The WHO estimates that the global prevalence of anaemia in children under 5 years of age is 47 %( 1 ). In Brazil, isolated studies carried out in the past two decades showed anaemia prevalences of up to 80 % in this same age group( Reference Batista-Filho, Souza and Miglioli 2 , Reference Vieira and Ferreira 3 ). Also according to WHO, 50 % of anaemia cases are due to diet-related Fe deficiency, although this proportion can vary across different population groups and geographical areas( 1 ).
In an attempt to decrease the prevalence of Fe-deficiency anaemia, the Brazilian government made fortification of wheat and corn flours mandatory in July 2004. Statute no. 344/2002( 4 ) determined that 4·2 mg of Fe be added to every 100 g of flour, which corresponds to approximately 50 % of the recommended daily value for pre-school children( 5 ), considering only the ingestion flour.
We conducted a time series analysis consisting of three population-based surveys of children under 6 years of age residing in the urban area of the city of Pelotas, Southern Brazil( Reference Assunção, Santos and Barros 6 ). Hb levels were measured in May/June 2004 (baseline study, prior to mandatory fortification) and during these same months in 2005 and 2006. To assess the long-term impact of fortification, a fourth survey was carried out in 2008.
Here, we report the results of this fourth survey which, in addition, investigated the role of Fe deficiency as a cause of anaemia and estimated the bioavailability of the Fe ingested by children in the sample. The study was complemented by a nationwide survey of flour mills that investigated the types of Fe compounds added to flour in Brazil.
Experimental methods
The 2008 survey used a methodology comparable to another three previous studies we conducted in 2004, 2005 and 2006( Reference Assunção, Santos and Barros 6 ). In the 2004 baseline study, we sorted the 404 census enumeration tracts (clusters with approximately 300 households) in Pelotas (Brazil) urban area according to mean family income based on data from the 2000 Demographic Census( 7 ) and selected a systematic sample of twenty tracts with probability proportional to size. In each tract, we visited a number of households sufficient to obtain an average of thirty children under 6 years of age, which varied depending on the size of the tract. Sample size was calculated to enable detection of a minimum difference of 0·5 g/dl in mean Hb levels between time points prior to and after the intervention, assuming 95 % confidence (two-tailed), 90 % power and a standard deviation of 1·7 g/dl in Hb concentration( Reference Monteiro and Szarfarc 8 ). Based on the Brazilian literature, which indicates there is little variability in Hb levels between different social strata( Reference Osório, Lira and Batista-Filho 9 ) and therefore between different geographical clusters, sample size calculations were not corrected for intra-cluster correlation in the 2004 survey. According to these calculations, a sample of 600 children would be sufficient for the baseline survey. However, contrary to our expectations, the 2004 survey revealed the presence of geographical clustering of anaemia cases( Reference Assunção, Santos and Barros 10 ). Therefore, in subsequent surveys the number of clusters was expanded to reduce the design effect, leading to samples of 900 children in forty census tracts. A different set of tracts was investigated in each survey because children found to be anaemic were referred for treatment, potentially affecting prevalence if assessed in the same tract in subsequent years. Systematic samplings with probability proportional to size and sorting of selected sectors according to family income were common features of all surveys. Other methodological aspects of these surveys have been published elsewhere( Reference Assunção, Santos and Barros 6 ).
The 2008 survey also consisted of home visits carried out by nutritionists, who interviewed the mothers or caregivers of the selected children. We obtained information on demographics (sex and age in years), family income and the child's diet (24 h recall, answered by the mother or caregiver, and referring to weekdays, not holidays or weekends). Hb levels were determined from peripheral blood samples obtained by digital puncture using a HemoCue portable haemoglobinometer (HemoCue, Angelholm, Sweden). Quality control assessments for the HemoCue using standard solutions were performed daily according to the manufacturer's specifications and all checks were found to be satisfactory. Hb levels were treated both as a continuous variable, expressed in g/dl, and as a categorical variable, children with Hb concentrations below 11 g/dl being regarded as anaemic( 11 ). Severe anaemia was defined as a serum Hb concentration below 7·0 g/dl( 12 ).
The bioavailability of ingested Fe was calculated based on the data obtained from the 24 h recall questionnaire, using the algorithm proposed by Monsen et al.( Reference Monsen, Hallberg and Layrisse 13 ). This algorithm takes into account the ingestion of total Fe, haem Fe, non-haem Fe and vitamin C, and the amount of meat (red meat, poultry or fish) ingested per meal. As recommended, Fe stores of 500 mg were assumed( 14 ). Based on the estimated bioavailability per meal, we calculated the estimated percentage of the daily Fe ingestion absorbed by each child. Diets were then classified as of low, medium or high Fe bioavailability according to WHO criteria( 14 ). The adequacy of daily Fe ingestion was then determined considering the median estimated requirement for the child's age group( 5 ).
To measure body Fe reserve parameters so as to determine the aetiology of anaemia, we obtained blood samples from a subset of children by venepuncture, also at the child's home. Because our baseline survey revealed greater prevalence of anaemia among lower-income children( Reference Assunção, Santos and Barros 10 ), we chose to examine all of the children aged 2 years or older residing in seven census tracts selected randomly from among tracts with family income below two minimum wages per month. This survey was limited to children aged 2 years or older because, according to our prior experience, the refusal rate for drawing blood samples from children below this age in our settings is high. Blood samples were collected after a fasting period of at least 4 h. Collection was done by diagnostic laboratory staff and supervised by the team's biochemist. We measured serum concentrations of Hb (MICROS 60) and ferritin (immunoenzymatic assay), as well as transferrin saturation (automated colorimetric method).
Body Fe reserves were estimated based on serum levels of ferritin and transferrin saturation. Ferritin levels below 12 ng/ml are indicative of Fe deficiency. However, since these may increase in the presence of inflammatory of infectious processes, we also relied on transferrin saturation as a second indication of Fe deficiency( 11 ). Fe-deficiency anaemia was thus defined as Hb concentration below 11 g/dl accompanied by ferritin concentration below 12 ng/ml or transferrin saturation below 20 %.
During the same period, we carried out a survey in the wheat mills of the country in order to obtain information on which Fe compounds were being used. We restricted our analysis to wheat mills due to the low consumption of cornmeal-based products and high consumption of wheat flour products, mostly bread, in Southern Brazil( 15 ). Companies and their representatives were contacted by email and telephone and asked to respond to a brief questionnaire on the sources of Fe employed in fortification. We interviewed personnel involved in the fortification process, most of whom were laboratory or quality control employees.
Data were entered twice and entry consistency was verified using Epi Info 6·04 software (Centers for Disease Control and Prevention). The nutritional composition of foods and preparations was determined using ADSNutri software version 9·0( 16 ), which calculates the nutritional composition of foods according to the Tabela Brasileira de Composição de Alimentos (Brazilian food composition table)( 17 ) and the US Department of Agriculture food composition table( 18 ). Data analysis was carried out using the STATA statistical software package version 11·0 (Stata Corporation), taking into account the sample design. Analysis included χ 2 tests and ANOVA. Differences were considered as significant when reaching a probability level of P < 0·05, two-sided.
Written consent was obtained from the child's mother or caregiver before information and blood samples were collected. The parents of children identified as anaemic were advised to seek assistance from a health-care provider. The study protocol was approved by the Research Ethics Committee of the Federal University of Pelotas/Brazil School of Medicine.
Results
Between October and December 2008, we were able to identify 850 children aged less than 6 years in the forty census tracts surveyed. Of these, 799 were included in the study, or 94 % of the total sample. Characteristics of children whose parents refused to participate did not differ from those of participants. Of these 799, forty-five had participated in at least one of our previous studies, having changed address between surveys. Since exclusion of these children did not change the results obtained, they were maintained in the sample. As shown in Table 1, the samples studied in each year were similar with respect to sex, skin colour, age and income.
Table 1 Demographic and socio-economic characteristics of the four surveys. Pelotas, Southern Brazil
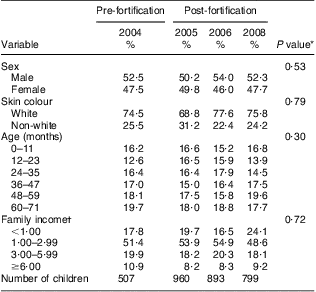
*χ 2 test.
†Monthly minimum wages.
The first issue we investigated was whether we could detect an impact of flour fortification on anaemia four years after its implementation. Of the 799 children, 92·2 % (n 737) provided digital puncture samples for HemoCue analysis. Mean Hb concentration with its standard error in 2008 for all children under 6 years of age analysed as a whole was 11·1 (se 0·09) g/dl, compared with 11·3 (se 0·13) g/dl in 2004, 11·2 (se 0·09) g/dl in 2005 and 11·3 (se 0·09) g/dl in 2006 (P < 0·001, ANOVA for linear trend). Table 2 presents the prevalence of anaemia according to age in all four surveys. Prevalences of anaemia and severe anaemia remained high across all four surveys. There was an increase in prevalence during the period among children under 24 months of age. Prevalence of anaemia among children aged 24 months or older was similar in all four surveys. Prevalence of severe anaemia (Hb <7·0 g/dl) among all children in the sample was 0·7 %, 0·9 %, 0·6 % and 1·6 %, respectively, in the 2004, 2005, 2006 and 2008 surveys (P = 0·15, χ 2 test for heterogeneity, and P = 0·12, χ 2 test for linear trend).
Table 2 Prevalence of anaemia (Hb < 11 g/dl) according to age group in the four surveys. Pelotas, Southern Brazil
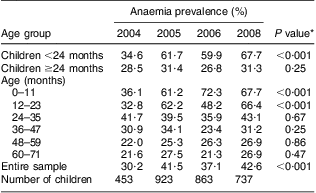
*χ 2 test for heterogeneity.
Mean Hb levels with their standard errors for children under 24 months were 10·9 (se 0·14) g/dl in 2004, 10·6 (se 0·14) g/dl in 2005, 10·4 (se 0·13) g/dl in 2006 and 10·0 (se 0·14) g/dl in 2008 (P = 0·02, ANOVA for linear trend). For children 24 months or older these levels were 11·5 (se 0·14) g/dl, 11·5 (se 0·09) g/dl, 11·7 (se 0·08) g/dl and 11·5 (se 0·10) g/dl, respectively (P < 0·001, ANOVA for linear trend).
We investigated the type of Fe added to wheat flour produced in Brazil. The legislation( 4 ) allowed the following compounds: ferrous sulfate; ferrous fumarate; reduced iron (325 Tyler mesh); electrolytic iron (325 Tyler mesh); sodium iron EDTA (NaFeEDTA); and iron bisglycinate chelate. After contacting a number of institutions and representatives in the industry, we identified 130 wheat mills, of which 118 were surveyed. According to the reports of their representatives, the majority (51 %) of mills used reduced Fe to fortify wheat flour. Ferrous fumarate was used by 13 % of mills, and ferrous sulfate by 7 %. The remaining 29 % of mills were unable to provide us with this information despite repeated attempts.
We also estimated the bioavailability of the Fe ingested by the children in the population-based survey. Initially, we estimated wheat flour intake based on the ingestion of flour-based foods reported in the 24 h recall questionnaires (Table 3). Intake was higher in 2005 and 2008 and lower in 2004 and 2006. The most striking trend observed was an increase with time in mean wheat flour intake among children aged 24–71 months. Taking into account the intake of flour and other Fe-containing foods reported in the 24 h recall, 88·6 % of the children were ingesting the recommended amount of Fe. Nevertheless, the daily bioavailability of the ingested Fe was estimated at roughly 5 % (Table 4), calculated based on 774 children after the exclusion of exclusively breast-fed babies (n 25). This estimate classifies the diets of children in the study as of low bioavailability( 14 ).
Table 3 Daily intake of wheat flour according to age in the four surveys. Pelotas, Southern Brazil
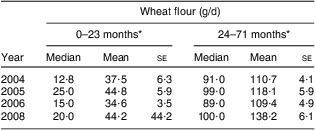
*In each age group, mean values were significantly different (ANOVA for linear trend): P < 0·001.
Table 4 Daily intake of iron per child (n 744Footnote *). Pelotas, Southern Brazil, 2008
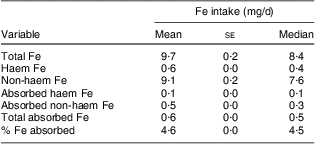
* Excluding twenty-five children under exclusive breast-feeding.
Finally, we investigated whether anaemia was indeed due to Fe deficiency in children aged 24–71 months. Of 145 children living in the seven tracts selected, 114 provided venous blood samples. Table 5 shows that, in 46·7 % (n 21/45) of cases of anaemia, Hb <11 g/dl was accompanied by ferritin concentration <12 ng/ml or by transferrin saturation <20 %, indicating Fe-deficiency anaemia.
Table 5 Prevalence of anaemia, iron deficiency and iron-deficiency anaemia in the studied sample (n 114). Pelotas, Southern Brazil, 2008

TSAT, transferrin saturation.
Discussion
We found no evidence of a decrease in prevalence of anaemia across the four surveys. The same was true for mean Hb levels, which remained essentially constant. Even though virtually all temporal comparisons reached statistical significance, this is likely due to the large number of children surveyed, which resulted in very high statistical power and contributed to render significant even clinically small differences. The most striking result in our survey was an increase in prevalence of anaemia among children under age 24 months, a trend that is opposite to what would be expected had the programme been effective. Mean Hb levels in this age group fell by 0·9 g/dl between 2004 and 2008.
It is estimated that two to three years are required to stabilize Fe balance and reserves following changes in the amount of bioavailable Fe in the diet. However, based mostly on results from studies in adults, 80 % of the impact is expected to be detectable within the first year( Reference Hallberg, Hulthen and Garby 19 ). This statement does not necessarily apply to children, but our data show that fortification of flour with Fe has failed to impact the prevalence of anaemia among pre-school children in Pelotas, Brazil, even as late as four years after its implementation.
Given their dietary characteristics, with low intake of foods containing flour, breast-fed babies are not part of the target population for whom flour fortification could have a positive impact( Reference Hurrell, Ranum and de Pee 20 ). However, if the fortification programme had been effective for adults, after four years the impact on Fe status of pregnant women would be expected to improve the Fe status of infants. Nevertheless, restricting our analysis to children aged 24–71 months also failed to show an impact of flour fortification on anaemia, despite an average daily intake of wheat flour 100 g or more in this age group. This level of Fe-fortified wheat flour consumption would be sufficient to supply half of the recommended daily values of Fe (Estimated Average Requirement, EAR)( 5 ) for this age group.
Therefore, the lack of impact of mandatory fortification on anaemia cannot be explained by insufficient consumption of flour. On the other hand, much of the flour produced in Brazil is fortified with reduced Fe, which has been shown to be ineffective in combating anaemia. Despite its low bioavailability, reduced Fe is frequently used as a food fortificant probably due to its low cost and negligible effect on the taste of flour( Reference Hurrell, Ranum and de Pee 20 ).
Also evident is the lack of interest of manufacturers regarding the fortification process, with 29 % of mill representatives being unable to inform us of the type of Fe they added to the flour. We believe it is fair to assume that the majority of these mills must be either adding reduced Fe or not adding Fe at all to their products. In the 2004 survey, we analysed samples of flour collected in the city of Pelotas and five of the twenty-three commercial brands did not contain any form of Fe, even though the labels stated that the product had been fortified( Reference Assunção, Santos and Barros 10 ). In the 2008 national survey, we were unable to obtain samples of the over 100 brands of flour investigated. There is no publicly available data on compliance of millers collected by a government agency, suggesting the lack of a well-designed monitoring system.
A limitation of the present study was the use of a 24 h recall questionnaire to estimate food intake, given the day-to-day variability in these results. However, dietary variability in the studied age group is known to be low, and recall questionnaires allow us to estimate the intakes of energy and nutrients distributed across all foods ingested( Reference Vitolo 21 ). Moreover, the algorithms used for these calculations should be applied to the foods effectively consumed in each meal, which prevents the use of more long-term FFQ. Another limitation is that although measurements of serum ferritin and transferrin receptor provide the best approach to measuring the Fe status of populations( 22 ), our analyses only included transferrin saturation and ferritin.
In addition, whereas the first three surveys were conducted in the winter, the fourth occurred in the spring, due to delayed funding. This is unlikely to have markedly affected our results because this is an urban, middle-income population with a low burden of seasonal infectious diseases; the infant mortality rate due to infectious diseases in 2004 was only two per thousand live births( Reference Santos, Menezes and Mota 23 ).
Despite the limitations of the 24 h recall, our results suggest that the diets of the children in the survey were found to contain very little bioavailable Fe, probably as a consequence of the low intake of dietary sources of haem Fe and of vitamin C during meals, which increases the bioavailability of non-haem Fe( Reference Hallberg and Hulthen 24 ). Nevertheless, in the sub-sample of 114 low-income children with venous blood samples, it was possible to obtain a more precise estimate of bioavailability by adjusting for ferritin levels according to the algorithm proposed by Hallberg and Hulthén( Reference Hallberg and Hulthen 24 ). This method resulted in an estimate of 7·4 % for bioavailability, a value which is comparable to the 5 % estimate obtained with the Monsen method for the whole sample (data not shown).
Together, the finding that children eat food that prevents the absorption of ingested Fe and the likely low bioavailability of the Fe used in fortification could be the reasons for the lack of impact of mandatory fortification.
Finally, the impact of flour fortification would be greater if a larger proportion of cases of anaemia were due to Fe deficiency. However, our combined Hb, ferritin and transferrin analyses suggest that half of the cases of anaemia did not result from deficiency in body Fe reserves, which is in agreement with international estimates( 1 ).
Since body Fe status was not determined in the surveys prior to 2008, we were unable to determine whether fortification affected the prevalence of Fe deficiency while having no effect on the overall prevalence of anaemia or on mean Hb levels.
Other nutrient deficiencies may be associated with anaemia in our settings, such as deficiencies in vitamins A, B6, B12, riboflavin and folic acid. Less prevalent causes, such as genetic haemoglobinopathies, could also play a role( 25 ). Acute or chronic infections may also cause anaemia; however, the ferritin and transferrin analyses carried out in 2008 consider this possibility when diagnosing Fe-deficiency anaemia. Parasitic infections by ancylostomes, schistosomes or malaria, all of which may lead to anaemia, are rare in this area of the country. It will be important to replicate the present results in other regions of the country where prevalence of Fe-deficiency anaemia is higher, such as the North and Northeast, since fortification is likely to have greater impact in such settings.
Food fortification may be an important strategy to improve the nutritional status of a population( Reference Hurrell, Ranum and de Pee 20 ). However, several of the characteristics of the Brazilian programme contribute towards its lack of impact on a population such as that of the present survey, including use of diets and Fe compounds with low bioavailability, failure to monitor commercial flour brands and a high proportion of non Fe-deficiency anaemia. Our main conclusion is that the national programme was ineffective as implemented. This does not mean that optimally implemented flour fortification programmes, using appropriate foods and bioavailable Fe compounds, could not be efficacious.
Hurrell et al. recently reviewed seventy-eight national Fe fortification programmes and judged that only nine national programmes (Argentina, Chile, Egypt, Iran, Jordan, Lebanon, Syria, Turkmenistan and Uruguay) were likely to have a significant positive impact on Fe status if coverage is optimized( Reference Hurrell, Ranum and de Pee 20 ). Although evaluations in Guatemala( Reference Imhoff-Kunsch, Flores and Dary 26 ) and Sri Lanka( Reference Nestel, Nalubola and Sivakaneshan 27 ) showed that flour fortification has little impact on anaemia, in Venezuela compulsory fortification of corn and wheat flours seems to have restored the Fe status of adolescents of low socio-economic status( Reference Layrisse, Garcia-Cassal and Méndez-Castellano 28 ).
In our opinion, adjustments in the mandatory Fe fortification programme are required. It should also be noted that any anaemia prevention programme must go beyond fortification, and include initiatives aimed at improving access to a healthy diet and nutritional counselling.
Acknowledgements
The study was supported by the Ministry of Health, Brazil. The authors have no conflict of interest. C.G.V., M.C.F.A., I.S.S., A.J.D.B. and D.P.G. designed the research; M.C.F.A. conducted the research and analysed the data; M.C.F.A. and C.G.V. wrote the paper. All authors read and approved the final manuscript.







