CVD is a major health problem in prosperous Western societies, causing considerable morbidity and mortality. The prevalence of CVD displays a well-known sex difference, being more common in men compared with women. Serum ferritin is an established biomarker of body Fe stores(Reference Milman1). In 1981 Sullivan(Reference Sullivan2) observed an increased risk of myocardial infarction in subjects with high serum ferritin levels and subsequently introduced the ‘iron hypothesis’, which claims that Fe depletion protects against IHD. This controversial hypothesis has been repeatedly discussed during the last 25 years(Reference Danesh and Appleby3–Reference You and Wang5). In biological terms it has been suggested that free Fe might increase the risk of heart diseases by supporting lipid peroxidation via catalysis of free radical production(Reference Heinecke6, Reference Reaven and Witztum7).
Several studies have investigated the influence of Fe stores on the risk of CVD. Their findings are, however, ambiguous. The first epidemiological study(Reference Salonen, Nyyssonen and Korpela8) published in 1992 found that ferritin represents a strong predictor of myocardial infarction (relative hazard per unit 1·002 (95 % CI 1·001, 1·003). Furthermore, this study reported a 2·2-fold increased risk of myocardial infarction among men with serum ferritin levels > 200 μg/l(Reference Salonen, Nyyssonen and Korpela8) compared with men with lower levels. Against the original Fe hypothesis, which did not precisely define the association between ferritin and IHD, this paper implied a linear relationship between ferritin and IHD. This altered Fe hypothesis was mostly the platform of further investigations. Several subsequent studies found positive associations between serum ferritin levels and arteriosclerosis or myocardial infarction(Reference Kiechl, Willeit and Egger9–Reference Wolff, Volzke and Ludemann14). In contrast, also no association between serum ferritin(Reference Galan, Noisette and Estaquio15–Reference Aronow and Ahn18), serum Fe(Reference Klipstein-Grobusch, Koster and Grobbee10, Reference Magnusson, Sigfusson and Sigvaldason17) or serum transferrin(Reference Klipstein-Grobusch, Koster and Grobbee10) and the risk of myocardial infarction or IHD were reported. In agreement with these investigations, a meta-analysis(Reference Danesh and Appleby3) revealed no association between serum ferritin, serum Fe or transferrin and CHD. However, serum Fe and transferrin are less sensitive measurements of body Fe stores(Reference Sempos and Looker19).
The evidence based on large cohort studies is limited(Reference Salonen, Nyyssonen and Korpela8, Reference Kiechl, Willeit and Egger9, Reference Galan, Noisette and Estaquio15, Reference Magnusson, Sigfusson and Sigvaldason17) and provided different and sometimes contradictory results. Furthermore, in the majority of these studies only one cardiovascular event such as arteriosclerosis(Reference Kiechl, Willeit and Egger9) or myocardial infarction(Reference Salonen, Nyyssonen and Korpela8, Reference Magnusson, Sigfusson and Sigvaldason17) was investigated. The cohort studies mentioned(Reference Salonen, Nyyssonen and Korpela8, Reference Kiechl, Willeit and Egger9, Reference Galan, Noisette and Estaquio15, Reference Magnusson, Sigfusson and Sigvaldason17) considered the full serum ferritin range. However, the effects of pathological serum ferritin levels are well investigated(Reference Fox, Cullen and Knuiman20–Reference Tuomainen, Kontula and Nyyssonen23). Therefore, the objective of the present study was to investigate the associations between serum ferritin levels within the reference range and the risks of non-fatal and fatal CVD as well as IHD in 2874 Danish subjects who were followed for 10 years.
Materials and methods
Subjects
The WHO initiated the ‘Monitoring of Trends and Determinants in Cardiovascular Disease’ (MONICA) survey of which the Danish part (DAN-MONICA) was conducted at the Research Centre for Prevention and Health in Copenhagen County. The DAN-MONICA study was performed in 1982–4 and was followed by a 1914 cohort survey performed in 1984, when the participants were 70 years of age. The participants of both studies were residents in the south Western part of Copenhagen County. The selection of the participants in DAN-MONICA I have been described in detail elsewhere(Reference Milman and Kirchhoff24). A random sample (n 4807) of 30-, 40-, 50- and 60-year-old men and women was drawn from the Census Registry. A total of 3785 individuals (response rate 79 %) participated in the present study. The selection of the participants in the 1914 cohort is described elsewhere(Reference Milman and Schultz-Larsen25). This cohort comprised a random sample of 540 men and women born in 1914; 389 subjects (response rate 72 %) responded to the invitation. In both studies, the investigation included a physical examination, a health questionnaire and blood samples. Both studies were approved by the Ethical Committee of Copenhagen, and all attendees had provided informed consent.
Subjects with missing data for serum ferritin (n 842), with serum ferritin levels > 300 μg/l (143 men; fifteen women) or < 15 μg/l (seventeen men; 174 women), with a history of myocardial infarction or stroke (n 95) at baseline or with missing data for selected confounding factors (n 14) were excluded. Altogether, 2874 subjects (DAN-MONICA I n 2534; 1914 cohort n 340) were included in the analyses and followed for 10 years.
Measurements
Serum ferritin was measured by an immunoradiometric assay (Phadebas Ferritin PRIST; Pharmacia Diagnostics, Uppsala, Sweden). Serum total cholesterol was measured using the cholesterol oxidase phenol 4-aminoantipyrene peroxidase (CHOD-PAP) enzymic method (Monotest R Cholesterol; Boehringer Mannheim, Germany). Blood pressure was measured twice with the participants in sitting position after at least 5 min of rest and the mean was calculated. BMI was calculated in kg/m2. Social class was determined using the method of the Danish National Institute of Social Research and classified into five social strata, with I as the highest and V as the lowest(Reference Enevoldsen, Michelsen and Friis-Hasche26). By means of a specific tobacco questionnaire the smoking status was categorised (non-smoker and smoker) and alcohol consumption was recorded in drinks per week. Myocardial infarction, stroke, hypertension and diabetes were defined as self-reported physician diagnoses (‘Has a doctor ever told you…?’).
Each person living in Denmark is assigned an individual ten-digit number in the census registration system (person number), which makes it possible to perform linkage across different registries in specific time periods. By means of their personal number the participants were linked to both The National Hospital Registry, which registers all diagnoses on all patients discharged from hospitals in Denmark and The Cause of Death Registry, which registers causes of deaths in all Danes. In order to identify subjects with non-fatal and fatal causes of IHD we linked the person number to the International Classification of Diseases (ICD) 8th edition diagnoses numbers 410–414 and ICD 10th edition numbers I20–I25. In order to identify CVD we used ICD 8th edition numbers 390–448 and ICD 10th edition numbers DI00–DI79. Thus the CVD diagnostic spectrum also comprised the IHD diagnoses.
Statistics
Continuous data are expressed as medians (25th quartile; 75th quartile). Nominal data are expressed as percentages. For group comparisons, the χ2 test (nominal data) or the Mann–Whitney U test (continuous data) was used. Multivariable Cox proportional hazard regression models were run separately in men and women to assess the associations between serum ferritin levels and CVD as well as IHD. The Cox proportional hazards model assumes that the log hazard function is linear in the covariates. Therefore, restricted cubic splines were used to detect a possible non-linear dependency of the log hazard function on serum ferritin levels(Reference Stone and Koo27). Four knots were pre-specified located at the 5th, 35th, 65th and 95th percentile as recommended by Stone & Koo(Reference Stone and Koo27), resulting in two components of the spline function: ferritin′ and ferritin″. The full models were adjusted for age, smoking, alcohol consumption, diabetes, hypertension, serum cholesterol levels and systolic blood pressure. Based on the results in women we performed multivariable Cox proportional hazard regression including serum ferritin levels categorised into three groups (low, < 40; middle, 40–80; high, > 80 μg/l). Hazard ratios (HR) with 95 % CI were calculated. The model assumption for the Cox proportional hazards regression model was checked with Schoenfeld residuals, plots of hazard function for stratified models and estimations in models including time-dependent versions of variables. The functional form of continuous variables was checked with Martingale residuals and in models including quadratic terms of the continuous variables, which resulted in considering quadratic terms of continuous variables in different models. The proportional hazard assumption was met for all variables. Furthermore, the interaction between serum ferritin and cholesterol levels was tested by a sequence of backward and forward stepwise regression analyses.
A value of P < 0·05 was considered statistically significant. Statistical analyses were performed with SAS 9.1 (SAS Institute Inc., Cary, NC, USA).
Results
During the 10-year follow-up CVD events occurred in 13·6 % (n 201) of men and 7·8 % (n 109) of women (P < 0·05), whereas IHD occurred in 7·9 % (n 117) of men and 3·2 % (n 44) of women (P < 0·05).
Men had a higher BMI, were more often smokers and consumed more beer and spirits than women (Table 1). On the other hand, men reported less often physician-diagnosed hypertension though both systolic and diastolic blood pressure was higher in men than in women. With respect to Hb and serum ferritin, men exhibited higher levels compared with women, which was also seen in individuals older than age 49 years. Further, we found that women with at least one childbirth had significant lower serum ferritin levels than women without a prior childbirth. This result was independent of the number of births.
Table 1 Selected characteristics of women and men
(Medians and 25th and 75th quartiles or percentages)
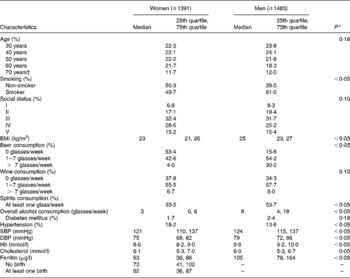
SBP, systolic blood pressure; DBP, diastolic blood pressure.
* χ2 Test (nominal data) or Mann–Whitney test (continuous data).
† 1914 Cohort (n 340).
Our analyses revealed no statistically significant associations between serum ferritin levels and CVD as well as IHD in men and women (Table 2). However, given the predicated log hazard as a function of ferritin (Figs. 1 and 2) the present results argue for a U-shaped relationship between serum ferritin levels and the risk of CVD as well as IHD in women. Serum ferritin levels of about 60 μg/l appear to be optimal, whereas higher and lower levels were associated with an increasing risk of CVD and IHD. A similar pattern was not found in men; instead the lowest log hazard was given for low serum ferritin levels. We found no significant interaction between serum ferritin and cholesterol levels in men and women.
Table 2 Parameter estimates of proportional hazard models for CVD and IHD including ferritin (modelled with restricted cubic splines) in women and men

* P value for the variable as a whole.
† Full model adjusted for age, smoking, alcohol consumption, diabetes or hypertension, cholesterol and systolic blood pressure. Ferritin′ and ferritin″ are the components of the spline function that were fitted in the adjusted model.
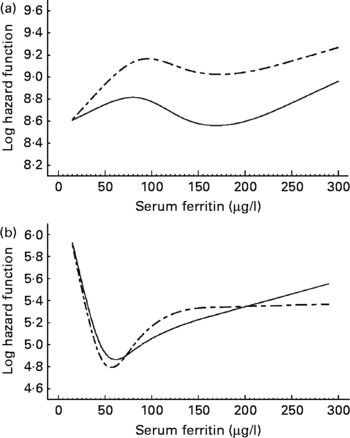
Fig. 1 Predicated log hazard function for CVD as a function of serum ferritin in men (a) and women (b). Results of Cox proportional hazard regression models with restricted cubic splines adjusted for age (- – -) and fully adjusted (—). The full model was adjusted for age, smoking, alcohol consumption, diabetes, hypertension, serum cholesterol levels and systolic blood pressure.
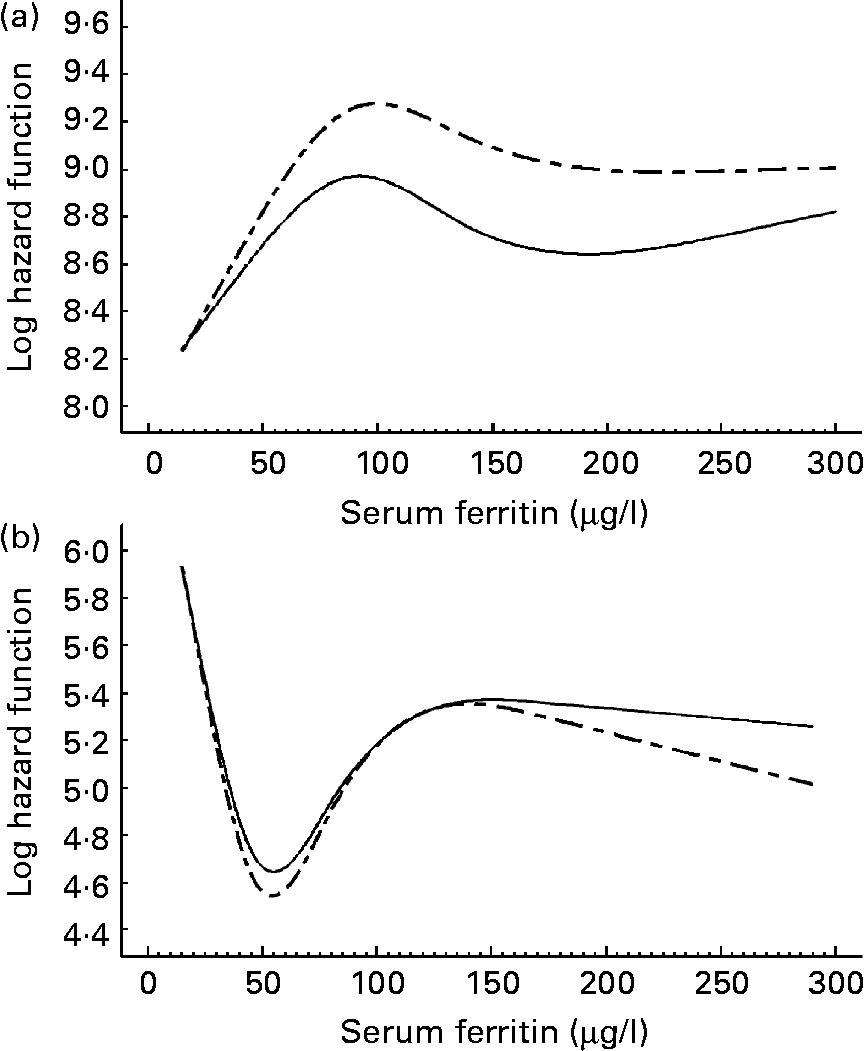
Fig. 2 Predicated log hazard function for IHD as a function of serum ferritin in men (a) and women (b). Results of Cox proportional hazard regression models with restricted cubic splines adjusted for age (- – -) and fully adjusted (—). The full model was adjusted for age, smoking, alcohol consumption, diabetes, hypertension, serum cholesterol levels and systolic blood pressure.
Based on these results, additional analyses in women with serum ferritin levels categorised into three groups (low, < 40; middle, 40–80; high, > 80 μg/l) confirmed the assumption of a U-shaped relationship. Women with low or high serum ferritin levels tended to have a higher risk of CVD (low serum ferritin, HR 1·92 (95 % CI 1·03, 3·59); high serum ferritin, HR 1·33 (95 % CI 0·85, 2·08)) and IHD (low serum ferritin, HR 1·90 (95 % CI: 0·68, 5·28); high serum ferritin, HR 1·55 (95 % CI: 0·75, 3·21)) compared with women in the intermediate ferritin group. However, only one of these estimates was statistically significant.
Sensitivity analyses were run for 773 women older than 49 years to evaluate the effect of menopausal status on the relationship between serum ferritin levels and CVD and IHD. CVD and IHD events occurred in 13·2 % (n 102) and 5·3 % (n 41) of postmenopausal women, respectively. Although these analyses also suggested a U-shaped association, comparisons did not attain statistical significance. The optimal serum ferritin value was about 65 μg/l. In further analysis using a categorised exposure variable (low serum ferritin, < 45; middle, 45–85; high serum ferritin, > 85 μg/l) the U-shape relationship was no longer apparent (CVD: low serum ferritin, HR 1·52 (95 % CI 0·80, 2·90); high serum ferritin, HR 1·10 (95 % CI 0·71, 1·71); IHD: low serum ferritin, HR 1·06 (95 % CI 0·36, 3·14); high serum ferritin, HR 1·19 (95 % CI 0·61, 2·33); reference: middle group).
Moreover in women, we included the number of childbirths (none, at least one birth) in the model. No significant effect of number of births on the risk of CVD as well as IHD and no significant changes in estimates for ferritin spline components were found. Further, we tested other potential confounding factors such as social class, physical activity and serum HDL-cholesterol levels in the models. All these analyses confirmed our main result, which demonstrated no significant role of serum ferritin on the development of CVD, even though we could not exclude the possibility of an association between serum ferritin and CVD in women.
Discussion
To the best of our knowledge this is the first study to investigate the association between normal serum ferritin levels and CVD as well as IHD. Overall, the present results showed no significant effect of serum ferritin levels on the risk of CVD or IHD. However, in women, the results suggested a U-shaped association between serum ferritin levels and CVD as well as IHD.
The background of the present study was the ‘iron hypothesis’, which claims in the original form that Fe depletion, a state in which ferritin generally is not available, protects against IHD. This hypothesis did not make any assumption about the quantitative association between ferritin and IHD(Reference Sullivan28). However, this hypothesis has been modified since 1992, when a Finnish cohort study demonstrated a higher risk of myocardial infarction in men with elevated serum ferritin levels(Reference Salonen, Nyyssonen and Korpela8). The altered hypothesis indicated that serum ferritin is directly related to the risk of CVD and was the basis of most of the following studies including the present one. Sullivan(Reference Sullivan28) extensively discussed this problem and indicated two possible ways of ferritin acting as a risk factor of CVD. In case of previous Fe depletion, relatively small amounts of Fe may lead to maximal promotion of CVD. Thus, extremely low ferritin levels may be a strong cardiovascular risk factor. On the other hand, if high serum ferritin is a marker of persistent Fe repletion, high levels might act as a risk factor. This concept is in good agreement with the present results of a U-shaped association in women. Likewise, The National Health and Nutrition Examination Survey (NHANES) II mortality study also did not find any significant influence of serum ferritin levels on the risk of mortality(Reference Sempos, Looker and Gillum29). However, their results also suggest a U-shaped association between serum ferritin and death from CVD in black men, though the risk estimates and the quadratic trend tests did not attain statistical significance. Also in concordance with the present results is a French cohort study(Reference Galan, Noisette and Estaquio15) on 9917 subjects with a median follow-up of 7·5 years, which found no significant association between serum ferritin and the risk of IHD in men and women. These results have been confirmed by further studies(Reference Manttari, Manninen and Huttunen16–Reference Aronow and Ahn18, Reference Moore, Folsom and Barnes30–Reference Regnstrom, Tornvall and Kallner32) including a meta-analysis(Reference Danesh and Appleby3), which reported no association between serum ferritin levels and the risk of cardiovascular events. Finally, a clinical trial based on the Iron (Fe) and Atherosclerosis Study (FeAST) has tested the impact of a reduction in body Fe by phlebotomy on mortality and non-fatal cardiovascular events(Reference Zacharski, Chow and Howes33). The results indicated no decrease of all-cause mortality as well as of myocardial infarction and stroke among subjects who underwent a reduction of body Fe stores by phlebotomy.
However, there are also contradictory results showing significant associations between serum ferritin and CVD(Reference Salonen, Nyyssonen and Korpela8–Reference Klipstein-Grobusch, Koster and Grobbee10, Reference Haidari, Javadi and Sanati12–Reference Wolff, Volzke and Ludemann14). A case–cohort study(Reference van der A, Grobbee and Roest34) of postmenopausal women showed that women with serum ferritin levels in the highest tertile had a 2-fold higher risk of ischaemic stroke compared with women with ferritin levels in the lowest tertile. Regarding atherosclerosis, a cohort study(Reference Kiechl, Willeit and Egger9) demonstrated that an increase in serum ferritin was associated with incident atherosclerosis and promoted the extension of atherosclerotic lesions. These findings were confirmed by further studies(Reference Klipstein-Grobusch, Koster and Grobbee10, Reference Haidari, Javadi and Sanati12–Reference Wolff, Volzke and Ludemann14). Moreover, in high-frequency blood donors with reduced serum ferritin levels a decreased oxidative stress and improved vascular function were found compared with low-frequency donors(Reference Zheng, Cable and Spencer35). These results also approve an association between body Fe stores and CVD.
The above-mentioned studies(Reference Salonen, Nyyssonen and Korpela8–Reference Manttari, Manninen and Huttunen16, Reference Sempos, Looker and Gillum29–Reference Regnstrom, Tornvall and Kallner32) provide a lack of clear evidence. However, cross-sectional studies(Reference Kiechl, Aichner and Gerstenbrand11–Reference Wolff, Volzke and Ludemann14, Reference Rauramaa, Vaisanen and Mercuri31) are generally not suitable to prove causal relationships. Moreover, the considered case–control studies(Reference Klipstein-Grobusch, Koster and Grobbee10, Reference Manttari, Manninen and Huttunen16, Reference Moore, Folsom and Barnes30, Reference Regnstrom, Tornvall and Kallner32) usually investigated small study populations. Furthermore, the included confounding factors displayed considerable variations, which might be a further reason for the different results. The inconsistency in the reported studies is probably also due to the different investigation regions ranging from Europe, USA, India to Iran and thereby comprising different ethnic groups. Worldwide variations in cardiovascular events-rates and mortality caused by CVD are well documented(Reference Mackay and Mensah36–Reference Verschuren, Jacobs and Bloemberg39). None of the above-mentioned longitudinal studies(Reference Salonen, Nyyssonen and Korpela8, Reference Kiechl, Willeit and Egger9, Reference Galan, Noisette and Estaquio15, Reference Magnusson, Sigfusson and Sigvaldason17, Reference Aronow and Ahn18, Reference Sempos, Looker and Gillum29) have looked at the shape of the log hazard curve to interpret the relationship between serum ferritin levels and the risk of cardiovascular events.
The assumed underlying medical theory suggests that Fe due to the catalysis of the production of free radicals indirectly stimulates the peroxidation of LDL-cholesterol, which promotes the development of atherosclerosis(Reference Heinecke6, Reference Reaven and Witztum7). This might explain the higher risk of CVD in women with high ferritin levels. However, as previously reported(Reference Sempos, Looker and Gillum29), the evidence for this hypothesis is inconsistent and the supposed association between serum ferritin and oxidised LDL-cholesterol levels is questionable. In the present female population we found that women with no childbirths had significantly higher serum ferritin levels than women with at one or more childbirths. This result, together with findings of a German study(Reference Wolff, Volzke and Robinson40) which demonstrated a positive association between number of children and carotid intima-media thickness, might explain the higher risk of CVD in women with low serum ferritin levels.
The strengths of the present study are the large population sample and the extended follow-up period. Furthermore, we tested for a broad range of confounding factors. However, the present study shares with other cohort studies the limitation that we had no repeated measurements of serum ferritin. Therefore, we were not able to investigate the impact of intra-individual variations in serum ferritin levels on the mortality risk. Serum ferritin levels are affected by inflammatory processes and Fe medication as well as by biological variation(Reference Cooper and Zlotkin41–Reference Pilon, Howanitz and Howanitz43). Given the long duration between baseline data collection and the occurrence of end points in the present study, this limitation becomes particularly relevant. Furthermore, the number of events (particularly among women) might be too low to detect possible association and thus represents a further limitation of the present study.
In concordance with previous prospective studies, the present results did not show a significant association between serum ferritin and cardiovascular mortality. However, our findings suggest that serum ferritin levels in the extreme low and high range may represent a cardiovascular risk factor in women.
Acknowledgements
The present study was supported by the National Research Council (grant no. 22-00-0174) and the Danish Health Insurance Foundation.
N. F. and T. J. were responsible for the study conception and design; N. F. was responsible for the data analysis and article drafting. All authors were involved in the interpretation of data and revision for important intellectual content. All authors gave their final approval.
There are no conflicts of interest to declare.






