Dietary proteins have been shown to be more effective at prolonging satiety and suppressing food intake than carbohydrates and fats(Reference Anderson and Moore1, Reference Weigle, Breen and Matthys2). Proteins' influence on postprandial levels of insulin, ghrelin, cholecystokinin and/or glucagon-like peptide-1 may account for some of the influence on satiety and food intake(Reference Bowen, Noakes and Clifton3, Reference Bowen, Noakes and Trenerry4). Insulin is thought to be a satiety hormone(Reference G5), with increased insulin levels in the brain eliciting a net catabolic response influencing food intake regulatory mechanisms(Reference Woods6). Dietary proteins have been shown to stimulate insulin release and influence glycaemia differentially(Reference Nilsson, Stenberg and Frid7, Reference von Post-Skagegård, Vessby and Karlström8), both over the longer term(Reference Layman, Shiue and Sather9) and postprandially(Reference Bowen, Noakes and Clifton10). Identifying specific types of dietary proteins which have the greatest influence on appetite and satiety and which stimulate a greater insulin release has a potential application for appetite control and food intake regulation in overweight and obese populations.
Evidence is inconclusive in relation to which types of proteins are more effective at decreasing appetite and subsequent food intake; however, some studies indicate that dairy whey proteins have a greater effect on appetite control than other protein sources such as egg and casein(Reference Hall, Millward and Long11, Reference Anderson, Tecimer and Shah12). Anderson et al. (Reference Anderson, Tecimer and Shah12) found that whey proteins reduced food intake at a later meal when compared with soya and egg albumin. Hall et al. (Reference Hall, Millward and Long11) found that whey reduced food intake, and had a greater subjective satiety rating than casein. However, other studies have found no differences in appetite and food intake when comparing whey with lactose and casein(Reference Bowen, Noakes and Trenerry4) or when comparing whey with soya and gluten(Reference Bowen, Noakes and Clifton3). Whey proteins account for 20 % of the total protein in bovine milk and are a by-product of cheese manufacturing, which is rich in essential amino acids(Reference Aimutis13).
Dairy whey proteins may influence gut hormones involved in satiety such as cholecystokinin-A, glucagon-like peptide-1 and glucose-dependent insulinotropic polypeptide(Reference Nilsson, Stenberg and Frid7, Reference Hall, Millward and Long11, Reference Frid, Nilsson and Holst14). Whey proteins and their bioactive components such as lactalbumin and branched-chain amino acids (BCAA) have also been found to have an insulinotropic effect(Reference Nilsson, Stenberg and Frid7, Reference Frid, Nilsson and Holst14–Reference Ostman, Liljeberg Elmstahl and Bjorck17). Whey proteins have a faster rate of digestion and absorption than other proteins, producing a rapid peak in plasma amino acids which may contribute to their effect on satiety(Reference Hall, Millward and Long11, Reference Boirie, Dangin and Gachon18). Whey proteins also have one of the highest concentrations of BCAA than other protein sources(Reference Nilsson, Stenberg and Frid7). Although there is some evidence on whey's effect on satiety and insulin response, there are few conclusive studies comparing whey with other proteins from whole foods. Also, comparisons between other protein sources reveal that some types of fish may have insulinotropic effects that may be better than those possessed by dairy products. Ouellet et al. (Reference Ouellet, Weisnagel and Marois19) found that the ingestion of cod protein may improve insulin sensitivity compared with that of beef, pork, veal, eggs, milk and dairy products. Similarly, von Post-Skagegård et al. (Reference von Post-Skagegård, Vessby and Karlström8) found that the cod meal produced a higher glucose response and a lower insulin/glucose ratio than cottage cheese and soya protein isolate in healthy women, especially after the first hour postprandially. Interestingly, Uhe et al. (Reference Uhe, Collier and O'Dea20) found that satiety was greater after the consumption of a tuna meal than after the consumption of chicken or beef meals. In contrast, other studies have shown no differences when comparing various protein sources with respect to insulin response(Reference Claessens, Calame and Siemensma21) and satiety and food intake(Reference Lang, Bellisle and Oppert22). Therefore, further studies are required to conclusively determine which proteins have a greater effect in suppressing appetite and reducing food intake.
The present study aims to determine the acute effects of four different protein sources, namely whey, tuna, turkey and egg albumin, on glycaemia and insulinaemia as well as on appetite and food intake at a subsequent meal. We hypothesise that the whey protein meal will stimulate insulin release to a greater extent when compared with the tuna, turkey and egg meals, and will have a greater effect in reducing appetite and food intake at a buffet meal 4 h later.
This clinical trial has been registered with the Australian New Zealand Clinical Trials Registry. The registration number is ACTRN12609000538246, and the trial web address is https://www.anzctr.org.au/registry/trial_review.aspx?ID=83835
Methods
Subjects
Healthy men between the ages of 18 and 30 years and with a BMI between 18·5 and 25 kg/m2 were recruited from the community using poster advertising, local radio and newspapers. Participants were excluded if they were smokers; had diabetes, renal disease, hypothyroidism and hypertension; consumed more than two standard alcoholic drinks per day; had more than 30 % energy intake from protein; used steroids; had a cardiovascular event or surgery within the last 6 months; had a major systemic illness, gastrointestinal surgery or dumping syndrome; were on warfarin; or had an allergy to test foods. The present study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects were approved by the Curtin University ethics committee, approval number HR 77/2005. All participants gave informed, written consent before participating in the study.
Experimental protocol
This was a randomised, single blind, cross-over design study, which the study participants attended at the Curtin University on four separate occasions, following a screening visit, with a 1-week washout period between each visit. Subjects were required to follow their normal dietary habits and complete a food diary of their usual dietary intake 3 d before each intervention. Those who consumed more than two standard drinks of alcohol per day were excluded from the study to limit any confounding metabolic effects from alcohol, and those who consumed more than 30 % of their total energy from protein were excluded to minimise variation in previous meals affecting glucose and insulin metabolism as well as appetite during the intervention day. Throughout the study period, the subjects were asked to maintain their normal diet and refrain from vigorous physical activity. Subjects were supplied with a standard frozen meal to be consumed the night before the intervention day. On each of the four occasions, the subjects arrived at the Curtin University at 07.00 hours by car (to minimise physical activity) after a 10–12 h fast. After subjects voided and were weighed, they rested in a supine position on a bed for 30 min. Fasting blood samples were collected into EDTA vacutainer tubes, via venepuncture. The liquid test meal was then consumed, and blood samples were collected thereafter at 30 min intervals for 2 h and then hourly for the last 2 h. Timing began after the subjects had finished consuming the liquid test meal. Subjects remained in a semi-recumbent position during the study, and were allowed bathroom and water access. Blood samples were centrifuged at approximately 2500 g at 4°C for 10 min, and plasma samples were stored at − 80°C before subsequent analysis. Plasma was collected for the determination of insulin and blood glucose.
Dietary protocol
On each of the four occasions, the subjects consumed a different liquid test meal, containing either tuna, turkey, whey or egg albumin (Table 1). The protein sources consisted of drained, canned tuna in springwater (Greenseas; HJ Heinz Company, Southbank, VIC, Australia); pre-cooked, sliced turkey (Primo Smallgoods, Chullora, NSW, Australia); whey protein isolate (Murray Goulburn Nutritionals, Melbourne, VIC, Australia) and liquid egg albumin (Cutting Edge, Golden Egg Farms, WA, Australia). The four types of proteins were each finely blended using a food processor and mixed with chocolate powder, rapeseed oil, polyjoule and water to ensure that all the meals had the same amount of protein (50 g), fat, carbohydrate and energy content and the same weights. The test meals were matched as closely as possible for appearance, texture and taste, and were tested for palatability by the laboratory staff. The subjects consumed the test meal as a blended drink, which was flavoured with chocolate powder in order to blind the subjects to the source of the protein. All the meals were well tolerated, and all the subjects finished the 600 ml liquid meal within 5 min.
Table 1 Content, energy and macronutrient composition of the breakfast meals

* Essential amino acids.
† Branched-chain essential amino acids.
Four hours after the consumption of the liquid test meal, subjects were offered a buffet meal to be consumed ad libitum, consisting of a platter of sandwiches (containing fillings such as tuna, cheese and chicken), fruits (apple, orange and banana), fruit juice and yogurt. Participants were asked to eat only until they felt comfortably full and were given 30 min to consume the meal. Each food was weighed before the subject had consumed the buffet meal and then again afterwards. The amount consumed was calculated, and the energy (kJ) content of the food consumed was determined using Foodworks 2006 (Xyris Software; Highgate Hill, QLD, Australia) based on the Australian food composition tables.
Appetite rating
Participants completed the visual analogue scale (VAS) ratings of their appetite before breakfast (t = 0) and throughout the morning (t = 30, 60, 120, 180, 210 and 240 min). VAS questionnaires were used to measure subjective sensations of appetite, and they consisted of 100 mm lines anchored at each end with opposing statements. Participants placed an X on the line to indicate their feeling at that point in time. The VAS score is calculated by measuring the distance in millimetres from the beginning of the line to the position of the X (from left to right). Subjective appetite was assessed using three VAS that measured hunger (‘not hungry at all’ to ‘as hungry as I have ever felt’), fullness (‘not full at all’ to ‘very full’) and prospective food consumption (‘nothing at all’ to ‘a large amount’)(Reference G5, Reference Flint, Raben and Blundell23).
Laboratory analysis
Plasma glucose levels were measured using the Randox glucose GOD-PAP kit (Antrim, UK), and plasma insulin was measured using an ELISA kit (Dako Diagnostic, Osaka, Japan).
Statistics
Statistical analysis was done using SPSS 17 for Windows (SPSS, Inc., Chicago, IL, USA). Data are expressed as means with their standard errors, and assessed for normality. The area under the curve (AUC) data for glucose, insulin and appetite responses were analysed using the General Linear Model to assess the effects of the groups after adjusting for baseline values. When significant between-group effects were present, post hoc comparisons were made using Tukey's test. ANOVA was used to determine significant differences between dietary treatments at a given time point. Pearson's correlation analyses were performed to identify the relationship between the appetite responses, energy intake and hormonal response. An α level of P < 0·05 was considered statistically significant.
Results
Thirty subjects were selected to commence the study with eight withdrawals after randomisation, six due to personal reasons and two due to loss to follow-up. Twenty-two subjects completed the study. The clinical characteristics of the twenty-two subjects are shown in Table 2. Table 3 shows the mean 3 d dietary intake of subjects before each postprandial visit. There was no significant difference in energy or macronutrient intake 3 d before each postprandial day. Table 1 shows the composition of the four breakfast meals. The concentrations of protein, fat and carbohydrate were matched in all the breakfast meals.
Table 2 Subjects' characteristics (n 22)
(Mean values with their standard errors)

Table 3 Reported dietary intake data assessed by weighed food records*
(Mean values with their standard errors)

* The nutritional data were recorded in 3 d food diaries before each intervention day. There was no significant difference in dietary intake before each postprandial visit.
The postprandial blood glucose concentrations are shown in Fig. 1(A). Postprandial plasma glucose levels increased after the intake of tuna, turkey and egg, and peaked at 30 min. However, at 30 min, the whey protein meal caused a significant decrease in glucose response (4·59 (sem 0·23) mmol/l) compared with the tuna (5·39 (sem 0·21) mmol/l; P < 0·001), turkey (5·49 (sem 0·22) mmol/l; P < 0·001) and egg (5·45 (sem 0·18) mmol/l; P < 0·001) meals. At 60 min postprandially, the glucose response with the whey protein meal (3·70 (sem 0·16) mmol/l) was still significantly lower than that with the turkey and egg meals (4·56 (sem 0·19) mmol/l; P < 0·034 and 4·47 (sem 0·16) mmol/l; P < 0·040, respectively), but it was not lower than that with the tuna meal (3·87 (sem 0·14) mmol/l; P>0·454). The blood glucose response after the consumption of the test meal, expressed as AUC, was significantly lower with the whey meal (8·69 (sem 0·32)) than with the turkey (9·62 (sem 0·35); P < 0·023) and egg (9·63 (sem 0·54); P < 0·001) meals, but it was not lower than that with the tuna meal (9·24 (sem 0·33); P < 0·34) (Fig. 1(B)).
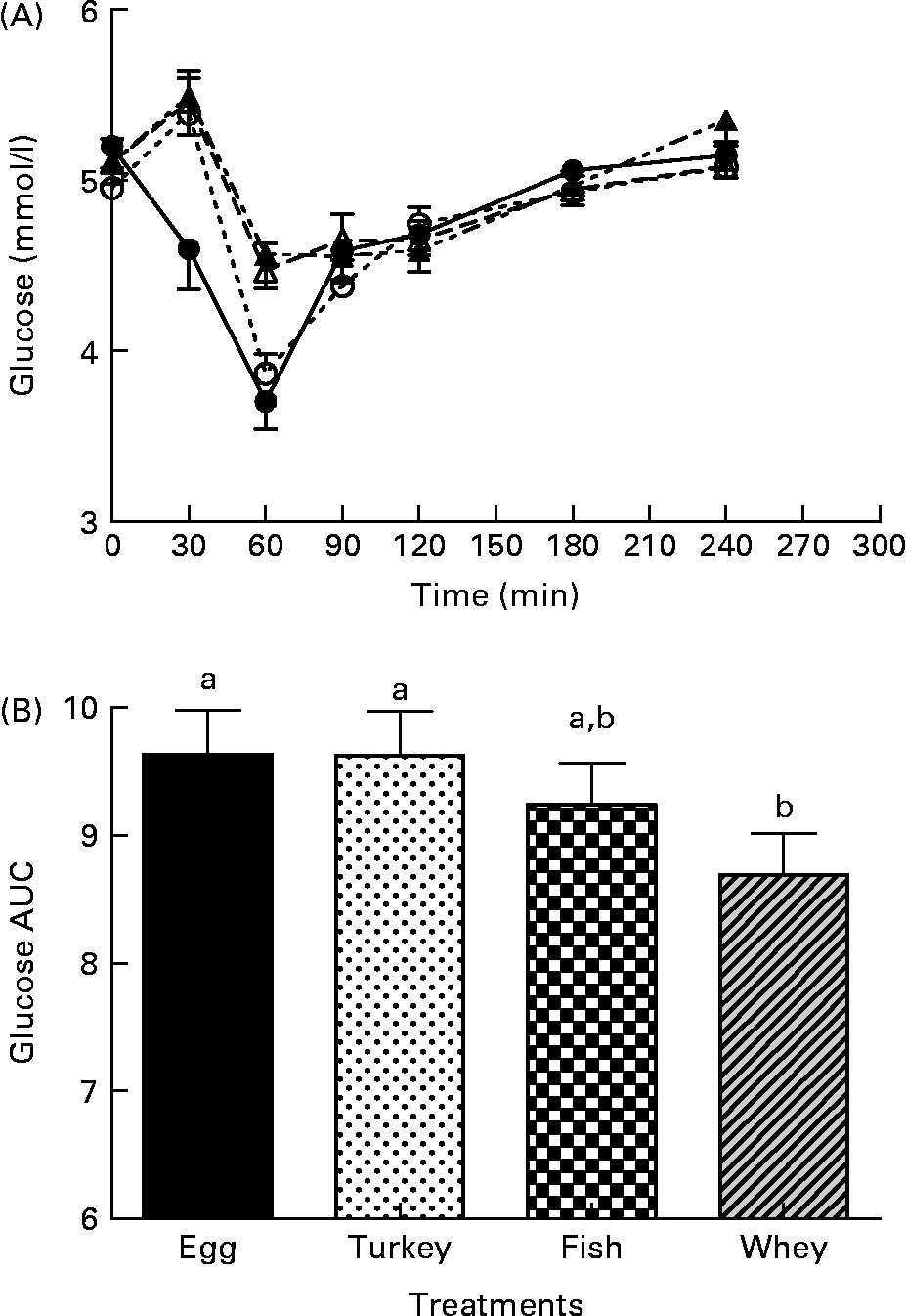
Fig. 1 The effect of four different protein test meals on postprandial glucose. (A) Postprandial glucose response (0–240 min) to the whey, tuna, turkey and egg breakfast meals. (B) Postprandial incremental area under the curve for glucose (0–240 min). Data are expressed as means with their standard errors. a,b Mean values with different letters above bar graphs were significantly different between the groups (P < 0·05). (A) - -○- -, Fish; –●–, whey; ![]() , egg white; - -▲- -, turkey. (B)
, egg white; - -▲- -, turkey. (B) ![]() , Egg;
, Egg; ![]() , turkey;
, turkey; ![]() , fish;
, fish; ![]() , whey. AUC, area under the curve.
, whey. AUC, area under the curve.
The postprandial insulin concentrations are shown in Fig. 2(A). Postprandial plasma insulin levels increased after the intake of whey, tuna, turkey and egg, and peaked at 30 min and then decreased thereafter. The postprandial insulin response at 30 and 60 min with the whey protein meal (48·6 (sem 5·7) and 20·6 (sem 2·4) pmol/l, respectively) was significantly higher than that with the tuna (36·4 (sem 4·7) and 11·7 (sem 0·9) pmol/l; P < 0·033 and 0·024, respectively), turkey (21·7 (sem 2·3) and 14·8 (sem 1·4) pmol/l; P < 0·001 and 0·001, respectively) and egg (21·8 (sem 3·5) and 10·1 (sem 0·9) pmol/l; P < 0·001 and 0·001, respectively) meals, but at 90 min, only the whey protein meal (15·7 (sem 1·2) pmol/l) showed significantly higher values than the turkey (12·5 (sem 1·2) pmol/l; P < 0·031) and egg (9·1 (sem 0·78) pmol/l; P < 0·021) meals. The postprandial insulin response with the tuna meal (36·4 (sem 4·7) pmol/l) was significantly higher at 30 min than that with the turkey (21·7 (sem 2·3) pmol/l; P < 0·001) and egg (21·8 (sem 3·5) pmol/l; P < 0·001) meals, but it was not higher at any other time points. The postprandial insulin response with the turkey meal was significantly higher than that with the egg meal at 60 (14·8 (sem 1·4) v. 10·1 (sem 0·9) pmol/l; P < 0·021), 90 (12·5 (sem 1·2) v. 9·1 (sem 0·78) pmol/l; P < 0·037) and 120 (12·3 (sem 1·1) v. 9·8 (sem 0·84) pmol/l; P < 0·044) min. The blood insulin response after the consumption of the test meal, expressed as AUC, was significantly higher with the whey meal (46·73 (sem 2·4)) than with the tuna (34·41 (sem 1·9); P < 0·001), turkey (29·69 (sem 1·9); P < 0·001) and egg (23·09 (sem 1·5); P < 0·001) meals (Fig. 2(B)). The AUC for the insulin response after the consumption of the test meal was also significantly higher with the tuna meal (P < 0·001) than with the turkey (P < 0·001) and the egg meals (Fig. 2(B)), with the turkey meal showing significantly higher values than the egg meal (P < 0·001).
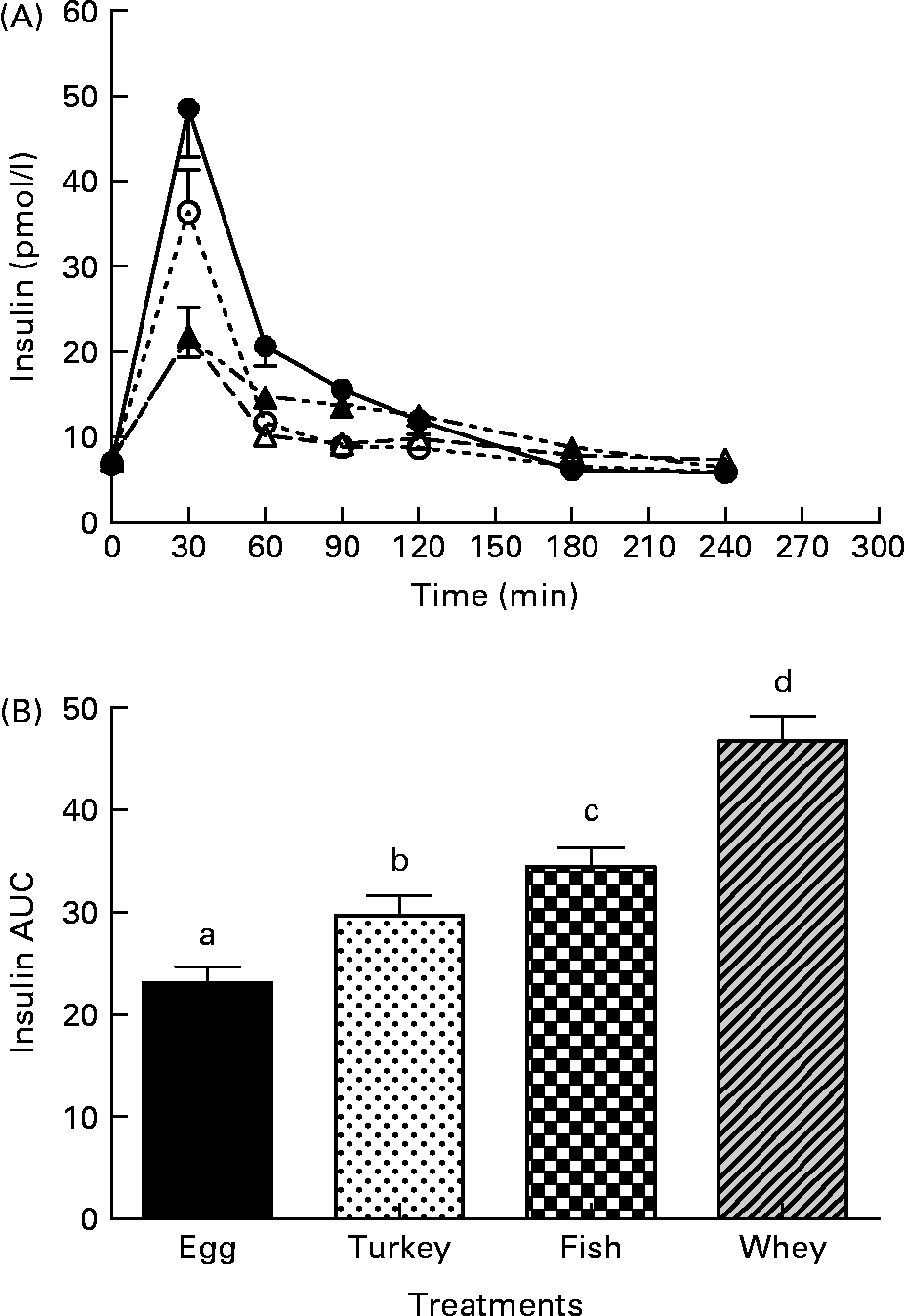
Fig. 2 The effect of four different protein test meals on postprandial insulin. (A) Postprandial insulin response (0–240 min) to the whey, tuna, turkey and egg breakfast meals. (B) Postprandial incremental area under the curve for insulin (0–240 min). Data are expressed as means with their standard errors. a,b Mean values with different letters above bar graphs were significantly different between the groups (P < 0·05). (A) - -○- -, Fish; –●–, whey;![]() , egg white; - -▲- -, turkey. (B)
, egg white; - -▲- -, turkey. (B) ![]() , Egg;
, Egg; ![]() , turkey;
, turkey; ![]() , fish;
, fish; ![]() , whey. AUC, area under the curve.
, whey. AUC, area under the curve.
Table 4 shows the differences in the incremental AUC between the intakes for VAS ratings of appetite. The AUC for the VAS rating of hunger was significantly lower with the whey meal (174·6 (sem 16)) than with the tuna (201·6 (sem 17); P < 0·033), turkey (237·0 (sem 18); P < 0·001) and egg (253·6 (sem 18); P < 0·001) meals, with the tuna meal also showing significantly lower values than the turkey (P < 0·043) and egg (P < 0·001) meals. Similarly, the AUC for the VAS rating of prospective consumption was significantly lower with the whey meal (162·1 (sem 13)) than with the tuna (192·5 (sem 14); P < 0·023), turkey (225·7 (sem 15); P < 0·001) and egg (244·9 (sem 15); P < 0·001) meals, with the tuna meal also showing significantly lower values than the turkey (P < 0·015) and egg (P < 0·001) meals. The AUC for the VAS rating of fullness was significantly higher with the whey (238·75 (sem 15)) and tuna (234·32 (sem 15)) meals than with the turkey (205·36 (sem 13); P < 0·023 and 0·027, respectively) and egg (198·1 (sem 13); P < 0·001 and 0·001, respectively) meals, with no differences being observed between the whey and tuna meals or between the egg and turkey meals.
Table 4 Ad libitum food intake at a buffet lunch and postprandial total area under the curve (AUC) of visual analogue scale rating of hunger, fullness and prospective food consumption after the ingestion of equienergetic preloads containing egg, turkey, tuna and whey
(Mean values with their standard errors)

a,b,c Mean values with unlike superscript letters were significantly different between the groups (P < 0·05).
The mean energy intake at the ad libitum buffet meal (Table 4) was significantly lower (P < 0·001) with the whey protein meal than with the tuna, egg and turkey meals (2950·1 (sem 98·1) kJ compared with 3275·2 (sem 104·4), 3513·7 (sem 110·7), 3534·8 (sem 113·6), respectively). The mean energy intake at the ad libitum buffet meal was significantly lower with the tuna protein than with the egg and turkey proteins.
Correlation analyses indicated that postprandial hunger was positively associated with desire to eat (r 0·620; P < 0·001) and energy intake (r 0·840; P < 0·001), and was negatively associated with fullness (r − 0·625; P < 0·001) and insulin (r − 0·577; P < 0·001). Fullness was positively associated with postprandial insulin (r 0·711; P < 0·001) and negatively associated with postprandial desire to eat (r − 0·535; P < 0·001) and energy intake (r − 0·721; P < 0·001). Desire to eat was negatively associated with energy intake (r − 0·521; P < 0·001) and insulin (r − 0·521; P < 0·001). Correlation analysis showed a significant correlation between insulin and energy intake measurements (r − 0·934; P < 0·001).
Discussion
The present study demonstrated that the whey protein meal had a superior effect in significantly reducing appetite and food intake and produced significantly greater insulin responses in healthy, lean men when compared with the tuna, turkey and egg albumin meals. The tuna meal was shown to be more effective than the turkey and egg meals in significantly reducing appetite and food intake, and in significantly stimulating a greater insulin response. The whey protein meal produced a significantly higher AUC for insulin when compared with the tuna, turkey and egg meals. The whey protein meal also produced a significantly lower AUC for postprandial glucose when compared with the egg and turkey meals. Energy intake at the buffet lunch 4 h after the consumption of the test meal was significantly lower after the consumption of the whey protein meal than after the consumption of the tuna, turkey and egg meals. This result was supported by self-reports of hunger, fullness and prospective consumption which indicated significantly lower perceived appetite after the consumption of the whey meal than after the consumption of the other protein meals. The study results suggest that whey protein's influence on appetite suppression and reduced energy intake, and its insulinotropic properties, may have a potential application for appetite control and food intake regulation in overweight or obese individuals.
There was a strong relationship between self-rated appetite and energy intake at lunch. Differences in energy intake observed after the consumption of various dietary protein meals were similar to those observed in the previous studies, with whey protein meal resulting in reduced energy intake compared with the other protein meals(Reference Hall, Millward and Long11, Reference Anderson, Tecimer and Shah12). Anderson et al. (Reference Anderson, Tecimer and Shah12) showed that whey proteins consistently suppressed food intake compared with soya and egg albumin. Hall et al. (Reference Hall, Millward and Long11) found that a whey liquid preload resulted in a greater subjective satiety rating and a reduced energy intake at a buffet meal 90 min later when compared with the casein meal. In the present study, a lower hunger and prospective consumption rating and a higher fullness rating were recorded for whey meal than for the other protein meals. Interestingly, Bowen et al. (Reference Bowen, Noakes and Trenerry4) found no differences in food intake and appetite when comparing whey with lactose and casein, and when comparing whey with soya and gluten(Reference Bowen, Noakes and Clifton3). However, there are few conclusive studies comparing whey protein with proteins from whole foods. The differences in appetite observed between the various protein test meals in the present study may be related to their amino acid composition. Previous studies have shown that phenylalanine and tryptophan have potent effects on appetite(Reference Ballinger and Clark24, Reference Muurahainen, Kissileff and Pi-Sunyer25). In the present study, the whey protein test meal had the highest phenylalanine and tryptophan contents than any other test meal (Table 1). The tuna meal had higher amounts of these amino acids than the turkey and egg protein test meals. Therefore, the higher content of phenylalanine and tryptophan in the whey and fish meals may be linked with the suppression of appetite and reduced energy intake at lunch (Table 3). In the present study, the tuna test meal resulted in a greater reduction of energy intake after 4 h, a greater insulin response, and lower ratings of appetite and prospective consumption when compared with the turkey and egg meals. Uhe et al. (Reference Uhe, Collier and O'Dea20) demonstrated that satiety was greater after the consumption of the tuna meal than after the consumption of chicken or beef meal; however, they did not observe any difference in postprandial glucose or insulin responses. They suggested that satiety after the consumption of the tuna meal in their study may be correlated with serotonergic activity, or may be attributable to differences observed in the postprandial tryptophan to large neutral amino acid ratio and/or differences in digestibility reflected in the longer time required for the plasma amino acids to peak after the consumption of the tuna meal than after the consumption of the other protein meals. In the present study, egg and turkey resulted in the highest energy intakes and stronger appetite ratings. Similar to this, Anderson et al. (Reference Anderson, Tecimer and Shah12) found that egg albumin did not suppress food intake and led to a greater energy intake when compared with the whey and soya proteins.
In the present study, whey elicited a lower postprandial glucose response than the other proteins consumed. These effects of whey are consistent with a previous study done by Frid et al. (Reference Frid, Nilsson and Holst14), in which it was found that when whey was added to a high-glycemic index breakfast and lunch, the postprandial blood glucose was reduced after the consumption of the lunch meal than after the consumption of the same meals without the whey in diabetic subjects. Petersen et al. (Reference Petersen, Ward and Bastian26) found that consumption of a blend of whey peptides and intact whey protein reduced blood glucose levels in a dose-dependent manner in healthy subjects. The reduced blood glucose after the consumption of the whey meal may be due to the action of the increased insulin secretion(Reference Zawadzki, Yaspelkis and Ivy27), although other mechanisms may also be responsible.
The effect of whey on energy intake may be related to its effects on satiety hormones. Whey protein ingestion results in the release of several gut peptides involved in satiety including cholecystokinin-A, glucagon-like peptide-1 and glucose-dependent insulinotropic polypeptide(Reference Hall, Millward and Long11). Nilsson et al. (Reference Nilsson, Stenberg and Frid7) found that the greatest AUC for glucose-dependent insulinotropic polypeptide occurred after the consumption of the whey test meal than after the consumption of milk, cheese, cod and wheat gluten. Frid et al. (Reference Frid, Nilsson and Holst14) also found an increase in glucose-dependent insulinotropic polypeptide after the consumption of the meals containing whey protein than after the consumption of the meals without whey. Whey may affect satiety by suppressing ghrelin, an appetite regulatory hormone(Reference Bowen, Noakes and Clifton10). Insulin is also thought to be a satiety hormone(Reference G5) which influences food intake regulation(Reference Woods6). Recent evidence suggests that high levels of insulin may be associated with a reduced rate of body weight gain in overweight adults, at least in the early stages of hyperinsulinaemia, possibly due to its effect on food intake regulatory mechanisms(Reference Samra, Wolever and Anderson28, Reference Swinburn, Nyomba and Saad29). The results of the present study show that whey proteins have one of the strongest insulinotropic properties, with the tuna protein also being a more efficient insulin secretagogue than the turkey and egg proteins. This rise in plasma insulin after the whey protein ingestion may have resulted in reduced energy intake at the buffet meal. Tuna ingestion produced the next highest insulin response, with energy intake at the buffet meal being lower than that at the turkey and egg meals. Although turkey had a slightly higher insulin response than egg, the energy intake at lunch was not significantly different.
Schmid et al. (Reference Schmid, Schulte-Frohlinde and Schusdziarra30) have suggested that gut factors are only of minor importance, and that amino acids are the major insulin secretagogue in the absence of carbohydrates. Whey protein has been shown to be digested quickly than other proteins, resulting in a rapid increase in plasma amino acids(Reference Hall, Millward and Long11, Reference Boirie, Dangin and Gachon18). Specific amino acids have been shown to influence insulin release both acutely and chronically by their effect on pancreatic β-cells(Reference Newsholme, Brennan and Bender31), and different amino acids appear to influence insulin release differently(Reference von Post-Skagegård, Vessby and Karlström8, Reference Schmid, Schulte-Frohlinde and Schusdziarra30). The higher insulin response elicited by the whey protein meal may be due to its high content of BCAA. Nilsson et al. (Reference Nilsson, Stenberg and Frid7) found that dairy whey proteins contained more BCAA than other protein and non-protein sources, although cod fish also contains a significant amount. The BCAA leucine, isoleucine and valine have been shown to increase insulin release to a greater extent compared with the non-branched amino acids(Reference Nilsson, Stenberg and Frid7, Reference von Post-Skagegård, Vessby and Karlström8). Table 1 shows that the whey meal contained higher amount of BCAA, such as isoleucine and leucine, than the tuna, turkey and egg test meals. The tuna meal also contained higher amounts of these amino acids than the turkey and egg test meals, but not as high as those contained by the whey test meal. Whey protein has been shown to produce the greatest amino acid response postprandially when compared with the milk powder, cheese, cod and other test meals(Reference Nilsson, Stenberg and Frid7). It was suggested that the rise in amino acids after the consumption of the cod meal was not sufficient to stimulate an amino acid-induced insulin response as did the dairy proteins(Reference Nilsson, Stenberg and Frid7). Nilsson et al. (Reference Nilsson, Stenberg and Frid7), however, noted that different digestion and absorption rates between various protein sources could impact on the amino acid responses postprandially, which may explain to some extent why whey proteins, which are digested quickly, had a greater insulinotropic effect than the tuna meal in the present study. Interestingly, chronic consumption of whey protein may result in reduced plasma insulin concentrations, indicating an increase in insulin sensitivity(Reference Belobrajdic, McIntosh and Owens15). A previous study done in our laboratory found that supplementation of whey protein for 12 weeks produced a lower insulin response, in the absence of weight loss, in overweight individuals when compared with that of casein and glucose(Reference Pal, Ellis and Dhaliwal32). Further studies are required to investigate the different insulin responses to whey ingestion acutely and chronically.
One of the limitations of the present study was that postprandial amino acid profiles and satiety hormone responses were not investigated, which may have provided greater insight into the effects of whey protein on appetite suppression, reduced food intake and insulin release when compared with tuna and the other protein meals. More research is required in this area. Further investigations examining the effects of different types of fish on insulin response, appetite and food intake may also be appropriate. Another study limitation was that liquid protein meals were examined, and it is unclear if the effects on subjective appetite, energy intake, insulin and glucose would be similar if the effects of the solid forms of food were used. Future studies should explore whether these findings can be extrapolated to whole foods rather than to liquid meals by comparing foods such as yogurt, fish, beef and chicken. Other population groups such as women and overweight and obese individuals also need to be examined to determine if the responses in lean men, as observed in the present study, can be translated to these groups. Overweight/obese individuals may exhibit different metabolic responses due to their greater adiposity and low-grade systemic inflammation often associated with obesity and chronic disease(Reference Egger and Dixon33). The amount of insulin secreted is in proportion to the total body fat in the absence of elevated glucose(Reference Woods6); therefore, overweight individuals who may be insulin resistant or hyperinsulinaemic may produce different responses compared with the lean individuals due to the effect of different insulin concentrations on appetite regulation(Reference Samra, Wolever and Anderson28, Reference Swinburn, Nyomba and Saad29). The effect of short-term food intake of these different protein sources on body weight regulation also remains to be determined.
The most important finding of the present study is that subjects who consume a meal rich in whey proteins feel less hungry and are fuller directly thereafter, and therefore eat less during the subsequent meal than when fed other types of proteins. This may be due to whey protein's insulinotropic influence and/or its influence on other satiety hormones. The tuna meal provided the next highest insulin response, and provided significant reductions in appetite and food intake when compared with the turkey and egg meals. Obesity is assumed to be caused in many cases by overfeeding, so dietary measures that influence food intake regulation and appetite offer a potential for weight reduction in overweight/obese populations. Whether chronic consumption of whey protein can regulate long-term appetite and therefore lead to weight loss remains to be elucidated.
Acknowledgements
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors. The authors declare that they have no conflicts of interest. The authors wish to thank Siew Liem for the collection of data for the study. The authors' contributions are as follows: S. P. conceived and designed the study, wrote the manuscript, supervised the study and the statistical analysis, and had primary responsibility for the final content. V. E. provided input for the manuscript, and co-wrote the manuscript.








