Personality, defined broadly as a dynamic and organised set of characteristics that uniquely influences an individual's cognition, motivation and behaviour, Reference Ryckman1 is a complex and challenging concept. Even the definition and quantification of personality traits is problematic. The ‘five factor’ model of personality has met with wide acceptance due to its robustness across ages, genders and cultures. Reference Goldberg2,Reference McCrae and Costa3 According to this model, personality results from the stable balance of five key traits, extraversion, agreeableness, conscientiousness, neuroticism and openness to new experiences, often called the ‘big five’. Reference Peabody and Goldberg4 However, although this model suggests a framework for the study of personality, the neurobiological basis of personality remains poorly understood. Personality traits are likely to be mediated by distributed brain networks Reference Kaasinen, Maguire, Kurki, Brck and Rinne5,Reference Deckersbach, Miller, Klibanski, Fischman, Dougherty and Blais6 and commonly implicated areas include prefrontal, anterior cingulate and orbitofrontal cortices. Reference Snowden7,Reference Rushworth, Behrens, Rudebeck and Walton8 These areas are likely to play a key role in the cognition of interpersonal and other social behaviours. Reference Adolphs9
Frontotemporal lobar degeneration (FTLD) is an important cause of young-onset dementia that frequently manifests with progressive behavioural abnormalities and social dysfunction, as recognised in consensus diagnostic criteria. As acknowledged in current definitions, Reference Neary, Snowden, Gustafson, Passant, Stuss and Black10 behavioural characteristics and their modulation in social contexts powerfully influence the maintenance of personality, implying that significant or persistent behavioural abnormalities may alter personality structure. Although much work in FTLD has focused on particular aspects of disturbed behaviour, there is evidence that more pervasive changes in personality are indeed a hallmark of diseases in the FTLD spectrum. Reference Mychack, Rosen and Miller11,Reference Sollberger, Stanley, Wilson, Gyurak, Beckman and Growdon12 From a neurobiological perspective, FTLD therefore presents an opportunity to assess the critical brain substrates that maintain a stable personality structure. Studies in healthy individuals have revealed neuroanatomical substrates overlapping with brain regions that are selectively damaged in FTLD. Reference Deckersbach, Miller, Klibanski, Fischman, Dougherty and Blais6,Reference Canli, Zhao, Desmond, Kang, Gross and Gabrieli13–Reference Sugiura, Kawashima, Nakagawa, Okada, Sato and Goto15 Whereas behavioural change has been linked with structural brain damage in Alzheimer's disease and FTLD, Reference Johnson, Head, Kim, Starr and Cotman16,Reference Farber, Rubin, Newcomer, Kinscherf, Miller and Morris17 particularly the subgroup with right temporal lobe atrophy, Reference Chan, Anderson, Pijnenburg, Whitwell, Barnes and Scahill18,Reference Josephs, Whitwell, Knopman, Boeve, Vemuri and Senjem19 studies assessing personality change per se are fewer. Agreeableness in FTLD has been correlated with changes in the volume of orbitofrontal cortex, Reference Rankin, Rosen, Kramer, Schauer, Weiner and Schuff20 whereas traits clustered according to properties such as affiliation and agency have been shown to correlate with patterns of distributed atrophy across a range of neurodegenerative diseases. Reference Sollberger, Stanley, Wilson, Gyurak, Beckman and Growdon12 However, little information is available concerning the cerebral correlates of longitudinal change in particular personality traits in neurodegenerative disease. From a clinical perspective, it has been shown that profiles of personality change can distinguish neurodegenerative diseases; analysis of personality change might potentially provide tools for early diagnosis of particular neurodegenerative diseases, and FTLD in particular. Reference Galvin, Malcom, Johnson and Morris21
Here we investigated cerebral correlates of longitudinal change in key personality traits in FTLD (as measured on a validated caregiver questionnaire) using voxel-based morphometry (VBM). We hypothesised that change in particular personality traits in FTLD would correlate with distinct profiles of brain atrophy and these profiles would overlap frontotemporal networks previously implicated in social cognition.
Method
Participants
Thirty individuals (mean age 64.6 years (s.d. = 8.5); 21 males) attending the tertiary cognitive disorders clinic at the National Hospital for Neurology and Neurosurgery with clinical diagnoses representing each of the major canonical syndromic subgroups of FTLD (10 with behavioural variant frontotemporal dementia, 10 with semantic dementia and 10 with progressive non-fluent aphasia) according to current consensus criteria Reference Neary, Snowden, Gustafson, Passant, Stuss and Black10,Reference Adlam, Patterson, Rogers, Nestor, Salmond and Acosta-Cabronero22 participated in the study. The diagnosis in each individual was supported by detailed neuropsychological assessment. Depression and anxiety symptoms exhibited by the participant cohort were indexed using the Neuropsychiatric Inventory (NPI). Reference Cummings, Mega, Gray, Rosenberg-Thompson, Carusi and Gornbein23 Executive function was assessed using Part B of the Trail Making Test Reference Reitan24 and the task-switching component of the Trail Making subsection of the Delis–Kaplan Executive Functions System. Reference Delis, Kaplan and Kramer25 Clinical and demographic details of all participants are summarised inTable 1. The mean age of the progressive non-fluent aphasia subgroup was significantly older (P<0.05) thgan the behavioural variant frontotemporal dementia subgroup; syndromic subgroups did not differ in gender distribution, mean Mini-Mental State Examination (MMSE) Reference Folstein, Folstein and McHugh26 score or disease duration. The mean NPI depression score was 1.6 (s.d. = 2.5) and mean anxiety score was 1.5 (s.d. = 2.4) (maximum score 12) for the combined FTLD cohort; in a subgroup analysis the mean anxiety score in the behavioural variant frontotemporal dementia subgroup (mean 3.6 (s.d. = 3.2)) was significantly higher (P = 0.01) than for other subgroups. Two-thirds of participants (n = 20) scored below the tenth centile on tests of executive function.
Table 1 Characteristics of participants with frontotemporal lobar degeneration (FTLD) and controls
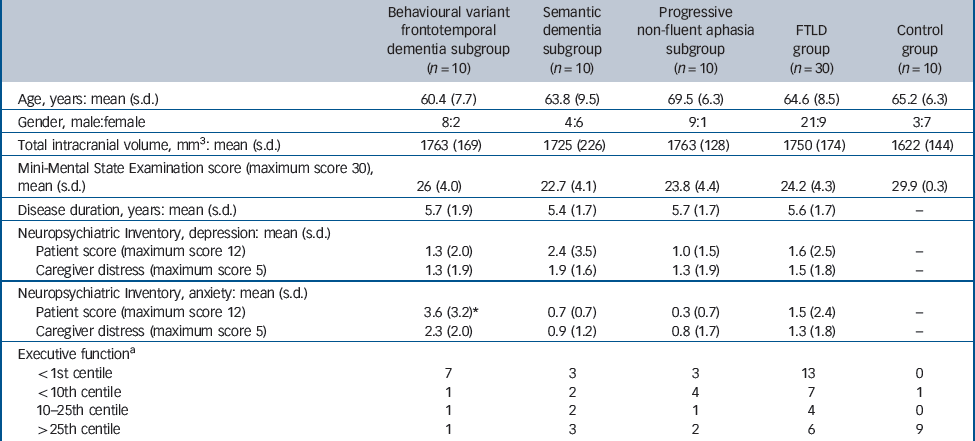
| Behavioural variant frontotemporal dementia subgroup (n = 10) | Semantic dementia subgroup (n = 10) | Progressive non-fluent aphasia subgroup (n = 10) | FTLD group (n = 30) | Control group (n = 10) | |
|---|---|---|---|---|---|
| Age, years: mean (s.d.) | 60.4 (7.7) | 63.8 (9.5) | 69.5 (6.3) | 64.6 (8.5) | 65.2 (6.3) |
| Gender, male:female | 8:2 | 4:6 | 9:1 | 21:9 | 3:7 |
| Total intracranial volume, mm3: mean (s.d.) | 1763 (169) | 1725 (226) | 1763 (128) | 1750 (174) | 1622 (144) |
| Mini-Mental State Examination score (maximum score 30), mean (s.d.) | 26 (4.0) | 22.7 (4.1) | 23.8 (4.4) | 24.2 (4.3) | 29.9 (0.3) |
| Disease duration, years: mean (s.d.) | 5.7 (1.9) | 5.4 (1.7) | 5.7 (1.7) | 5.6 (1.7) | – |
| Neuropsychiatric Inventory, depression: mean (s.d.) | |||||
| Patient score (maximum score 12) | 1.3 (2.0) | 2.4 (3.5) | 1.0 (1.5) | 1.6 (2.5) | – |
| Caregiver distress (maximum score 5) | 1.3 (1.9) | 1.9 (1.6) | 1.3 (1.9) | 1.5 (1.8) | – |
| Neuropsychiatric Inventory, anxiety: mean (s.d.) | |||||
| Patient score (maximum score 12) | 3.6 (3.2)* | 0.7 (0.7) | 0.3 (0.7) | 1.5 (2.4) | – |
| Caregiver distress (maximum score 5) | 2.3 (2.0) | 0.9 (1.2) | 0.8 (1.7) | 1.3 (1.8) | – |
| Executive functiona | |||||
| <1st centile | 7 | 3 | 3 | 13 | 0 |
| <10th centile | 1 | 2 | 4 | 7 | 1 |
| 10–25th centile | 1 | 2 | 1 | 4 | 0 |
| > 25th centile | 1 | 3 | 2 | 6 | 9 |
The study was conducted in accord with the Declaration of Helsinki and ethics approval was obtained from the Joint Research Ethics Committees of the National Hospital for Neurology and Neurosurgery and the Institute of Neurology.
Assessment of personality change
The Big Five Inventory (BFI), a 44-point personality assessment tool, was completed by a caregiver respondent for each participant. Reference John, Donahue and Kentle27 Caregivers have been shown to be a reliable source of longitudinal personality questionnaire information in dementia. Reference Strauss, Pasupathi and Chatterjee28,Reference Rankin, Baldwin, Pace-Savitsky, Kramer and Miller29 For the purposes of this study, the caregiver respondent was a first-degree relative with excellent personal knowledge of the participant over at least a 10-year period. The respondent was asked to rate each personality trait based on their knowledge of the participant currently and 10 years previously (i.e. before the clinical onset of disease: see disease duration data inTable 1). The BFI is structured to allow independent analysis of the traits of agreeableness, conscientiousness, extraversion, neuroticism and openness to new ideas. Patient data were validated in comparison with non-self-respondent data for a group of 10 neurologically healthy individuals age matched to the FTLD group. Examples of questions for each trait from the BFI are provided in the Appendix. All answers were scored on a linear scale, from ‘disagree strongly’ to ‘agree strongly’. A previously developed scoring syntax Reference John, Donahue and Kentle27 was used to derive a numerical score for each trait, and a change score (the difference between current and premorbid scores for that trait) was computed for each participant. Changes in personality scores were assessed statistically for the entire FTLD group and for each syndromic subgroup using analysis of variance methods implemented in STATA version 8.0 for Windows XP.
MRI acquisition
Each participant had brain magnetic resonance imaging (MRI) on a 1.5 Tesla GE Signa scanner (General Electric, Milwaukee, Wisconsin, USA) using a standard quadrature head coil. The T 1-weighted volumetric images were obtained with a 24 cm field of view and 256 × 256 matrix to provide 124 contiguous 1.5 mm thick slices in the coronal plane (echo time (TE) = 5 ms, repetition time (TR) = 512 ms, inversion time (TI) = 5650 ms).
Image analysis
Voxel-based morphometry of brain images was performed using the DARTEL toolbox of SPM5 (www.fil.ion.ucl.ac.uk/spm) running under Matlab 7.0 (Mathworks, Sherborn, Massachusetts, USA). An optimised VBM protocol was used. Reference Ridgway, Henley, Rohrer, Scahill, Warren and Fox30 Normalisation, segmentation, modulation and smoothing of grey and white matter images were performed using default parameter settings. In order to adjust for individual differences in global grey matter volume, total intracranial volume was calculated for each participant (Table 1) by summing grey matter, white matter and cerebrospinal fluid volumes following segmentation of all three tissue classes. Linear regression models were used to examine regional grey matter volumes correlated with the BFI change score for each trait (β1 = BFInow–BFI10years). Voxel intensity (grey matter volume), V, was modelled as a function of the BFI change score across the group, including participants age, gender and total intracranial volume (TIV) as nuisance covariates:
(where μ is a constant and ϵ is error). Personality traits were analysed both in a common design matrix including all five trait scores (in order to assess brain correlates of each trait independently of other traits) and also in separate design matrices for each individual trait (a trait-specific design). Separate design matrices were constructed for the combined FTLD group and for each syndromic subgroup. To protect against voxel drop-out because of marked regional atrophy in particular participants, we implemented a customised explicit brain mask using a specified ‘consensus’ voxel threshold intensity criterion: Reference Ridgway, Omar, Ourselin, Hill, Warren and Fox31 using this consensus mask, a voxel was included in the analysis if grey matter intensity at that voxel was >0.1 in >70% of the participants (rather than in all participants, as with the default statistical parametric map mask).
Statistical parametric maps of regional grey matter volume correlating with score on each trait were examined at a threshold of P<0.001 uncorrected and at a threshold of P<0.05 after voxel-wise false discovery rate correction over the whole brain. A cluster extent threshold of 50 voxels was applied when reporting significant brain regions. Maps were displayed as overlays on a study-specific template, created by warping all native space whole-brain images to the final DARTEL template and calculating the average of the warped brain images. In order to assess any grey matter correlates of personality change in relation to the overall distribution of disease-related brain damage, we derived a map of FTLD-related brain atrophy in a separate VBM subanalysis comparing magnetic resonance images from the FTLD group with images (obtained using the same scanner and acquisition parameters) from a group of 20 healthy age- and gender-matched controls (mean age 65.2 years (s.d. = 7.3); 8 females; total intracranial volume 1620 ml (s.d. = 152)).
Results
Personality data
The participants with FTLD when considered as a single group showed significant change in each personality trait when current were compared with estimated premorbid characteristics. There was a decrease in mean scores for agreeableness (–0.45 (s.d. = 0.66)), conscientiousness (–1.03 (s.d. = 1.21)), extraversion (–1.11 (s.d. = 1.14)) and openness to new ideas (–1.05 (s.d. = 0.8)), and a mean increase in neuroticism score (+ 0.83 (s.d. = 1.2)) (all P<0.001). The healthy control group showed no significant changes between current trait score and score 10 years previously.
The FTLD subgroups showed broadly similar patterns of personality change. Raw BFI data for FTLD subgroups and controls are presented inFig.1, and change scores are shown inFig. 2. The behavioural variant frontotemporal dementia subgroup showed a statistically significant (P<0.05) decrease in mean scores for agreeableness (0.74 (s.d. = 0.84)), conscientiousness (2.0 (s.d. = 1.44)) and openness (1.24 (s.d. = 0.87)) and a non-significant trend towards decreased extraversion (1.0 (s.d. = 1.58) (P = 0.08). The semantic dementia subgroup showed a significant decrease in mean scores for agreeableness (0.49 (s.d. = 0.66)), extraversion (1.0 (s.d. = 0.93)) and openness (1.03 (s.d. = 0.81)) and a trend towards decreased conscientiousness (0.53 (s.d. = 0.89)) (P = 0.09); this subgroup also showed an increase in mean score for neuroticism (+1.2 (s.d. = 1.22)). The progressive non-fluent aphasia subgroup showed a significant decrease in mean scores for extraversion (0.34 (s.d. = 0.87)), conscientiousness (0.55 (s.d. = 0.54)) and openness (0.88 (s.d. = 0.74)) and a significant increase in mean score for neuroticism (+ 0.63 (s.d. = 0.79)). Comparing subgroups, change in conscientiousness score differed significantly (P<0.01) between the behavioural variant frontotemporal dementia subgroup and both language (semantic dementia and progressive non-fluent aphasia) subgroups. Change scores for other traits did not differ significantly between subgroups.

Fig. 1 Personality trait scores in participants with frontotemporal lobar degeneration (present and premorbid) and controls.
(a) Behavioural variant frontotemporal dementia subgroup; (b) progressive non-fluent aphasia subgroup; (c) semantic dementia subgroup; (d) control group. BFI, Big Five Inventory; E, extraversion; A, agreeableness; C, conscientiousness; N, neuroticism; O, openness.
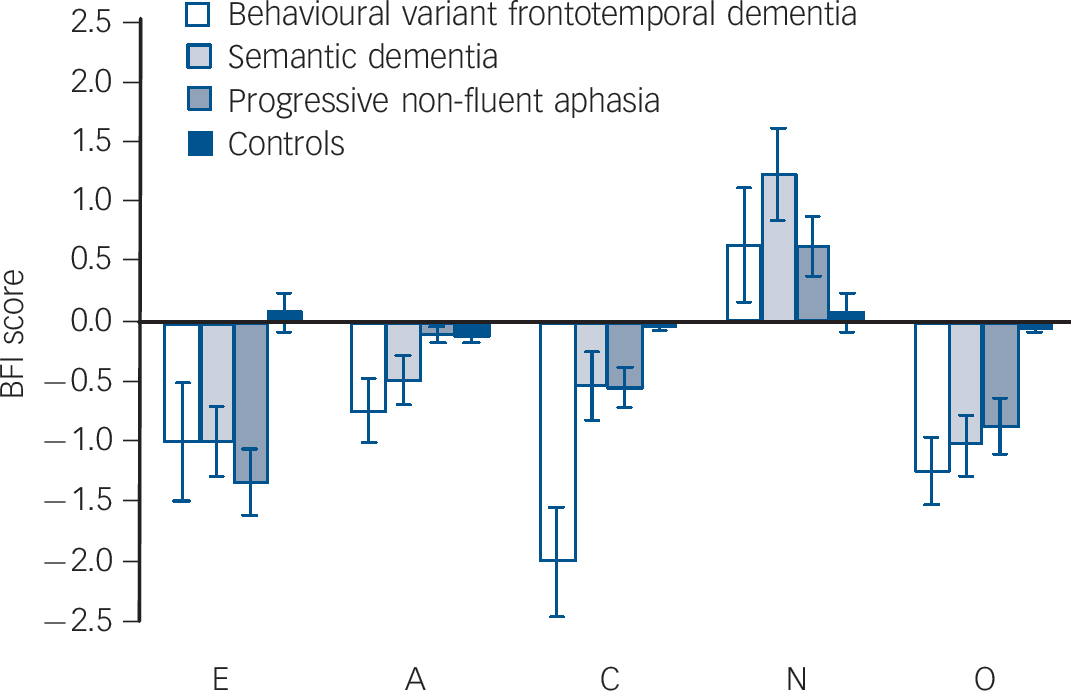
Fig. 2 Personality change data in participants with frontotemporal lobar degeneration and controls.
BFI, Big Five Inventory; E, extraversion; A, agreeableness; C, conscientiousness; N, neuroticism; O, openness.
VBM data
Correlations between grey matter and personality change were investigated over the entire FTLD group and for each syndromic subgroup. Here we report cortical areas where trait change scores were associated with grey matter loss and areas where change scores were inversely associated with grey matter volume. The separate contrast between the FTLD group and a healthy control group (thresholded at P<0.05 after false discovery rate correction overt the whole brain volume) revealed a typical profile of extensive disease-related atrophy involving frontal, temporal and parietal lobes bilaterally. These regions of disease-related grey matter loss included the areas identified as correlates of personality change in FTLD, allowing us to conclude that any inverse association between regional grey matter and personality change in these areas signifies relative preservation of grey matter (rather than an absolute increase in local grey matter).
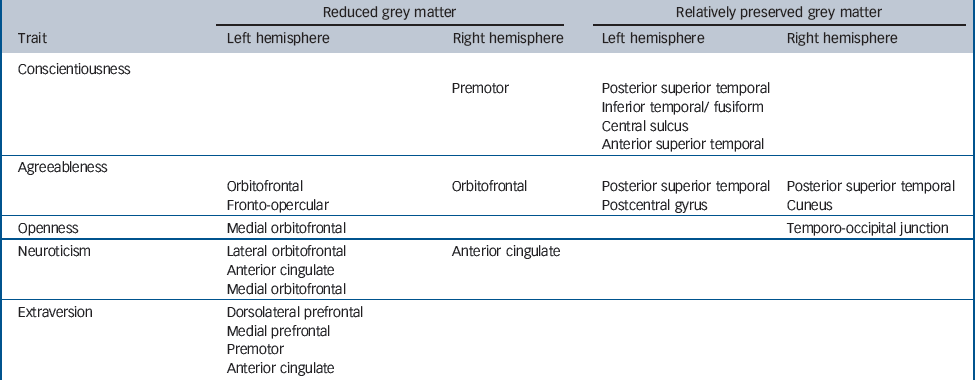
For the combined FTLD group, VBM correlates of personality change are presented inTable 2 andFig. 3 (a colour version ofFig. 3 can be found online (Fig. DS1)). Reduced conscientiousness was associated with grey matter loss in right premotor cortex (P<0.001 uncorrected) and associated with relative preservation of grey matter in left posterior superior temporal sulcus and superior temporal gyrus (P<0.05 false discovery rate corrected over the whole brain volume when analysed in a trait-specific design; P<0.001 uncorrected after covarying for other personality traits), left middle, inferior temporal and fusiform gyri and left central sulcus (all P<0.001 uncorrected). Less robust cerebral correlates (all P<0.001 uncorrected) of personality change were identified for other traits, analysed both in a combined design and separately for each trait. Reduced agreeableness was associated with grey matter loss in bilateral orbitofrontal cortices and left fronto-opercular cortex and associated with relative grey matter preservation in bilateral posterior superior temporal gyrus, right cuneus and left postcentral gyrus. Reduced extraversion was associated with grey matter loss in left medial prefrontal cortex, left dorsolateral prefrontal cortex, left premotor cortex and left anterior cingulate cortex; no areas of relative grey matter preservation correlated with reduced extraversion were identified. Reduced openness was associated with grey matter loss in left medial orbitofrontal cortices and associated with relative grey matter preservation in the region of the right temporo-occipital junction. Increased neuroticism was associated with grey matter loss in left lateral and medial orbitofrontal cortex and bilateral anterior cingulate cortex; no areas of relative grey matter preservation correlated with increased neuroticism were identified.
Table 2 Summary of voxel-based morphometry regional grey matter correlates of personality change in the frontotemporal lobar degeneration groupa
| Reduced grey matter | Relatively preserved grey matter | |||
|---|---|---|---|---|
| Trait | Left hemisphere | Right hemisphere | Left hemisphere | Right hemisphere |
| Conscientiousness | ||||
| Premotor | Posterior superior temporal | |||
| Inferior temporal/ fusiform | ||||
| Central sulcus | ||||
| Anterior superior temporal | ||||
| Agreeableness | ||||
| Orbitofrontal | Orbitofrontal | Posterior superior temporal | Posterior superior temporal | |
| Fronto-opercular | Postcentral gyrus | Cuneus | ||
| Openness | Medial orbitofrontal | Temporo-occipital junction | ||
| Neuroticism | Lateral orbitofrontal | Anterior cingulate | ||
| Anterior cingulate | ||||
| Medial orbitofrontal | ||||
| Extraversion | Dorsolateral prefrontal | |||
| Medial prefrontal | ||||
| Premotor | ||||
| Anterior cingulate | ||||

Fig. 3 Voxel-based morphometry correlates of personality change in frontotemporal lobar degeneration (FTLD).
See Fig. DS1 for a colour version of this figure. For each personality trait, panels show statistical parametric maps of associated grey matter loss (GM loss) or relative grey matter preservation (GM). Maps have been rendered on the mean T -weighted normalised brain image for the FTLD group; the left hemisphere is presented on the left. For display purposes, maps are thresholded at P<0.001 uncorrected for all results. T-scores of grey matter change are coded as indicated on the colour bar (lower left). T-scores of grey matter change are coded as indicated on the greyscale bar (lower left).
In the separate VBM analyses for each syndromic subgroup, grey matter correlates of personality change were similar to that of the combined FTLD group: no additional or syndrome-specific grey matter correlates were identified.
Discussion
Here we have demonstrated profiles of personality change and their neuroanatomical associations in FTLD. Participants with FTLD exhibited widespread alterations in personality compared with healthy age-matched controls: disease onset was associated with significant decline in the traits of extraversion, agreeableness, conscientiousness and openness, but an increase in neuroticism. Our findings corroborate previous studies of personality alteration in FTLD Reference Mychack, Rosen and Miller11,Reference Sollberger, Stanley, Wilson, Gyurak, Beckman and Growdon12 and further underline that personality change is a cardinal symptom of diseases in the FTLD spectrum. Previous work focusing on measurement of personality change in Alzheimer's disease has shown a qualitatively similar although less marked profile of personality alteration, with increased neuroticism and decreased conscientiousness, openness and extraversion. Reference Siegler, Dawson and Welsh32 Although some evidence suggests that the trait of agreeableness is largely stable, Reference Siegler, Welsh, Dawson, Fillenbaum, Earl and Kaplan33 other work has reported a modest decline. Reference Chatterjee, Strauss, Smyth and Whitehouse34 Little quantitative information is available concerning personality alterations in other neurodegenerative diseases. In one study, participants with Parkinson's disease had a profile of personality change similar to Alzheimer's disease, with a slight emphasis on decreased extraversion. Reference Glosser, Clark, Freundlich, Kliner-Krenzel, Flaherty and Stern35
Neuroanatomical substrates for personality traits
The most robust neuroanatomical correlate of personality change identified here was the association of decreased conscientiousness with relative grey matter preservation in the region of the left superior temporal sulcus and superior temporal gyrus. This region has previously been linked to a diverse range of cognitive (including social behavioural) functions. Reference Hein and Knight36–Reference Kipps, Nestor, Acosta-Cabronero, Arnold and Hodges38 A recent large VBM study of brain structure and the BFI in healthy adults Reference DeYoung, Hirsh, Shane, Papademetris, Rajeevan and Gray39 showed a profile of regional volume change similar to the profile we observed in the FTLD group in our study: conscientiousness was positively associated with brain volume in prefrontal (and superior parietal) cortex but negatively associated with volume in other areas including fusiform and paracentral cortices. Superior temporal cortex has been implicated in the pathogenesis of personality disorders and sociopathy. Reference Goldstein, Hazlett, New, Haznedar, Newmark and Zelmanova40,Reference de Oliveira-Souza, Hare, Bramati, Garrido, Azevedo Ignácio and Tovar-Moll41 As lack of concern for others’ well-being and with the consequences of one's own behaviour are prominent features of sociopathy, it is unsurprising that those scoring highly on scales of sociopathy should have correspondingly reduced scores for the trait of conscientiousness. Reference Zuckerman, Kuhlman and Camac42 This line of evidence suggests that temporal lobe cortices may have an important role in regulating conscientiousness. In the present study, a number of additional cortical areas were identified as correlates of reduced conscientiousness, including regions previously implicated in analysis of social signals, programming of social behaviours and representation of others’ emotional states. Reference McCloskey, Phan and Coccaro43–Reference Bastiaansen, Thioux and Keysers46
The cerebral correlates of change in other personality traits in our study implicated distributed frontal, temporal and parietal lobe areas. Our findings are consistent with previous evidence concerning the brain substrates of particular traits. Reduced agreeableness had a correlate in orbitofrontal cortex bilaterally, as demonstrated previously in FTLD. Reference Rankin, Rosen, Kramer, Schauer, Weiner and Schuff20 Reduced extraversion had a correlate in medial and dorsolateral prefrontal cortex, consistent with previous structural and functional imaging evidence in healthy populations Reference Kaasinen, Maguire, Kurki, Brck and Rinne5,Reference Johnson, Wiebe, Gold, Andreasen, Hichwa and Watkins47–Reference Wright, Feczko, Dickerson and Williams49 and in neurodegenerative disease. Reference Sollberger, Stanley, Wilson, Gyurak, Beckman and Growdon12 Increased neuroticism had a correlate in anterior cingulate cortex, which has previously been implicated in conflict monitoring and avoidance, Reference Allman, Hakeem, Erwin, Nimchinsky and Hof50,Reference Botvinick, Nystrom, Fissell, Carter and Cohen51 and in the pathogenesis of obsessive–compulsive disorder, a condition with high levels of neuroticism. Reference Radua and Mataix-Cols52
A broad neuroanatomical pattern that emerges from this study is the association of personality change with grey matter loss in more anterior and dorsal cortical areas and relative preservation of grey matter in more posterior and ventral areas. These findings suggest that personality change in FTLD results from dysfunction within a distributed network of cortical areas; besides the effects of disease-related brain damage per se, personality alterations may arise from the abnormal interaction of more severely damaged with less severely damaged cortex. The concept of neural network dysfunction in the pathogenesis of FTLD and other neurodegenerative diseases has gained currency, Reference Seeley, Crawford, Zhou, Miller and Greicius53 and network dysfunction is likely to be particularly relevant to complex behavioural symptoms. Frontomedial cortices (orbitofrontal cortex, prefrontal cortex) and their connections (in particular, superior temporal gyrus and sulcus) have been linked to mentalising and other social cognitive skills and behaviours in previous structural and functional imaging studies in both healthy and clinical populations. Reference Völlm, Taylor, Richardson, Corcoran, Stirling and McKie54–Reference Carrington and Bailey59
A multidimensional neural construct such as personality is unlikely to bear any simple unitary relation to macro-anatomical parameters such as regional brain volume. Evidence from healthy participants Reference DeYoung, Hirsh, Shane, Papademetris, Rajeevan and Gray39 supports the present clinical findings, demonstrating that anatomical correlates of personality traits are both distributed and bidirectional: a particular trait associates with increased brain volume in some regions and reduced volume in others. Although our understanding of the neural mechanisms that sustain personality remains too rudimentary to specify how these volume changes are related, one plausible hypothesis is that personality traits represent the net output of a balance of competing processes. For example, from a social cognition perspective, traits such as agreeableness or neuroticism might reflect an altered interaction between self (inwardly directed) and mentalising (externally directed) perspective-taking processes. Reference Vogeley, Bussfeld, Newen, Herrmann, Happé and Falkai60,Reference Nettle and Liddle61 We do not of course suggest that any single such simple dichotomy will capture the richness of human personality structure or its erosion by disease, although we speculate that ‘reduced conscientiousness’ might require (in addition to diminished regard for the consequences of one's behaviour) preserved understanding of one's own selfish interests. Furthermore, in the present study, the cortical areas implicated in particular personality alterations showed substantial overlap. This suggests that regional anatomical specificity for particular traits should be regarded as relative rather than absolute: any neuroanatomical specificity for personality change in FTLD is likely to emerge as a profile of brain damage distributed among areas in one or more cerebral networks that mediate personality.
Directions for future work
This study has several methodological limitations. In particular, change in personality was based on retrospective indices, and neuroanatomical correlation was performed at a single time point. A further important limitation of this study is that diagnoses were clinical, without neuropathological correlation: this leaves open the possibility that at least a proportion of participants in this series had a histopathological substrate outside the FTLD spectrum. Moreover, detailed correlation with neuropathological data will ultimately be required in order to define the neurobiological basis for personality change in FTLD. These limitations notwithstanding, the findings provide initial evidence that the clinical signal of personality alteration in FTLD may be associated with signature patterns of structural brain damage. From a neurobiological perspective, FTLD presents a unique ‘experiment of nature’ that allows identification of brain substrates critical for maintenance of stable personality traits. Important issues for future work will include the corroboration of these findings using prospectively acquired personality indices and longitudinal measures of brain atrophy (including measures of earliest clinical change in genetic mutation carriers), and evaluation of altered personality in FTLD in relation both to other neurodegenerative diseases and to different pathological subtypes within the heterogeneous FTLD spectrum.
Funding
This work was undertaken at UCLH/UCL who received a proportion of funding from the Department of Health's NIHR Biomedical Research Centres funding scheme. The Dementia Research Centre is an Alzheimer's Research Trust Co-ordinating Centre. This work was also funded by the Medical Research Council UK. C.J.M. is supported by an MRC programme grant. J.D.R. was supported by a Wellcome Trust Research Training Fellowship and R.O. by a Royal College of Physicians/Dunhill Medical Trust Research Fellowship. M.N.R. is an NIHR senior investigator. J.D.W. is supported by a Wellcome Trust Senior Clinical Fellowship.
Appendix
Examples of questions used to assess personality traits in the Big Five Inventory
Extraversion (contrasts talkativeness or assertiveness with taciturnity or passivity): Is he/she talkative? Is he/she reserved? Agreeableness (contrasts helpfulness or altruism with hostility or selfishness): Is he/she helpful with others? Does he/she easily find fault with others? Conscientiousness (contrasts organisation or attention to detail with negligence or carelessness): Is he/she a reliable worker? Does he/she tend to be disorganised? Neuroticism (features of nervousness or temperamentality): Can he/she be moody? Does he/she remain calm in intense situations? Openness to new ideas (contrasts creativity with narrow-mindedness): Is he/she sophisticated with art or music? Does he/she prefer to work in a routine?
Acknowledgements
We are grateful to the individuals who participated.








eLetters
No eLetters have been published for this article.