A substantial proportion of chronic benzodiazepine users are suffering from depression (Reference Rickels, Case and SchweizerRickels et al, 1986). For them, benzodiazepines are not considered adequate treatment. The therapeutic value of chronic use is doubtful and benzodiazepines are not recognised as effective antidepressants (Reference Birkenhaeger, Moleman and NolenBirkenhaeger et al, 1995). It has been clinical routine to treat depression first and then taper off the benzodiazepine while anti-depressive treatment is continued (Reference LaderLader, 1994). Such procedures have never been tested in well-designed studies with substantial patient numbers. Moreover, reports evaluating antidepressants in the treatment of benzodiazepine withdrawal show ambiguous results (Reference Rickels, Case and SchweizerRickels et al, 1990; Reference Ansseau and De RoeckAnsseau & De Roeck, 1993; Reference Tyrer, Ferguson and HallströmTyrer et al, 1996).
We therefore designed a study testing the feasibility of a discontinuation programme. Our goals were: to compare the efficacy of paroxetine and placebo in the treatment of major depression in a group of chronic users with depression; in the successfully treated patients, to compare paroxetine and placebo in tapering off the benzodiazepine; and to evaluate the long-term efficacy of the programme.
METHODS
Design
Following approval of the medical ethics committee of the University Hospital Nijmegen, The Netherlands, this multicentre, double-blind, placebo-controlled study was carried out between August 1994 and September 1996. Because this study made use of an electronic case report form, we preferred to use general practitioners (GPs) experienced in the use of such a device. The company that had developed the electronic case report form compiled a list containing the names of 200 GPs. All were invited by means of a letter to consider participation. During the study period additional centres were recruited. A total of 45 GPs agreed to participate. The practices were well distributed across The Netherlands, representing the average Dutch practice in size and type. The decision to do a follow-up study was taken in December 1997 and the study was executed between March and July 1998. It consisted of complete screening by a research psychologist of psychopharmacological treatment of all participating patients (n=230) based on medical records. Retrospectively, GPs were asked by means of a questionnaire to estimate patient numbers at the start of the discontinuation programme as well as the use of the programme in other patients after the study.
Sample size and randomisation
Sample size was estimated by means of power calculation based on the success rate in tapering off: 60-70 patients per group would detect a 25% difference between paroxetine (85%) and placebo (60%), assuming α=0.05 and β=0.20.
Patients were randomised to 20 mg of paroxetine or placebo in a 1:2 double-blind fashion, assuming paroxetine to be twice as effective on antidepressive treatment and to end up with equal numbers in phase III.
A randomisation list (1-330) in blocks of six was obtained by using the random number generator of SPSS/PC+ (SPSS, 1997). Based on this list, study medication (paroxetine, placebo) was blisterpacked and wrapped by Genfarma, The Netherlands. Blocks were sequentially distributed to GPs. Unused blocks were reallocated. The list was kept by the Medical Adviser on Safety of the medical department of SmithKline Beecham, The Netherlands. After the database was closed and basic descriptive analyses were done, the actual codes were added to the database.
Study population
Patients were eligible for the study if they met the following criteria: chronic benzodiazepineFootnote 1 use, defined as daily use for at least 3 months; a diagnosis of major depressive disorder according to DSM-III-R (American Psychiatric Association, 1987); at least 18 years of age; and written informed consent provided. For diagnostic psychiatric screening we used the Mini International Neuropsychiatric Interview (MINI, version 2.1; Reference Lecrubier, Sheehan and WeillerLecrubier et al, 1997), a semi-structured interview based on DSM-III-R. Excluded were patients with: depression caused by organic factors such as complicated mourning, psychosis, schizophrenia, pregnancy or lactation; child-bearing potential with a lack of adequate contraception; severe concomitant medical conditions; history of seizure disorders; use of other psychotropic medication during the 3 months prior to screening; clinically significant abnormalities in haematology or clinical chemistry; misuse of alcohol or illicit drugs; excessive use of benzodiazepines, defined as more than three times the maximal daily dose; and current suicidal risk.
General practitioners
All participating doctors were trained during interrater sessions in applying the MINI interview and the Hamilton Rating Scale for Depression (HRSD; Reference HamiltonHamilton, 1960) and in the use of the electronic case report form. During the study an additional ‘booster’ training session was performed.
Discontinuation programme
General practitioners were advised to screen all chronic benzodiazepine users for study eligibility. Many asked their pharmacy to prepare a list of chronic users. Eligible patients were entered into three phases.
Phase I: transfer to diazepam
For patients not using diazepam, an equivalent daily dose was calculated based on a conversion table taken from several sources (Reference BazireBazire, 1994). This table was built into the electronic case report form. Of patients treated with more than one benzodiazepine, the dosages were added. Ten milligrams of diazepam was considered equivalent to: 1 mg alprazolam, 10 mg bromazepam, 0.25 mg brotizolam, 20 mg chlordiazepoxide, 20 mg clobazam, 7.5 mg clorazepate, 1 mg flunitrazepam, 30 mg flurazepam, 1 mg loprazolam, 2 mg lorazepam, 1 mg lormetazepam, 15 mg midazolam, 10 mg nitrazepam, 40 mg oxazepam, 20 mg temazepam and 13 mg zopiclone. The dose could be adapted after 2 weeks. Phase I lasted for 4 weeks, to allow diazepam and its metabolites to reach steady state.
Phase II: antidepressive treatment
Patients were randomised and started treatment with 20 mg of paroxetine once daily or placebo once daily. After 6 weeks patients with a score of 7 or lower on the 17-item HRSD were entered into Phase III.
Phase III: tapering off diazepam
While study medication was continued, diazepam was withdrawn according to a scheme suggested by Rickels et al (1990a). Daily dose was reduced by 25% in the first and second week. The remaining 50% was tapered off in four steps of 12.5% in weeks 3 and 4. After stopping diazepam, patients continued treatment with the study medication for 2 weeks, followed by 3 weeks of no psychotropic medication. A successful taper-down was defined as benzodiazepine free at week 16.
Measurements
-
(a) The HRSD was assessed during the visits at weeks 0, 4, 6, 10, 14, 16 and 19 (3 weeks after the end of the taper-off period).
-
(b) Clinical Global Impression (CGI; Reference GuyGuy, 1976) was assessed on all visits except week 2.
-
(c) The Spielberger State-Trait Anxiety Inventory — state anxiety sub-scale (STAI-DY1 and 2; Reference van der Ploeg, Defares and Spielbergervan der Ploeg et al, 1981) was assessed on all visits except weeks 2, 11 and 13.
-
(d) The Benzodiazepine Withdrawal Symptom Questionnaire (BWSQ; Reference Tyrer, Murphy and RileyTyrer et al, 1990) was assessed on all visits except week 2.
-
(e) Safety assessments: adverse experiences were assessed on all visits except week 0 (screening). Basic haematology and clinical chemistry laboratory tests were taken at week 0 and week 16.
Statistical analyses
The department of Biostatistics and Epidemiology of the University of Utrecht did statistical analyses for the main study. Follow-up data were analysed by a research psychologist of the psychiatric department of the University of Nijmegen. In both analyses, SPSS/PC+ for Windows was used (SPSS, 1997).
All analyses were done on an intention-to-treat basis unless stated otherwise. Descriptive analyses, Pearson's χ2 test for independent samples, general linear model (GLM) repeated-measures analyses controlled for baseline values, multiple logistic regression analyses and survival analysis (log rank) were used, P < 0.05 was taken as the level of statistical significance.
All patients who went through Phase II (antidepressive treatment), irrespective of the outcome, were analysed as ‘Phase II patients’. Patients with an HRSD score of 7 or lower who continued into Phase III were coded ‘Phase III patients’. Thus, ‘Phase III patients’ are a subgroup of ‘Phase II patients’.
RESULTS
At screening patients had used benzodiazepine for an average of 6 years (range 0.3-27) with an average dose of diazepam equivalents of 9 mg (range 0.5-60 mg). Twenty six per cent of patients had used two benzodiazepines.
A comparison between different sub-groups concerning patient characteristics and prognostic factors is given in Table 1. No statistically significant differences between treatment groups were found, indicating a successful randomisation.
Table 1 Characteristics of patients per group
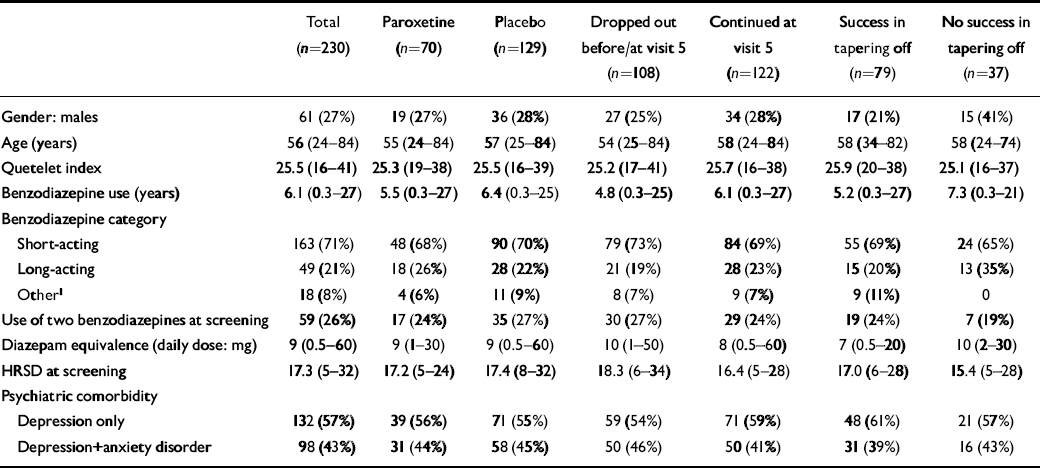
| Total (n=230) | Paroxetine (n=70) | Placebo (n=129) | Dropped out before/at visit 5 (n=108) | Continued at visit 5 (n=122) | Success in tapering off (n=79) | No success in tapering off (n=37) | |
|---|---|---|---|---|---|---|---|
| Gender: males | 61 (27%) | 19 (27%) | 36 (28%) | 27 (25%) | 34 (28%) | 17 (21%) | 15 (41%) |
| Age (years) | 56 (24-84) | 55 (24-84) | 57 (25-84) | 54 (25-84) | 58 (24-84) | 58 (34-82) | 58 (24-74) |
| Quetelet index | 25.5 (16-41) | 25.3 (19-38) | 25.5 (16-39) | 25.2 (17-41) | 25.7 (16-38) | 25.9 (20-38) | 25.1 (16-37) |
| Benzodiazepine use (years) | 6.1 (0.3-27) | 5.5 (0.3-27) | 6.4 (0.3-25) | 4.8 (0.3-25) | 6.1 (0.3-27) | 5.2 (0.3-27) | 7.3 (0.3-21) |
| Benzodiazepine category | |||||||
| Short-acting | 163 (71%) | 48 (68%) | 90 (70%) | 79 (73%) | 84 (69%) | 55 (69%) | 24 (65%) |
| Long-acting | 49 (21%) | 18 (26%) | 28 (22%) | 21 (19%) | 28 (23%) | 15 (20%) | 13 (35%) |
| Other1 | 18 (8%) | 4 (6%) | 11 (9%) | 8 (7%) | 9 (7%) | 9 (11%) | 0 |
| Use of two benzodiazepines at screening | 59 (26%) | 17 (24%) | 35 (27%) | 30 (27%) | 29 (24%) | 19 (24%) | 7 (19%) |
| Diazepam equivalence (daily dose: mg) | 9 (0.5-60) | 9 (1-30) | 9 (0.5-60) | 10 (1-50) | 8 (0.5-60) | 7 (0.5-20) | 10 (2-30) |
| HRSD at screening | 17.3 (5-32) | 17.2 (5-24) | 17.4 (8-32) | 18.3 (6-34) | 16.4 (5-28) | 17.0 (6-28) | 15.4 (5-28) |
| Psychiatric comorbidity | |||||||
| Depression only | 132 (57%) | 39 (56%) | 71 (55%) | 59 (54%) | 71 (59%) | 48 (61%) | 21 (57%) |
| Depression+anxiety disorder | 98 (43%) | 31 (44%) | 58 (45%) | 50 (46%) | 50 (41%) | 31 (39%) | 16 (43%) |
Comorbid anxiety disorders (defined as panic disorder, agoraphobia, social phobia, simple phobia, obessive—compulsive disorder, general anxiety disorder and post-traumatic stress disorder) were diagnosed in 50% of patients, with panic disorder (16.1%), agoraphobia (18.7%), general anxiety disorder (18.7%) and social phobia (14.8%) in the highest frequencies.
Already evident while carrying out the study was the considerable amount of patients refusing consent, as indicated by the fact that 9 (20%) of the 45 practices did not manage to recruit any patients despite considerable numbers of chronic benzodiazepine users.
Estimated retrospectively, each practice had an average of 75 (range 17-250) chronic benzodiazepine users, of whom 12 (16%, range 6-40) had a major depressive disorder. Thus, given our recruitment numbers, 47% of eligible patients did not enter the programme (Fig. 1). A flow diagram of the discontinuation programme and followup study, with exact patient numbers, reason for withdrawal/drop-out and efficacy assessments, is shown in Fig. 1.

Fig. 1 Flow diagram of discontinuation programme and follow-up study, patient numbers, reasons for withdrawal/drop-out, primary efficacy assessments and intervention outcome. 1. Ratio of randomisation was 2:1 in favour of placebo. 2. Non-randomised patients (n=31) were also included in the follow-up study. BWSQ, Benzodiazepine Withdrawal Symptom Questionnaire; CGI, Clinical Global Impression; GP, general practitioner; HRSD, Hamilton Rating Scale for Depression; STAI-DYI, Spielberger State—Trait Anxiety Inventory — state anxiety sub-scale.
Phase I: transfer to diazepam (weeks 1-4)
Of the 230 patients entering the study, 40 (17%) used diazepam already. Of the remaining 190 patients, 162 (85%) were successfully transferred to diazepam (Fig. 1). For patients who dropped out during this phase, we checked at the level of individual drugs for differences in successful transfer. No differences were found: alprazolam, 2/13 patients; bromazepam, 4/16; brotizolam, 1/11; chlordiazepoxide, 1/3; clobazam, 0/3; clorazepate, 0/8; flunitrazepam, 0/2; flurazepam, 0/4; loprazolam, 0/5; lorazepam, 2/16; lormetazepam, 1/5; midazolam, 0/4; nitrazepam, 2/7; oxazepam, 12/72; temazepam, 2/18; zopiclone, 1/6. Three patients who already use diazepam dropped out during this phase.
Phase II: antidepressive treatment (weeks 5-10)
Analysis of responder rates (HRSD score ≤7) between treatment groups in the first 6 weeks of treatment is given in Table 2a . In the paroxetine group 74% of patients and in the placebo group 61% were treated successfully (P=0.067). However, at screening we found four patients with HRSD scores of ≤7, of whom two discontinued before randomisation. At randomisation, 18 patients had an HRSD score of ≤7. This group had an average of 12.4 (range 5-22) at screening, were rated mild to moderately ill and had used 7.5 mg of diazepam for 3 years. In a subsequent multiple logistic regression analysis, we excluded these ‘Phase I responders’. Using HRSD score (> 7 and ≤7) as a binary independent variable with correction for gender and diazepam dose, we again found no significant difference between treatment groups (P=0.07).
Table 2 Intention-to-treat analysis in number of patients (%): efficacy of paroxetine v. placebo

| (a) Treatment of depression | ||
| HRSD scores | ||
| ≤ 7 | > 7 | |
| Paroxetine (n=66) | 49 (74%) | 17 (26%) |
| Placebo (n=126) | 77 (61%) | 49 (39%) |
| Dichotomised Hamilton Rating Scale for Depression (HRSD) scores at the end of Phase II (n=192): Pearson's χ2 test, P=0.067. | ||
| (b) Discontinuation of diazepam | ||
| Taper off diazepam | ||
| Success | Failure | |
| Paroxetine (n=48) | 32 (67%) | 16 (33%) |
| Placebo (n=74) | 47 (64%) | 27 (36%) |
Figure 2a,b gives an overview of average HRSD and STAI-DY1 scores. The patient-rated STAI-DY1 shows a significant decrease in the paroxetine-treated group compared with placebo (P=0.009). The CGI did not show a statistically significant effect (P=0.10).
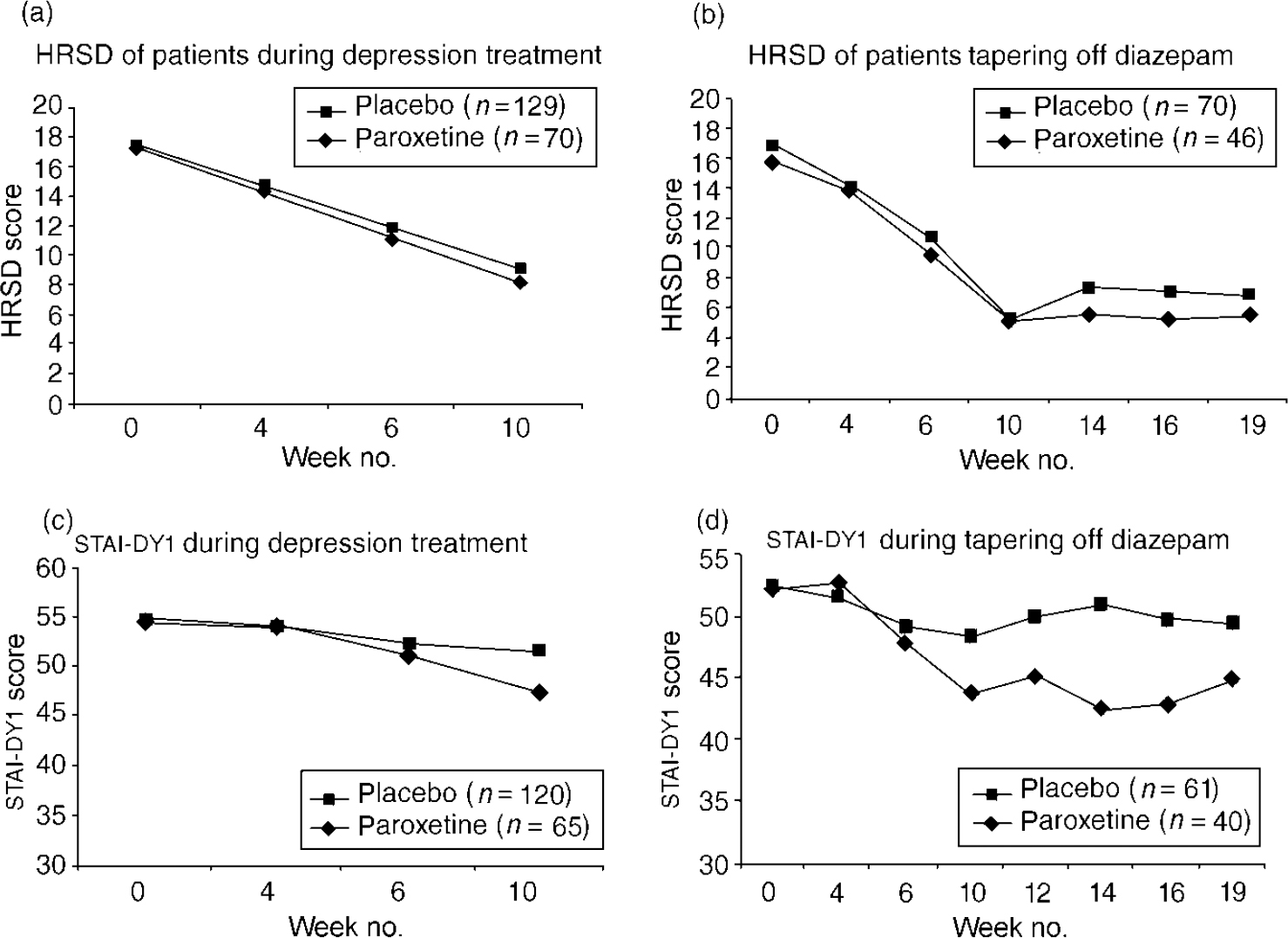
Fig. 2 Hamilton Rating Scale for Depression (HRSD) of patients during depression treatment (a) and of patients tapering off diazepam (b), and Spielberger State—Trait Anxiety Inventory (STAI-DYI) during depression treatment (c) and during tapering off diazepam (d). General linear model (GLM) repeated-measures analyses: (a) NS; (b,c) P=0.009; (d) P <0.001.
Thus, patient's depression score improved significantly during the first weeks of treatment, but we did not find paroxetine to be better than placebo. In contrast, the state anxiety score decreased significantly in patients treated with paroxetine but did not in placebo-treated patients.
In the analysis of the antidepressive effect for patients during the first 12 weeks of treatment, a GLM repeated-measures analysis with the HRSD at randomisation as a covariate showed a significant difference of HRSD between paroxetine and placebo (Fig. 2c ). The STAI-DY1 showed comparable results (Fig. 2d ). The CGI did not show a statistically significant effect (P=0.24). During or at the end of Phase II, 22 (31%) patients in the paroxetine group and 55 (43%) in the placebo group were withdrawn or dropped out (Fig. 1).
Phase III: diazepam withdrawal (weeks 11-16)
The main result during Phase III is that 32 (67%) of the patients on paroxetine and 47 (64%) of the patients on placebo successfully tapered off diazepam (Table 2b ; P=0.72). The withdrawal symptoms were analysed by two measures: an increase of BWSQ scores, with week 10 as point of reference, and reported adverse experiences.
Overall, 61% v. 65% of patients in the success and no-success groups, respectively, reported an increase in number and/or severity of symptoms during the diazepam withdrawal phase. In the no-success patients the average BWSQ scores showed an increase of three points (6.5-9.5) in the last phase of tapering down. In the success group, the score remained unchanged (mean=6.5, s.d.=6.6, P=0.036).
Adverse experiences had two peaks concerning the time of onset: one peak around visit 3 (transfer to diazepam and start of study medication) and another 3 weeks after taper-off start. The latter is of interest here. Adverse experiences during taper-down with an incidence of 5% or more were insomnia (13%), anxiety (6%), headache (5%) and agitation (5%). Interesting is the difference of reported insomnia/sleep disorder between paroxetine-treated patients (8%) and placebo-treated patients (23%). The difference, if any, between success and no-success patients focuses on agitation. Increases in the average HRSD score (from 5.6 to 9.6) and the STAI-DY1 score (45 to 54) were found in the non-success group but not in the success group.
No serious adverse experiences occurred during the taper-down phase.
Follow-up period
Follow-up assessments, achieved for 207 patients (90% of main study patients), were done on average 2.3 years (range 29 days to 3 years) after patients had ended the discontinuation programme.
Twenty-six patients (13%) who started the programme remained benzodiazepinefree throughout the follow-up period: of these, 19 (26%) were among the patients who successfully completed the programme; one was from the unsuccessful group (i.e. who still used diazepam at week 16); and six had dropped out before/at week 10.
Survival curves until benzodiazepine restart for the success v. no-success group show a significant difference (Fig. 3). Table 3 shows the characteristics of benzodiazepine use during the follow-up period. Patients who successfully finished the discontinuation programme were less likely to restart; if they did restart this was at a later stage, for shorter periods, in lower dosages and fewer patients tended to resume their old habit of chronic use (defined as benzodiazepine use >95% of follow-up period). Compared with the characteristics at baseline (Table 2) the successful taperoff group had used benzodiazepines in lower doses and for shorter periods. At the time of follow-up, 52% of patients used benzodiazepine. The difference between the successful (42%) and unsuccessful group (69%) was significant (P=0.028).

Fig. 3 Follow-up survival time until first use of benzodiazepine after the discontinuation programme. Log rank survival analysis; P <0.001.
Table 3 Characteristics of benzodiazepine use during follow-up (n=207)
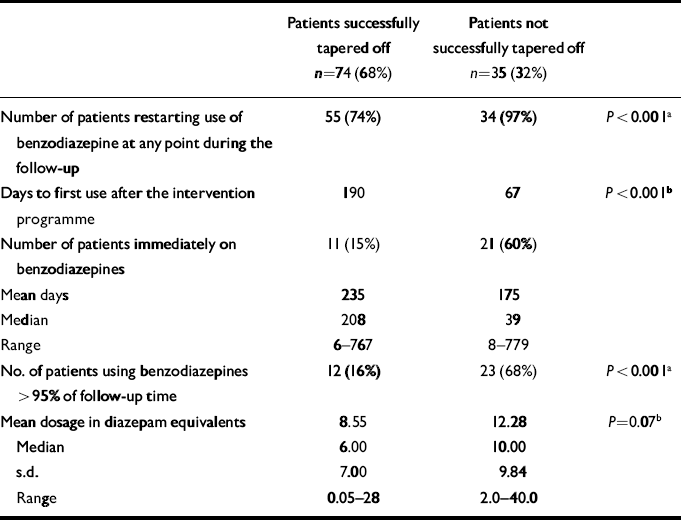
| Patients successfully tapered off n=74 (68%) | Patients not successfully tapered off n=35 (32%) | ||
|---|---|---|---|
| Number of patients restarting use of benzodiazepine at any point during the follow-up | 55 (74%) | 34 (97%) | P < 0.001a |
| Days to first use after the intervention programme | 190 | 67 | P < 0.001b |
| Number of patients immediately on benzodiazepines | 11 (15%) | 21 (60%) | |
| Mean days | 235 | 175 | |
| Median | 208 | 39 | |
| Range | 6-767 | 8-779 | |
| No. of patients using benzodiazepines > 95% of follow-up time | 12 (16%) | 23 (68%) | P < 0.001a |
| Mean dosage in diazepam equivalents | 8.55 | 12.28 | P=0.07b |
| Median | 6.00 | 10.00 | |
| s.d. | 7.00 | 9.84 | |
| Range | 0.05-28 | 2.0-40.0 |
After study completion 64% of GPs used the discontinuation protocol for other patients. On average, they had treated another five patients per practice: 71% reported to be satisfied with the results.
DISCUSSION
To our knowledge this is the first time a sample of chronic benzodiazepine users suffering from depression has been investigated separately in a benzodiazepine discontinuation programme. Moreover, it is the largest intervention study trying to deal with the problem of chronic use.
When we planned this study, it was our goal to develop and test a tool for GPs, that could help them in treating the depression as well as long-term benzodiazepine dependence in one programme.
Short-term efficacy of the programme
Transfer to diazepam
In the 13% of patients who dropped out during this phase, we failed to detect any differences between individual drugs in transferring to diazepam. There have been some reports that coming off lorazepam might be more difficult, but our data do not support this (Reference Murphy and TyrerMurphy & Tyrer, 1991).
Treatment of depression
Evaluating the depression treatment phase we found the mean HRSD score of our sample to be relatively low: 17.4 at screening and 14.8 at randomisation. We did not set a severity entry criterion in order to be as close as possible to the day-to-day setting of the general practice. The HRSD is not a diagnostic tool and a severity limit may have pushed up the scores. It is also known that training health care professionals in structured interviews like the MINI can anticipate some overrating. After all, setting a psychiatric diagnosis can be a difficult process in many patients. Given these results, one could argue that not all of our patients had major depressive disorder, but given the high comorbidity of anxiety disorders as well as the high anxiety scores on the STAI we are convinced that this group of patients needs adequate treatment and not just a daily dose of benzodiazepines.
Seventy-four per cent of patients with an HRSD score of 7 or lower after 6 weeks of treatment with paroxetine is comparable to that of efficacy studies in other populations of patients suffering from depression (Reference Dunner and KumarDunner & Kumar, 1998). Our results, however, differ from those studies with respect to a placebo response of 61%.
In the state anxiety measure (STAI-DY1) we did not find any placebo response. We would have expected that transfer from short-acting benzodiazepine in 70% of patients to diazepam would have given some anxiolysis during the day, but we found no decrease in anxiety during Phase I. Moreover, although these patients were treated for 6 years with benzodiazepines, their anxiety level of 54 during Phase I was comparable to that of psychiatric outpatients, whereas in normal populations scores are 30-40 on this scale (Reference van der Ploeg, Defares and Spielbergervan der Ploeg et al, 1981). Patients treated with paroxetine for 6 weeks were significantly less anxious. This confirms the results of many clinical studies that have shown the efficacy of selective serotonin reuptake inhibitors (SSRIs) in the treatment of anxiety disorders.
In summary, we found paroxetine to be more effective in treating anxiety than depression. Perhaps the relatively low depression scores versus the high anxiety scores at screening (‘Law of Initial Value’; Reference Uhlenhuth, Matuzas and WarnerUhlenhuth et al, 1997) might clarify this, but we are not able to explain this inconsistency completely.
Unfortunately, no MINI was done at the end of the programme to check for possible changes in psychopathology.
Discontinuation of diazepam
The short-term effect of the programme showed that after treating the depression adequately 65% of these primary care patients, either on paroxetine or placebo, were successful in stopping benzodiazepine use. This percentage is comparable to the 66% reported by Schweizer et al (Reference Schweizer, Rickels and Case1990), who used a comparable tapering-off procedure without concomitant treatment to ameliorate withdrawal symptoms, and in a non-selected group of chronic users. Several publications report that a substantial proportion of chronic benzodiazepine users are depressed, and that these patients have greater difficulty in stopping benzodiazepine use (Reference LaderLader, 1994). Our data suggest that if patients are treated for their depression successfully, they end up with comparable success rates of taper-off.
Of the patients who entered the tapering-off phase, 63% reported withdrawal symptoms. Although figures of prevalence of withdrawal symptoms across studies have been reported from zero to 100%, in low-dose, long-acting benzodiazepine use with gradual taper this is 40-80% (Reference Rickels, Case and SchweizerRickels et al, 1990; Reference Schweizer, Rickels and CaseSchweizer et al, 1990). As in the findings of Schweizer et al (Reference Schweizer, Rickels and Case1990), no serious withdrawal effects were noted, indicating that the procedure is safe in this group of chronic low-dose users. We estimated that 50% of patients refused informed consent. Although we did not assess the reasons for their unwillingness to participate, this result might indicate medication craving, as suggested by Linden et al (Reference Linden, Baer and Geiselmann1998), who found that two-thirds of chronic benzodiazepine users rejected a drug holiday.
Comparing paroxetine with placebo during discontinuation, we found that patients treated with paroxetine were less depressed, less anxious and complained less of insomnia/sleep disorder compared with placebo. Surprisingly, this did not result in higher taper-off success rates. In both groups, two-thirds of patients managed to taper off diazepam.
To our knowledge, lack of a beneficial effect of an antidepressant in successfully tapering off benzodiazepine in chronic users in general has been demonstrated in one study (Reference Tyrer, Ferguson and HallströmTyrer et al, 1996). Two other studies reported positive effects of imipramine and trazodone (Reference Rickels, Case and SchweizerRickels et al, 1990; Reference Ansseau and De RoeckAnsseau & De Roeck, 1993). The conclusions of these studies have been based on small patient numbers.
Diazepam dose, duration of chronic use, incidence and severity of withdrawal symptoms and concomitant effective anti-depressive treatment do not fully explain why a patient can or cannot taper off benzodiazepine. Other factors, not measured in this study, must be of importance. It has been well reported already that personality traits might be relevant in explaining the difficulties in stopping benzodiazepines (Reference Murphy and TyrerMurphy & Tyrer, 1991; Reference Seivewright, Tyrer and SeivewrightSeivewright et al, 1991).
Long-term efficacy of the programme
Our main result, showing the long-term effect of the programme, is that about 2 years after participation two-thirds of patients proved to have stopped their old habit of daily benzodiazepine use. Moreover, 13% managed to remain benzodiazepine free. If patients successfully completed the programme they had a better outcome, but a substantial number of patients who dropped out of the programme early or did not successfully taper off managed to change their benzodiazepine use. All this confirms the findings of Holton & Tyrer (Reference Holton and Tyrer1990), that a substantial number of patients who have been on benzodiazepine chronically in the past can restart and stop their medication without much difficulty.
The finding of 48% benzodiazepine free at the time of follow-up with a difference of 58% and 31%, respectively, between the success and no-success groups replicates the findings of the only comparable study reported by Rickels et al (Reference Rickels, Case and Schweizer1991). They found 73% v. 39%, respectively, of patients benzodiazepine free after 3 years.
The number of patients restarting benzodiazepine in the first 6 months might suggest the need for follow-up treatment to consolidate the reasons for stopping. In patients with panic disorder, for example, Spiegel & Bruce (Reference Spiegel and Bruce1997) found behavioural psychotherapy efficacious in preventing benzodiazepine restart.
The overall satisfaction of GPs who treated patients according to the discontinuation programme was demonstrated by its use in other patients and their appraisal of the programme after 2 or 3 years.
Clinical Implications and Limitations
CLINICAL IMPLICATIONS
-
▪ Benzodiazepine discontinuation is said to be difficult but half of chronic users with depression are willing to participate in changing their habit.
-
▪ Chronic benzodiazepine users need screening for psychiatric disorders in order to re-evaluate treatment; depression in long-term benzodiazepine use is accompanied by high levels of anxiety.
-
▪ This discontinuation programme is safe in these primary care patients; moreover, two-thirds of patients change the habit of chronic use.
LIMITATIONS
-
▪ Roughly half of the patients were not willing to take part in this study.
-
▪ We did not taper off the non-responders to treatment of the depression. Therefore, a comparison in taper-off rates between patients with a resolved depression and those with the depression still present was impossible.
-
▪ A considerable number of patients had low depression scores, which made the diagnosis of depression unconfirmed.
ACKNOWLEDGEMENTS
The authors wish to thank Carola Schurink-tenHorn, Marius Kuypers, Frank Lippens, Suzanne Loriaux, Sandra Boogaard-van der Wal and Manuela Timmermans for making this study possible.
The Dutch Chronic Benzodiazepine Working Group consisted of J.P.H. Aarts, H. Anderson Röed, J. A. D. Arnolds, A. B. A. Backer, M. E. J. M. Baggen, M. G. Bekker-Teekens, K. M. van den Berg, A. J. M. Boermans, J. A. A. M. Bolmers, J. H. Bonarius, G. Bruinsma-Stibbe, R. Brand, R. S. van Coevorden, G. H. J. van Dijk, R. Duursman, P. A. J. M. Emmers, C. Engel, H. Ferguson, J. H. A. H. Ferrée, P. W. J. M. Fuhring, M. D. de Graaf, F. G. W. M. Haase, A. Hamel, H. J. J. van der Heijden, J. Hoefman, A. J. Huygen, P. J. H. A. Jansen, G. J. de Jong, K. W. de Jong, A. L. M. Kiel, S. Kiel, J. Kingma, H. J. J. Klaassen, C. R. A. M. Knüppe, G. A. P. M. van Loon, K. M. Moes, W. B. M. Oomen, L. Osterholt, P. L. W. Pijman, P. H. van Putten, P. R. Salomé, I. K. Schut, G. C. J. M. van der Spank, L. T. Tan, S. Tiemersma, P. Veerman, J. J. C. M. Versteeg, D. J. M. van der Voort, G. F. R. Vosters, J. A. de Vries, R. L. Warnaar, A. J. W. M. Wijnen, E. H. R. Wins, P. Zee and H. C. F. Zwaal. Some of these GPs were collaborating in one practice.









eLetters
No eLetters have been published for this article.