Hirayama disease (HD) was first described in 1959;Reference Hirayama, Toyokura and Tsubaki 1 however, further elucidation of its pathophysiology was not fully understood until 1987, resulting in its eponym, Hirayama disease.Reference Hirayama, Tomonaga, Kitano, Yamada, Kojima and Arai 2 Cervical myelopathy, caused by repetitive flexion of the neck resulting in forward displacement of the posterior dural sack with compression and injury of the spinal cord, is the hallmark presentation of this rare entity.Reference Foster, Tsang, Kam, Storey, Day and Hill 3 It mainly affects people of Asian descent, with a male predominance of 7:1,Reference Zhou, Chen, Fan and Zhou 4 during their second or third decade of life.Reference Zhou, Chen, Fan and Zhou 4 , Reference Pradhan 5 Characteristically, the C7-T1 myotomes show weakness that results in muscular atrophy while sparing the lower extremities without loss of sensation.Reference Pradhan 5 Patients commonly display a tremor on finger extension.Reference Zhou, Chen, Fan and Zhou 4 In the majority of cases, the disease stabilizes within approximately 5 years of symptom onset.Reference Zhou, Chen, Fan and Zhou 4 However, neurological function can deteriorate in some instances, with unilateral symptoms progressing to bilateral ones in 10% of cases.Reference Pradhan 5
We present the first two Canadian case reports of HD, describing the presentation, investigations, and management of this rare condition, followed by a short discussion of the sparse body of literature to date.
Case 1 concerns a Caucasian left-hand-dominant male professional singer and musician who developed left-hand clumsiness and spasm at the age of 17. On exam, there was weakness with a British Medical Research Council (BMRC) grade of 4/5 for the intrinsic hand muscles, and the flexor and extensor pollicis longus, as well as the flexor digitorum profundus to his third, fourth, and fifth digits, with no loss of sensation. Investigations at that time found increased T2-weighted signal intensity centrally at C5-C7 on magnetic resonance imaging (MRI). These were felt to reflect post-infective transverse myelitis, as he had just been treated for a dental abscess. His symptoms were not responsive to steroids but remained stable for a number of years. At the age of 22, he subsequently presented with a 6-week history of spasms in the third, fourth, and fifth digits of his right hand that were aggravated with neck flexion, noticeably while playing the violin. Nerve conduction studies revealed C7-T1 motor neuropathy bilaterally. Follow-up MRI showed additional T2 signal change in his lower cervical spinal cord. Furthermore, flexion MRI demonstrated flattening of the cervical cord and engorgement of epidural veins (Figure 1). The patient was placed in a Miami J cervical collar to restrict neck mobility for a few weeks. Immobilization of the neck provided improvement in terms of increased strength and dexterity of his right hand. The patient ultimately underwent a two-level anterior cervical discectomy and fusion (ACDF) of C5-C6 and C6-C7. Surgery was without complications, as well as without injury to his recurrent laryngeal nerve, in particular. At his 3-month follow-up appointment, he maintained the preoperative improvements he had gained while wearing a cervical collar. He has resumed singing and playing the violin and other musical instruments, without significant compromise.
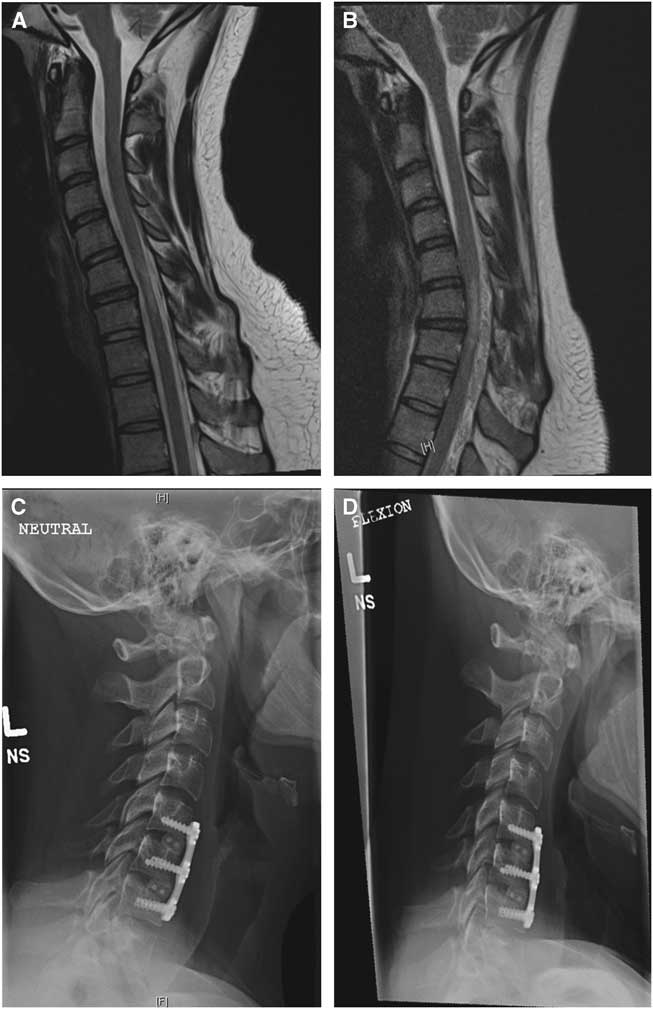
Figure 1 (A) Sagittal T2-weighted magnetic resonance imaging (MRI) in neutral position with no obvious cause for a T2-weighted signal change at C6-C7. (B) Sagittal T2-weighted dynamic flexion MRI with anterior displacement of the posterior dura resulting in cord compression and epidural venous distension in keeping with the diagnosis of Hirayama disease. (C) Lateral radiograph in neutral position after anterior cervical discectomy and fusion showing the cages and plate from C5-C7 in place. (D) Lateral flexion view demonstrating decreased flexion at the operative levels.
Case 2 concerns a 19-year-old, right-hand-dominant, female college student who awoke with spontaneous weakness in her right hand (BMRC grade of 3/5 for the intrinsic muscles), a year prior to presentation. She also reported decreased dexterity while there was no loss of sensation. Electromyogram (EMG) was suggestive of a right ulnar motor neuropathy. Unfortunately, her symptoms worsened, follow-up EMG was interpreted as multifocal motor neuropathy, and she was treated with intravenous immunoglobulin. As this was without effect, she subsequently underwent right ulnar nerve transposition and also received a right anterior intraosseous to ulnar motor nerve graft for presumed ulnar neuropathy. Following surgery, her symptoms stabilized with minimal improvement. The patient then underwent a dynamic MRI of her cervical spine that demonstrated T2-weighted signal hyperintensity in the spinal cord at C6-C7 with compression and flattening of the spinal cord in flexion with posterior distension of epidural veins (Figure 2). The patient declined a trial with a Miami J cervical collar and elected to undergo an ACDF at C6-C7. She tolerated the procedure well with no complications. At the 6-week follow-up appointment, she already showed improvement of her right-hand strength and function.
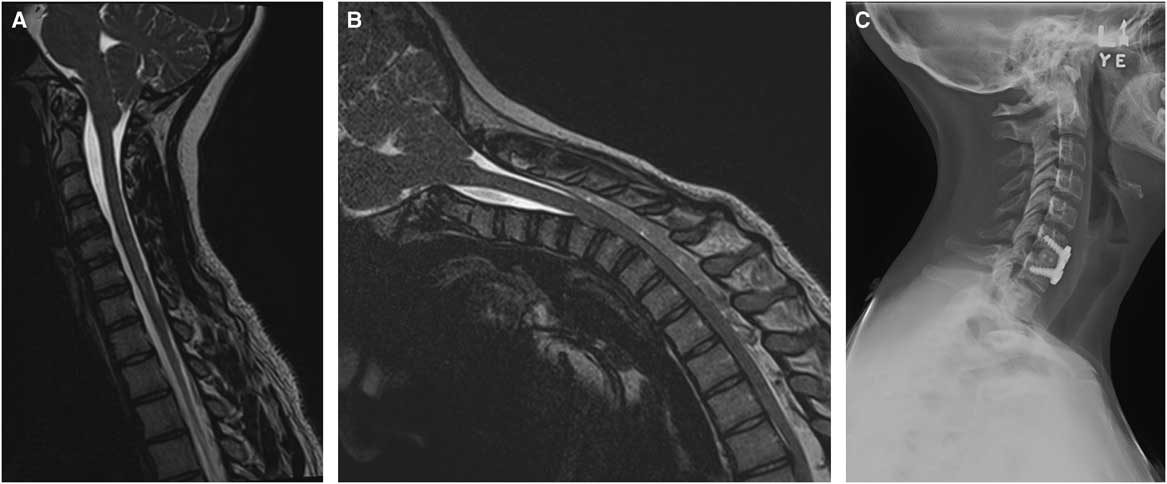
Figure 2 (A) Sagittal T2-weighted magnetic resonance imaging (MRI) in neutral position with minor T2-weighted signal change at C6-C7. (B) Sagittal T2-weighted flexion MRI with anterior displacement of the posterior thecal sac and cord compression, as well as epidural venous distension confirming the diagnosis of Hirayama disease. (C) Postoperative C6-C7 anterior cervical discectomy and fusion neutral lateral view.
The prolonged presentations and numerous diagnostic tests outlined in these cases illustrate how HD is a rare entity, presenting with vague symptoms that carry a broad differential for amyotrophy.Reference Lin, Kung, Chiu, Lyu, Chen and Chen 6 Typically, in HD there is no apparent underlying cause for the T2 signal changes on standard MRI sequences, thus emphasizing the importance for dynamic MRI to be performed when suspicious symptoms are present.Reference Gotkine, Abraham, Drory, Argov, Gomori and Blumen 7 Flexed position typically shows anterior shifting of the posterior dura and flattening of the cervical spinal cord against the vertebral bodies, as well as anterior horn T2-weighted signal change at the affected levels.Reference Foster, Tsang, Kam, Storey, Day and Hill 3
The management and surgical treatment of HD is uncertain. The lack of an obvious pathology to decompress and guide decision making for the best surgical approach represents a challenge. Cervical collar may be utilized for conservative management initially until a plateau is reached. If a progressive decline occurs in neurological status, surgery should be considered.Reference Kohno, Takahashi, Ide, Yamakawa, Saitoh and Inoue 8 The typical key feature of surgical treatment is to fuse the vertebrae that are causing flexion injury.Reference Kohno, Takahashi, Ide, Yamakawa, Saitoh and Inoue 8 This can be achieved via an anterior or posterior approach, as each of these have been shown to have a good outcome with improvement of symptoms.Reference Kohno, Takahashi, Ide, Yamakawa, Saitoh and Inoue 8 While fewer levels are targeted in anterior approaches, posterior approaches typically require fusion over multiple levels.Reference Kohno, Takahashi, Ide, Yamakawa, Saitoh and Inoue 8 - Reference Lu, Wang and Jiang 10 Anterior approaches have been proven to efficiently halt disease progression and, considering the technical advantages, are given preference over posterior approaches.Reference Watanabe, Hasegawa, Hirano, Endo, Yamazaki and Homma 9 , Reference Lu, Wang and Jiang 10 In addition, new data support duraplasty with tenting of the dura via laminoplasty as a successful treatment option.Reference Ito, Takai and Taniguchi 11 For the aforementioned reasons, we performed an ACDF in both cases, even though one patient was a singer and noted retained good function and range of motion postoperatively.
In conclusion, albeit rare, HD should be kept in mind when cervical spine signal cord changes cannot be explained by standard MRI in the absence of other plausible differentials. Dynamic MRI is imperative to correctly diagnose patients with a history suspicious for flexion myelopathy. Cervical fusion, as performed in these two Canadian cases, represents a surgical treatment option when management of HD fails with a collar.
Disclosures
London Health Sciences Centre receives grants from Medtronic Canada and DePuy Johnson and Johnson for use of their equipment during surgery. These grants did not fund this research. Neil Duggal reports grants from Medtronic Canada and from DePuy Johnson and Johnson during the conduct of the study. Stuart McGregor, Holger Joswig, and Thomas Miller have nothing to disclose.
Statement of Authorship
SM was responsible for drafting and finalizing the manuscript and for literature research. HJ managed patient consent and contributed to manuscript finalization/review and literature research. TM was responsible for study design and contributed to manuscript review. ND was involved in manuscript review and case report supervision.




