Autoimmune encephalitis after herpes virus simplex encephalitis (HSVE) is a relatively frequent occurrence with 30%–42% of patients developing neuronal cell surface antibodies, Reference Armangue, Spatola and Vlagea1 and up to 27% developing autoimmune encephalitis post-HSVE. Reference Armangue, Spatola and Vlagea1,Reference Armangué, Olivé-Cirera and Martínez-Hernandez2 Of those that develop autoimmune encephalitis, 82% in one large cohort had anti-N-methyl-D-aspartate receptor (NMDAR) antibodies and the remainder unidentified cell surface antibodies. Reference Armangué, Olivé-Cirera and Martínez-Hernandez2 Rarely GABA-A Receptor and Dopamine-Receptor 2 antibodies have been reported as well. Reference Armangue, Moris and Cantarín-Extremera3 Post-HSVE autoimmune encephalitis typically begins within 2 months of initial infection and frequently presents with neuropsychiatric symptoms. Reference Armangue, Spatola and Vlagea1 Accompanying seizures and more rarely, nonconvulsive status epilepticus (NCSE) has been reported in patients with autoimmune encephalitis post-HSVE. Reference Armangue, Moris and Cantarín-Extremera3
NCSE can be difficult to diagnose with certainty, and the Salzburg Consensus Criteria are used to determine probable, possible or no NCSE based on scalp electroencephalogram (EEG) findings. Reference Jaraba, Reynés-Llompart and Sala-Padró4 In patients with NCSE, single-photon emission computed tomography (SPECT) detects NCSE with a sensitivity of greater than 80%, even in the setting of indeterminate scalp EEG patterns. Reference Jaraba, Reynés-Llompart and Sala-Padró4,Reference Kutluay, Beattie and Passaro5 Repeating the scan after treating seizures allows ictal-interictal subtraction, revealing the seizure onset zone. Reference Kutluay, Beattie and Passaro5 Nuclear imaging is sensitive for autoimmune encephalitis as well, with positron emission tomography (PET) abnormalities estimated in up to 86%. Reference Lv, Pan and Zhou6,Reference Probasco, Solnes and Nalluri7 SPECT is less well studied for autoimmune encephalitis; however, perfusion abnormalities that normalize with immune therapy are reported, even in the absence of magnetic resonance imaging (MRI) lesions. Reference Ohta, Seki, Dalmau and Shinohara8,Reference Suárez, Domínguez, Gómez, Portilla, Gómez and Casado9 We present a patient with an unclear clinical presentation determined to have simultaneous post-HSVE autoimmune encephalitis and subsequent nonconvulsive status epilepticus with ictal subtraction SPECT.
A 55-year-old previously healthy woman initially presented with 10 days of fever and confusion. MRI revealed bilateral mesial temporal hyperintensities without enhancement (Figure 1), and cerebrospinal fluid polymerase chain reaction (PCR) was positive for HSV-1, with 200 white blood cells (WBC)/mm3 (100% lymphocytic), protein 0.71 g/L and normal glucose. The patient was treated with IV acyclovir for 14 days, and the admission was complicated by neuropsychiatric symptoms and focal seizures treated with increasing antiseizure medications. The patient was discharged after stabilization of seizures and other symptoms on oxcarbazepine, clobazam and lamotrigine 6 weeks following her admission.
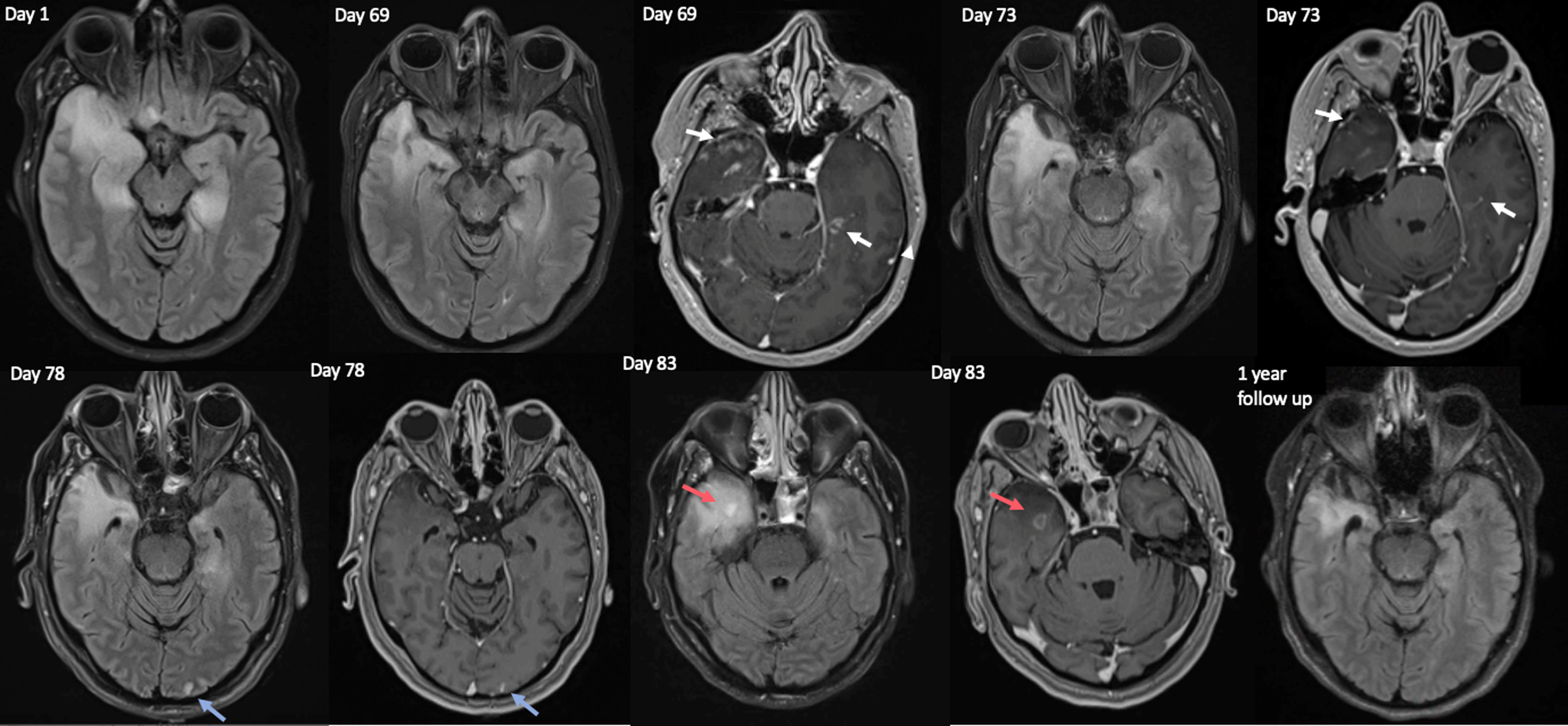
Figure 1. Serial magnetic resonance imaging (MRI) images from initial admission (Acute HSVE- day 1), and the second admission for autoimmune encephalitis (starting 6 weeks after the initial scan), on Days 69, 73, 78 and 83 since initial MRI with acute HSVE. At the time of acute HSVE bilateral mesial temporal and inferior frontal T2 hyperintenisites were noted, with no diffusion restriction, hemorrhage or gadolinium contrast enhancement (not pictured). On the initial scan of the second admission (Day 69) new areas of enhancement were present in bilateral basal temporal lobes (white arrows). These areas of enhancement were improved on day 73 (white arrows) and resolved on day 78. On day 78 a new area of occipital T2 hyperintensity was seen on FLAIR (left-most blue arrow) with associated enhancement (right-most blue arrow), which resolved on the next scan on Day 83. On day 83 a new area of FLAIR hyperintensity was noted in the anteromedial right temporal lobe (left-most red arrow), with associated ring enhancement (right-most red arrow). This abnormality improved on a subsequent 3-month scan, and enhancement had resolved by 1-year follow-up. 1-year follow-up revealed gliosis of the right more than left mesial temporal lobes.
She re-presented 2 weeks later with progressive agitation and confusion (Day 67 since first admission). Repeat EEG (day 68) was unremarkable, and MRI on day 69 showed new areas of temporal lobe enhancement compared to the prior scan during active HSVE (Figure 1). CSF on day 73 revealed 17 WBC/mm3 (82% lymphocytic), protein 0.63 g/L, negative HSV-1 and -2 PCR and 19 CSF specific bands. Comprehensive CSF and serum autoimmune encephalitis panels, which included testing for neural antibodies by mouse tissue indirect immunofluorescence as well as monospecific commercial assays, returned negative except for weak serum positivity for anti-CASPR2 cell-based assay (CBA) at 1:10 dilution only that was most in keeping with a clinically irrelevant/false-positive antibody result. Reference Garrido Sanabria, Zahid and Britton10
On day 76, the patient became suddenly obtunded without any witnessed preceding seizure activity, and was placed on ventilator support with sedation. Over the following four weeks, the patient remained unresponsive without a clearly identified cause. She was treated with a pulse of steroids with prednisone taper and IV Immunoglobulins (IVIG) for possible post-HSVE autoimmune encephalitis. During this time 3 additional MRIs were done (Figure 1), showing migrating enhancement with resolution of previously affected areas on sequential scans. Serial EEGs showed nonevolving 0.5–1.5 Hz generalized periodic discharges (GPD) (possible-NCSE per Salzburg Criteria), with rare periodic discharges centered over C4-P4 (Figure 2c). Repeat CSF on day 84 revealed normal cell counts, protein, glucose and microbiology including HSV PCR. Malignancy screening with computed tomography chest, abdomen and pelvis and pelvic ultrasound were unremarkable.
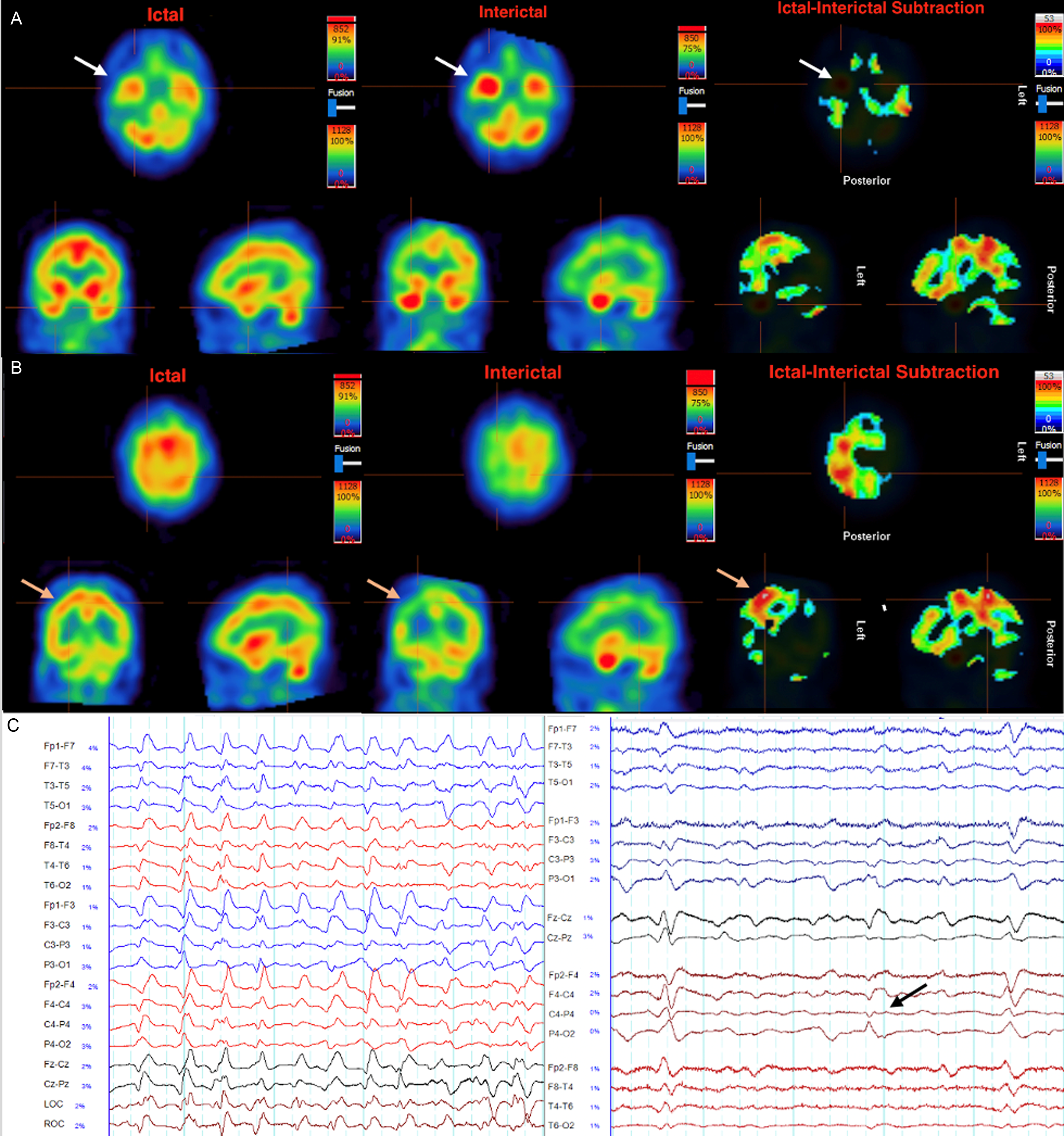
Figure 2. Ictal (A,B left), interictal (A,B middle) and ictal subtraction (A,B right) SPECT scans focusing on inflammation in the temporal lobes (A) and the seizure focus in the right parietal lobe (B). The ictal scan (Day 93) was performed after weaning anesthetic, with corresponding EEG revealing periodic generalized epileptiform discharges maximal at the biparietal regions at 1–2.5 Hz, not fulfilling Salzburg criteria for status epilepticus (C left). The interictal scan (A,B middle, Day 97) was done after achieving burst suppression. A. Significant hyperperfusion of the temporal lobes is evident in both ictal and interictal states, which is eliminated completely by ictal subtraction, reflecting inflammatory activity. B. The greatest area of discrepancy by ictal subtraction is revealed in the convexity of the right parietal lobe (orange arrows) reflecting the seizure focus. Unlike the temporal lobes, this area was not clearly hyperperfused in the ictal scan. In the interictal scan this region is hypoperfused relative to other structures, revealing an area of relative hyperperfusion when ictal subtraction is performed (B right). C. Left: Generalized periodic discharges at the time of ictal perfusion scan, which was seen for a majority of the patient’s course in intensive care. Right: Periodic lateralized discharges at C4-P4 (black arrow) briefly noted only on day 73, corresponding to where the seizure focus was identified 23 days later by ictal subtraction SPECT (B right).
After 3 weeks of treatment with corticosteroids, a course of IVIG, continued burst suppression and escalating antiseizure medications (oxcarbazepine, phenytoin, levetiracetam, lacosamide, lamotrigine and clobazam), there was no clinical or electrographic improvement. To reduce diagnostic uncertainty and determine whether further escalation of immunotherapy and antiseizure medication was warranted, a SPECT scan was done off sedation on Day 93 that revealed hyperperfusion in various regions including both anterior temporal lobes, suggestive of active inflammation (Figure 2a). An ictal subtraction SPECT was done with a repeat scan while burst-suppressed with isofluorane on day 97 (Figure 2b), revealing an area of discrepancy in the right parietal lobe, suggestive of an active seizure focus in this region.
These findings confirmed both autoimmune encephalitis and NCSE, thus clobazam dose was maximized and Rituximab induction was undertaken (Day 146). The malignant EEG pattern resolved on day 101, and clinical improvement was seen from day 103 onwards, which rapidly accelerated after Rituximab induction (nonverbal and visually tracking at time of induction, speaking and following commands 3 weeks after first dose). At 1-year follow-up there was substantial improvement with residual cognitive impairment (MOCA 17/30), and repeat imaging demonstrating resolution of all enhancement (Figure 1).
The diagnosis of post-HSVE autoimmune encephalitis can be challenging as the median onset is 32 days after initial infection, Reference Armangue, Spatola and Vlagea1 temporally overlapping with non-autoimmune complications of HSVE such as viral relapse or seizure disorder. Reference Armangué, Olivé-Cirera and Martínez-Hernandez2 At this juncture, a cohort of 14 patients with post-HSV autoimmune encephalitis had no difference in fluid attenuated inversion recovery (FLAIR) lesion volume or CSF pleocytosis compared to those that did not develop autoimmune encephalitis. New MRI enhancement occurred without any difference between these groups 1–3 months post-HSVE, Reference Armangue, Spatola and Vlagea1 although the migrating MRI enhancement distant from initial areas of injury seen in this patient may be a more specific finding for post-HSVE autoimmune encephalitis. Additionally contributing to diagnostic uncertainty, 25%–30% of patients develop neuronal surface antibodies but do not go on to develop autoimmune encephalitis. Reference Armangue, Spatola and Vlagea1,Reference Armangué, Olivé-Cirera and Martínez-Hernandez2 While nearly all patients with autoimmune encephalitis post-HSVE have a cell surface antibody, Reference Armangué, Olivé-Cirera and Martínez-Hernandez2 18%–26% will have an unidentified antibody indicated only by CSF or serum reactivity with live neuronal culture. Reference Armangue, Spatola and Vlagea1 Neural antibody detection using live cultured neurons is not routinely performed in many clinical laboratories, which may be the reason for negative neural antibody testing among a subset of patients suspected to have post-HSVE autoimmune encephalitis like in this case.
Despite its infrequent use in critical care, SPECT is an effective adjunctive test where NCSE is suspected despite unclear EEG findings, Reference Jaraba, Reynés-Llompart and Sala-Padró4,Reference Kutluay, Beattie and Passaro5 and in the setting of neuroinflammation. Reference Ohta, Seki, Dalmau and Shinohara8,Reference Suárez, Domínguez, Gómez, Portilla, Gómez and Casado9 Although most nuclear imaging studies done to detect autoimmune encephalitis and NCSE have used PET, Reference Lv, Pan and Zhou6,Reference Probasco, Solnes and Nalluri7 SPECT permits ictal subtraction which has greater sensitivity than PET for seizure detection Reference Spanaki, Spencer, Corsi, MacMullan, Seibyl and Zubal11 and allows the disentanglement of simultaneous inflammation and seizures. Ictal subtraction is effective in the setting of neurological comorbidities such as post-stroke epilepsy Reference Fukuma, Kajimoto and Tanaka12 but has rarely been used to detect status epilepticus. Reference Jaraba, Reynés-Llompart and Sala-Padró4,Reference Kutluay, Beattie and Passaro5,Reference Donnelly, Kasatwar and Hafeez13 While alternating burst suppression and lifting sedation to manage NCSE provides a natural opportunity to perform ictal subtraction SPECT, producing an interictal state by burst suppression may introduce limitations including global changes to brain perfusion. Further study is required to determine the sensitivity and specificity of SPECT findings for neuroinflammation and seizure activity when both are simultaneously present.
Author contributions
KG: conceptualization, writing manuscript, figure generation, RW: data acquisition, figure generation, editing, supervision, AB: writing manuscript, editing, supervision, JJ: data acquisition, figure generation, editing, supervision.
Funding statement
None.
Competing interests
Dr Adrian Budhram reports that he holds the London Health Sciences Centre and London Health Sciences Foundation Chair in Neural Antibody Testing for Neuro-Inflammatory Diseases, and that he receives support from the Opportunities Fund of the Academic Health Sciences Centre Alternative Funding Plan of the Academic Medical Organization of Southwestern Ontario. KG, RW, JJ have no disclosures.




