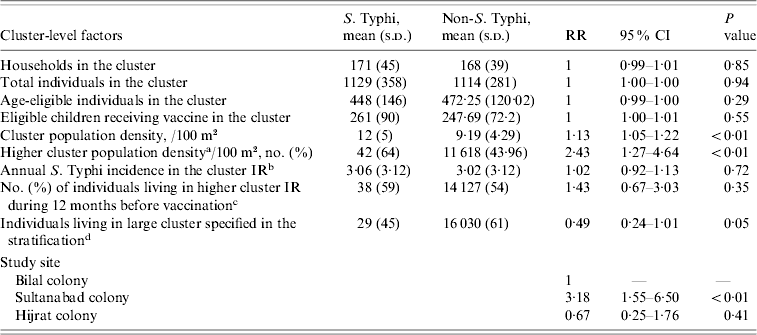
RR, Rate ratio; CI, confidence interval; IR, incidence rate.
a Higher cluster population density: more than the median cluster population density; median derived using the vaccinated population (0, low cluster population density; 1, high cluster population density).
b Cluster IR: calculated from the number of cases between 366 days and 1 day prior to the first day of vaccination in each site (1, Sultanabad and Hijrat; 2, Bilal) divided by the number of total population at the beginning of the study period.
c Higher cluster incidence rate during 12 months before vaccination: more than the median cluster incidence rate during 12 months before vaccination (0, low cluster IR; 1, high cluster IR).
d Larger/small cluster specified in stratification: (Sultanabad/Hijrat: large, >350; small, ⩽350) (Bilal: large: >450; small, ⩽450).
We performed log-binomial regression analysis for assessment of risk factors for typhoid fever in children. The independent relationships of individual, household and cluster factors were evaluated for statistical significance. Age [risk ratio (RR) 0·89, 95% confidence interval (CI) 0·83–0·95], population density (RR 1·13, 95% CI 1·05–1·22) and residing in Sultanabad compared to Bilal colony (RR 3·18, 95% CI 1·55–6·5) had a statistically significant independent relationship with typhoid fever. In univariate analysis we did not find a statistically significant association of socioeconomic factors with typhoid fever. We adjusted for effect of covariates and cluster correlation in the final model. After adjustment, the risk of typhoid decreased with increasing age (RR 0·89, 95% CI 0·83–0·95), was higher with an increase in population density (RR 1·13, 95% CI 1·05–1·22) and was lower in the households using a safe drinking-water source (RR 0·63, 95% CI 0·41–0·99) (Table 3).
Table 3. Adjusted model of risk of typhoid fever in children in Karachi using log binomial regression
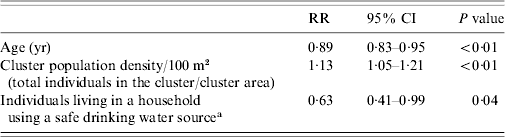
RR, Risk ratio; CI, confidence interval.
a Safe water source: consumption of tap (communal/personal) water or bottled water.
We also performed subgroup analysis by age, sex, and participation in the Vi vaccine trial (i.e. receipt of the control vaccine within the control clusters), assuming these variables affect typhoid fever incidence in the study population. However, we did not find statistically significant association of age, sex and receipt of control vaccine with typhoid fever incidence.
DISCUSSION
We identified three variables at three different levels for typhoid fever in children living in low socioeconomic settings in Karachi. Our results are consistent with findings from studies on typhoid fever in Bangladesh and India which indicate that younger children are at higher risk of contracting typhoid [Reference Brooks5, Reference Sinha14]. In subgroup analysis for age, we did not find a statistically significant effect of other covariates on typhoid fever in children younger than 5 years. Higher typhoid fever incidence at a younger age could be due to immunological reasons and not due to environmental factors [Reference Butler15]. Repeated S. Typhi exposure in such settings as children grow up, may result in lower incidence; however, the mechanism of protection is poorly understood. The non-specific clinical presentation of typhoid fever in young children results in prolongation of fever and hence hospitalization and sometimes results in fatal outcome [Reference Bhutta16].
Poor hygiene and housing are among other household factors suggested to increase the risk of typhoid fever [Reference Vollaard17, Reference Vollaard18]. Except for population density, we did not find any statistically significant association of other household variables after adjustment for the effect of the covariates. We found that with an increase of one person/100 m2 of land the risk of typhoid increased by 13% (95% CI 5–21). In Sultanabad the population density was much higher (15 vs. 11 and 6/100 m2 land area) than the other two colonies. High rates of typhoid fever infection in urban areas of South and South East Asia could be explained by increased migration from rural areas to these cities.
We found that children in households that reported use of a safe drinking-water source were less likely to have typhoid fever. In households reporting safe drinking-water practice, the risk of typhoid was 40% (95% CI 1–60) lower than in those households that did not use safe drinking-water methods. The water supply system in Karachi is disorganized and most households receive water through private water supply companies through various container types. In our study setting the median per capita income was PKR 629.0 (US$11.0) per month. The cost of one water tank ranges from PKR 100 to 300 (US$1.5–4). The poor population cannot afford to subscribe to safer water-supply vendors. Depending on the neighbourhood, the source of water supply varies. While water reaches the end user, it has been kept in different containers and the cleanliness of such water containers is frequently below standard. Water thus supplied to households may carry with it microorganisms that cause clinical disease once ingested.
We enrolled children in the study through study health centres especially established for disease surveillance. Health-seeking behaviour in Pakistan does not follow a recognized pattern and people usually prefer private practitioners. The study made every effort for a comprehensive surveillance, but it is possible that we may have missed cases that visited healthcare providers outside the study setting.
Blood culture-positive fever cases were defined as typhoid fever in our study. Sensitivity of blood culture is 30–50% that may have resulted in false negative typhoid fever in our study. We identified 65 blood culture-positive typhoid cases and assumed a similar number of typhoid fever cases were missed by our case identification method. However, the proportionate size of false negatives to true negatives is small (65 vs. 5416), therefore the effect of false negatives on effect estimates is negligible.
CONCLUSION
Our results estimate an increased risk of typhoid fever transmission with younger age, increased population density and use of unsafe drinking water in a homogenous population. Typhoid fever control in urban settings of Pakistan such as Karachi must focus on these factors. Water purification mechanisms and implementation of building regulations in the urban, low socioeconomic areas of Pakistan could prevent the population, especially children, suffering from typhoid fever. As a short-term strategy, as recommended by World Health Organization (WHO), typhoid fever vaccination in high-risk areas will have a synergistic effect with water and sanitation interventions in reducing typhoid fever incidence [19].
ACKNOWLEDGEMENTS
We are grateful to the community leaders and members of the study site for their participation in the project. We also thank J. Wain, A. L. Page, J. Farrar, R. Abu-Elyazeed, T. Pang, C. M. Galindo, D. Alam, and L. Jodar for their contributions at various levels of the project. We are grateful to the administrative staff of the International Vaccine Institute and Aga Khan University for their continuous support through the duration of the project.
This study was conducted as part of the Diseases of the Most Impoverished (DOMI) Programme's Typhoid Project. The DOMI Programme was funded by the Bill and Melinda Gates Foundation and coordinated by the IVI. Additional support was provided by the governments of Korea, Kuwait, and Sweden.
DECLARATION OF INTEREST
None.