Introduction
The Cerros del Sira is an isolated mountain range in Peru, home to a diverse and unique flora and fauna. The summits rise from the left bank of the Ucayali River, with rugged terrain that extends over five elevational zones (200–2,230 m). Such isolation predisposes the Cerros del Sira to host a large number of endemic species but also means that these species and their habitats are sensitive to human-driven forest disturbance and climatic change (Forero-Medina et al., Reference Forero-Medina, Terborgh, Socolar and Pimm2011). Although climatic changes are not of dramatic consequence for species residing in low-lying well-connected habitat, tropical species in isolated ranges, such as the Cerros del Sira, will have no suitable habitat to shift to, and could be outcompeted by low-elevation species moving to higher altitudes (Tewksbury et al., Reference Tewksbury, Huey and Deutsch2008).
Marginalized and difficult-to-access areas with little attraction for agriculture have historically been passively protected from anthropogenic disturbance. These lands are remote, nutrient poor, and steep, and are ideal for governments to assign for protection (Nelson & Chomitz, Reference Nelson and Chomitz2011; Harris et al., Reference Harris, Dwi Putra, Gregory, Brook, Prawiradilaga and Sodhi2014). However, the passive protection provided by such remoteness is increasingly being questioned (e.g. Poulsen et al., Reference Poulsen, Koerner, Moore, Medjibe, Blake and Clark2017). Evidence indicates that many remote protected regions are undergoing defaunation of medium-sized and large vertebrates (Fa et al., Reference Fa, Peres and Meeuwig2002; Galetti & Dirzo, Reference Galetti and Dirzo2013), and fragmented landscapes facilitate access for illegal activities in remote areas (Michalski & Peres, Reference Michalski and Peres2007). In the case of Sira, Novoa et al. (Reference Novoa, Finer and Snelgrove2016) reported the loss of 1,600 ha of forest to agriculture and grazing inside the Sira Communal Reserve during 2013–2016.
Although surveys on birds (Terborgh & Weske, Reference Terborgh and Weske1975; Mee et al., Reference Mee, Ohlson, Stewart, Wilson, Örn and Diaz Ferreyra2002; Corvacho et al., Reference Corvacho, MacLeod, Brooks and Hennessey2011; Forero-Medina et al., Reference Forero-Medina, Terborgh, Socolar and Pimm2011), amphibians and reptiles (Duellman & Toft, Reference Duellman and Toft1979; Aichinger, Reference Aichinger1991), and flowering plants (Wasshausen, Reference Wasshausen2007; Monteagudo Mendoza & Huamán Guerrero, Reference Monteagudo Mendoza and Huamán Guerrero2010) have been carried out in the Cerros del Sira, the mammalian fauna remains poorly documented. Large and medium-sized mammals play a fundamental role in the functioning of Amazonian ecosystems, including control of prey populations (Estes et al., Reference Estes, Terborgh, Brashares, Power, Berger and Bond2011; Ripple et al., Reference Ripple, Estes, Beschta, Wilmers, Ritchie and Hebblewhite2014, Reference Ripple, Abernethy, Betts, Chapron, Dirzo and Galetti2016) and seed dispersal (Miller et al., Reference Miller, Dugelby, Foreman, Martinez del Río, Noss and Phillips2001; Fløjgaard et al., Reference Fløjgaard, Bruun, Hansen, Heilmann-Clausen, Svenning and Ejrnaes2018), but their large home ranges and slow reproductive rates make them particularly sensitive to habitat fragmentation and hunting (Fisher & Owens, Reference Fisher and Owens2004; Cardillo et al., Reference Cardillo, Mace, Jones, Bielby, Bininda-Emonds and Sechrest2005).
Camera traps are well-suited for surveys in remote locations with poor local infrastructure and harsh terrain (e.g. Jiménez et al., Reference Jiménez, Quintana, Pacheco, Melton and Tello2010; Beirne et al., Reference Beirne, Pillco-Huarcaya, Serrano-Rojas and Whitworth2017), and we present the first camera-trap survey of the mammalian community of the Cerros del Sira. We used 45 terrestrial camera traps over 2 years along a previously unstudied elevational gradient, with the aim of laying a foundation for future research, monitoring and conservation planning efforts in one of the last intact forest landscapes in the central region of the Peruvian Amazon.
Study area
The Sira Communal Reserve is situated between the Ucayali River to the east and the Pachitea River to the west. It is the largest community reserve in Peru, forming part of the Oxapampa–Asháninka–Yánesha Biosphere Reserve, located in the departments of Pasco, Huánuco and Ucayali, with an area of 616,413 ha and a buffer area of 1,032,340 ha (INRENA, 2001). The core protected area encompasses the Cerros del Sira Mountains, and the surrounding buffer area comprises a local human population of ethnic groups of Ashaninka, Asheninka, Shipibo-Conibo and Yanesha, and rural communities of Andean migrants (Benavides et al., Reference Benavides, Barclay and Smith2006). Our study transect is to the east of Puerto Inca, in the province of Huánuco (Fig. 1), along a previously unstudied ridgeline on the north-western border of the Sira Communal Reserve. The transect covers both lower and upper montane forests, and elfin forest towards the highest elevations. Mean annual precipitation is c. 3,000 mm in the montane forest and up to 6,000 mm on the peaks (2,220 m). The remoteness of the area has attracted illegal extractive industries, including coca cultivation (for cocaine production), gold mining, poaching and logging. The construction of roads since 2004 has attracted the private sector, with timber and agricultural corporations replacing many of the local cattle farmers. Mining and oil concessions now cover almost the entire area (Finer et al., Reference Finer, Novoa, Cruz and Peña2016; Novoa et al., Reference Novoa, Finer and Snelgrove2016).
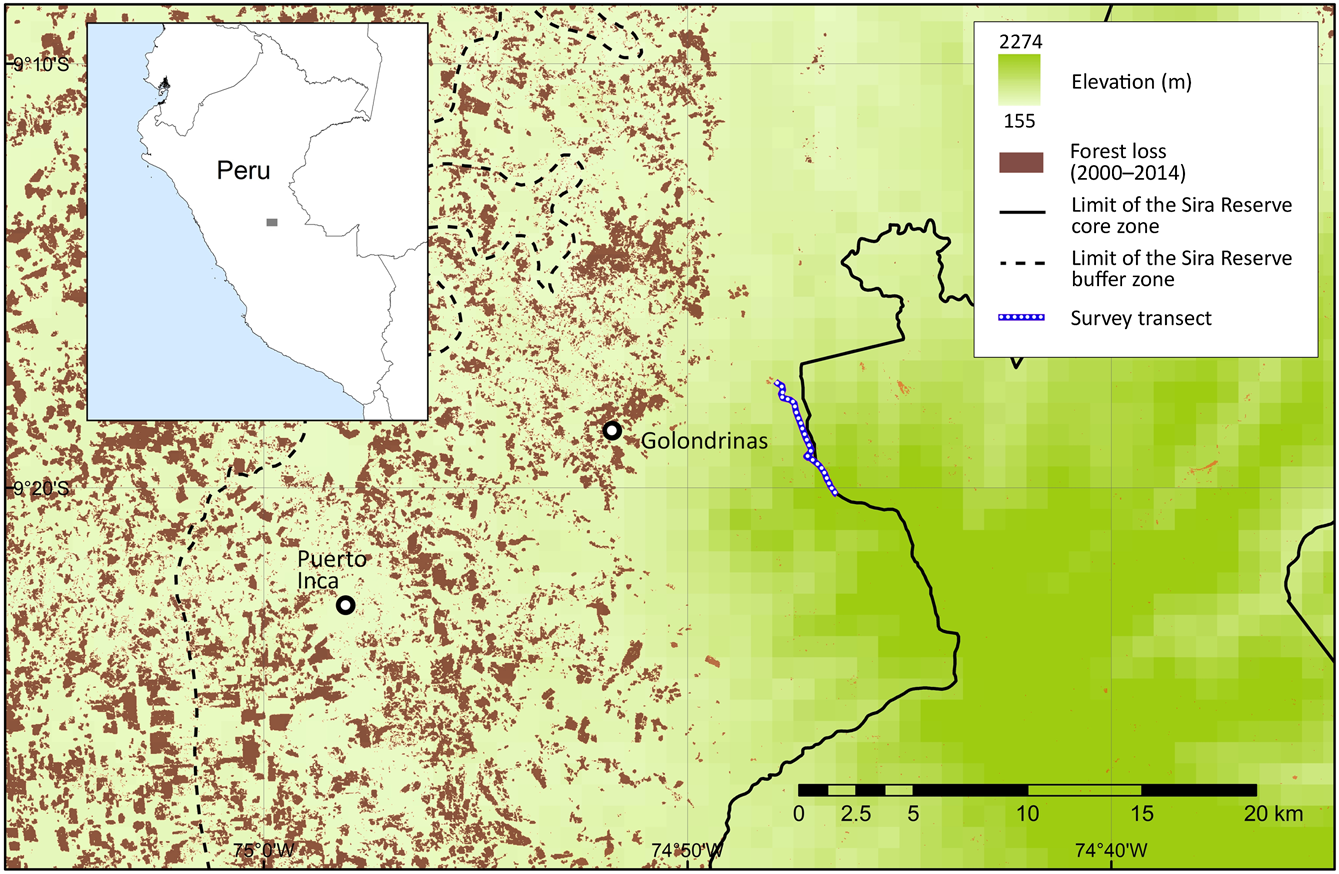
Fig. 1 Location of transect used in a camera-trap survey of medium-sized and large mammals in Sira Communal Reserve, Peru, and deforestation in the region during 2000–2014 (Hansen et al., Reference Hansen, Potapov, Moore, Hancher, Turubanova and Tyukavina2013).
Methods
Camera traps (Trophy Cam, Bushnell, Overland Park, USA) were deployed in mid to late March and removed at the beginning of September (dry season) in 2015 and 2016. Cameras were placed 40 cm above the ground, and all low vegetation within 3 m was cleared to standardize detection probabilities. Cameras were programmed to record a 14 s video, with intervals of 30 s between successive triggers (Meek et al., Reference Meek, Ballard, Claridge, Kays, Moseby and O'Brien2014; Beirne et al., Reference Beirne, Pillco-Huarcaya, Serrano-Rojas and Whitworth2017). Of the 21 cameras deployed in 2015, 19 were set to monitor medium-sized and large vertebrates (including the Critically Endangered Sira curassow Pauxi koepckeae; Beirne et al., Reference Beirne, Pillco-Huarcaya, Serrano-Rojas and Whitworth2017) and two were placed at the entry point to the ridgeline (865 m elevation) and at our principal campsite (900 m), to monitor hunting activity. Thirteen cameras were placed at elevational intervals of 50 m between 800 and 1,400 m, four were placed at intervals of 100 m between 1,500 and 1,800 m and two were placed at water sources (a clay lick at 1,056 m and a stream at 1,215 m). In 2016, 22 camera traps were placed at elevational intervals of 50 m between 950 and 1,920 m to monitor the vertebrate community, and two were placed at the same water source locations used in 2015. As people were also detected by the wildlife cameras along the ridgeline in 2015, camera traps were not set specifically to target hunting activity in 2016. The camera-trap rate was calculated for every given species as the number of videos/100 camera-trap days. Non-independent events, defined as videos of the same species at the same location within 30 minutes of a previous detection, were excluded from the calculation.
Cameras were interfered with by both people and wildlife: jaguars Panthera onca moved three cameras (destroying one completely), spectacled bears Tremarctos ornatus moved four cameras, and people moved two cameras into less effective positions for surveying (i.e. facing dense bushes or the ground). The survey team recorded all incidental audio and visual signs of medium-sized and large mammals while the team was present on the study ridge (6–24 March 2015 and 27 March–1 April 2016).
Results
Overall, we detected 34 medium-sized and large mammal species, belonging to eight orders and 18 families (Plate 1; Supplementary Table 1). Thirty mammal species were detected by camera traps, with 1,485 independent records over 6,490 camera-trap nights. Six species of mammals were detected via incidental records, four of which (all arboreal) were not detected by camera traps: the night monkey Aotus nigriceps, the tufted capuchin Sapajus macrocephalus, the burnished saki monkey Pithecia inusta and the kinkajou Potos flavus.

Plate 1 Threatened and rare mammals of the Sira Communal Reserve, Peru (Fig. 1), and direct evidence of hunting activity at high elevations within the Reserve, recorded on camera traps. (a) Panthera onca and (b) Tremarctos ornatus (both captured at the same camera trap, at 1,920 m), (c) Myrmecophaga tridactyla, (d) Tapirus terrestris, (e) Dinomys branickii, (f) Leopardus tigrinus, (g) Mustela frenata, (h) Atelocynus microtis, (i) Lagothrix cana, and (j) a hunter carrying a shotgun at 1,400 m within the core area of the Reserve.
The species accumulation curve from camera data for both 2015 and 2016 shows a clear plateau, indicating that the sampling effort was sufficient to characterize the community of medium-sized and large mammals (Fig. 2). During the first 2,000 camera-trap days most of the mammal community was recorded (S obs = 25), with only five more species added to the accumulation curve from an additional effort of > 4,000 camera-trap days. The distribution of species across the elevational bands indicates that diversity was highest at 1,000–1,250 m, with the highest observed species richness (S obs = 19) at 1,250 m. Only five species were detected at > 1,400 m: the oncilla Leopardus tigrinus, the spectacled bear, the long-tailed weasel Mustela frenata, the Andean white-eared opossum Didelphis pernigra and the pacarana Dinomys branickii. The pacarana was captured only once during the study (at 1,750 m) and the long-tailed weasel only on two occasions, at 1,450 and 1,500 m (Fig. 3; Supplementary Tables 1 & 2).
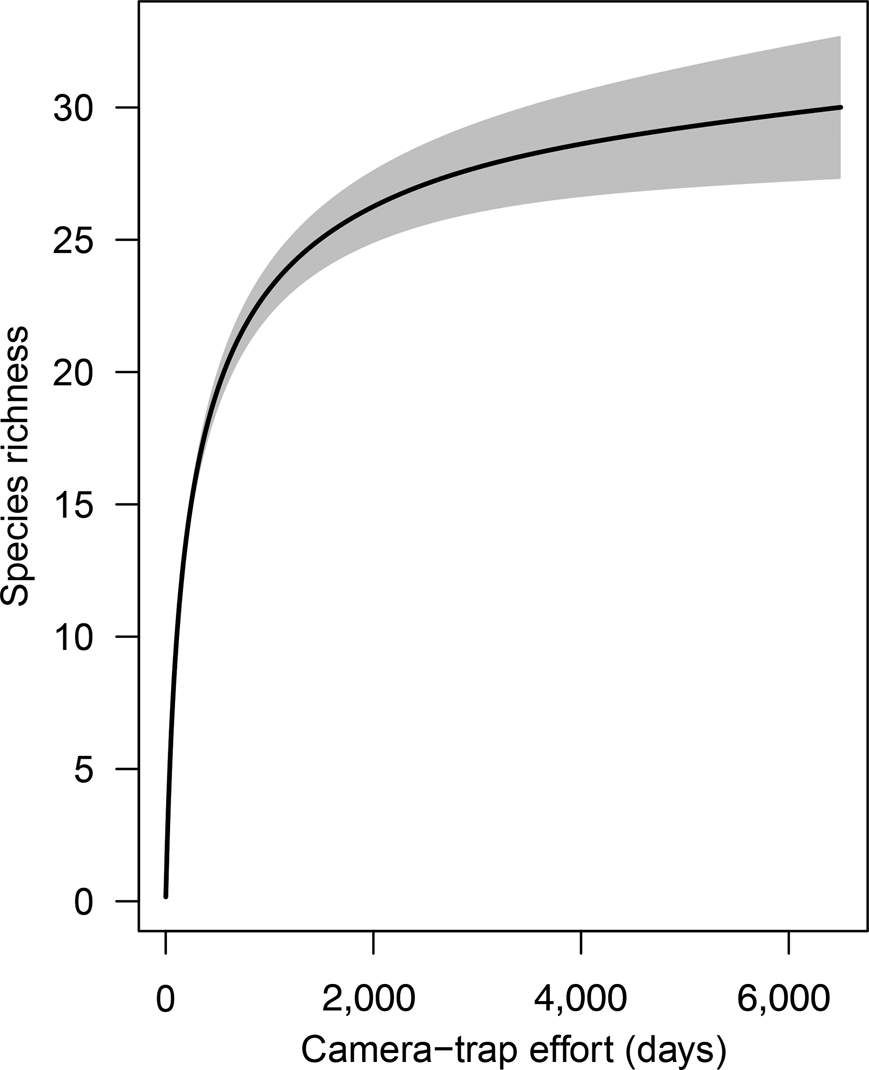
Fig. 2 Species richness accumulation curve with cumulative number of camera-trap nights, for medium-sized and large mammals in Sira Communal Reserve (Fig. 1). The grey shaded area indicates the 95% confidence interval.
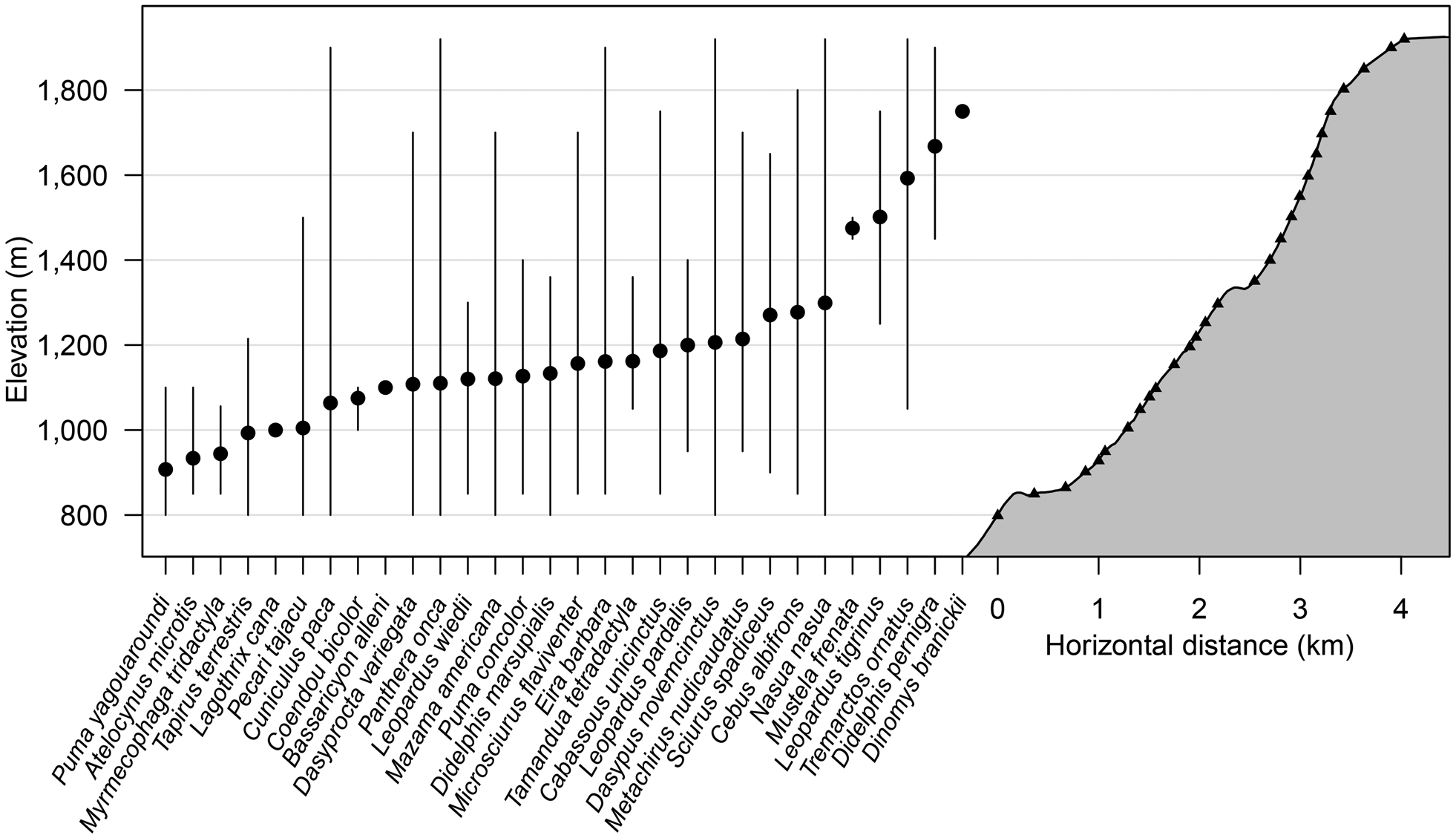
Fig. 3 Elevational distribution of mammal species recorded by camera traps along the survey transect in Sira Communal Reserve (Fig. 1). Cameras placed on trails and at specific habitat features are represented by triangles, mean elevation of detections is represented by filled circles, and maximum and minimum elevation records are represented by vertical lines.
Of the 34 species detected, one is categorized as Endangered on the IUCN Red List (the Peruvian woolly monkey Lagothrix cana), four as Vulnerable (the lowland tapir Tapirus terrestris, the spectacled bear, the oncilla and the giant anteater Myrmecophaga tridactyla), and three as Near Threatened (the short-eared dog Atelocynus microtis, the margay Leopardus wiedii and the jaguar). Three of the species detected are categorized as Data Deficient (the South American red brocket deer Mazama americana, the agouti Dasyprocta variegata and the Amazon dwarf squirrel Microsciurus flaviventer), and one is yet to be assessed (the burnished saki monkey). Nineteen of the species detected are listed in the CITES Appendices (CITES, 2017; Supplementary Table 1).
The two cameras placed specifically to monitor human presence within the study site in 2015 detected direct hunting activity (i.e. people with guns or carrying dead wildlife) on seven occasions, and shotgun shells were found frequently on the forest floor. According to our guides, some of the hunters observed in the videos were cocaleros (people hired to harvest coca) and some were working on inventorying trees for a timber concession. One of the videos shows a hunter carrying a dead razor-billed curassow Mitu tuberosum. While camping at 1,350 m we heard two gunshots. Shortly afterwards, camera-trap footage confirmed the presence of hunters carrying two dead woolly monkeys.
Discussion
The community of medium-sized and large mammals of the Cerros del Sira is exceptionally diverse, with a unique assemblage of species comprising typical lowland Amazonian species as well as high-elevation species. Illegal hunting activity was detected at 1,400 m elevation and within the protected area of the Sira Communal Reserve, despite the suggestion that the montane terrain of the Reserve probably receives little attention from hunters (Mee & Ohlson, Reference Mee and Ohlson2001).
The species richness of medium-sized and large mammals reported here is higher than that reported from other highland rainforest areas of Peru (Puno = 29, Apurimac River = 31, northern Peru = 13, and Kosñipata = 14; Pacheco et al., Reference Pacheco, Salas, Cairampoma, Noblecilla, Quintana and Ortiz2007, Reference Pacheco, Márquez, Salas and Centty2011; Jiménez et al., Reference Jiménez, Quintana, Pacheco, Melton and Tello2010; Medina et al., Reference Medina, Zeballos and López2012). Furthermore, we detected seven species additional to the 40 recorded at nearby lowland Panguana Biological Station (Hutterer et al., Reference Hutterer, Verhaagh, Diller and Podloucky1995). Four of these species have a high-elevation distribution (spectacled bear, oncilla, long-tailed weasel and Andean white-eared opossum); the other three are the pacarana, the short-eared dog and the agouti.
Of particular significance was the first detection (to our knowledge) of the two largest land predators in South America at the same camera location, the spectacled bear and the jaguar, on the highest camera, at 1,920 m. Bears were captured at elevations as low as 1,050 m, and both species were subsequently recorded at a camera station at 1,418 m in Soqtapata Reserve (13.350862°S, 70.84539°W); Rafael Pilares, pers. comm.). It has been suggested that the elevation range of these two species in Peru and Bolivia does not overlap anywhere within a single mountain slope, and overlaps only slightly at c. 900 m throughout the Cordillera Oriental (Servheen et al., Reference Servheen, Herrero and Peyton1999). The closest known previous records of the bear to our study area are from the Pachitea Basin, determined by interviews conducted with the inhabitants of the Sira Communal Reserve, and from products derived from bears avaialable for sale in the local markets of Puerto Inca, El Sira and Llullapichis (Figueroa, Reference Figueroa2016; Hurtado et al., Reference Hurtado, Pacheco, Fajardo and Uturunco2016). Compared to the range of the spectacled bear documented on the IUCN distribution map for the species (Velez-Liendo & García-Rangel, Reference Velez-Liendo and García-Rangel2017), our photographic records together with those of Figueroa (Reference Figueroa2016) and Hurtado et al. (Reference Hurtado, Pacheco, Fajardo and Uturunco2016) indicate an eastwards extension of c. 100 km. If the bear is distributed throughout the high elevations of the Sira range this could represent an increase in its known habitat of up to 540,000 ha, enough habitat for c. 325 adult individuals, based on previous density estimates from other locations (Kattan et al., Reference Kattan, Hernández, Goldstein, Rojas, Murillo and Gómez2004; Ríos-Uzeda et al., Reference Ríos-Uzeda, Gómez and Wallace2007). As various individual spectacled bears were recorded on several occasions and in different locations, these are unlikely to be records of vagrant individuals.
Another threatened species of note is the oncilla, one of the smallest cats and least known Neotropical mammals (Tortato & Oliveira, Reference Tortato and Oliveira2005; Hurtado et al., Reference Hurtado, Pacheco, Fajardo and Uturunco2016). A search of the literature and museum databases (Hurtado et al., Reference Hurtado, Pacheco, Fajardo and Uturunco2016) yielded only 10 records from the past 100 years, of which only three were since 2001, all within the montane forest of the Peruvian Yungas region (Hurtado et al., Reference Hurtado, Pacheco, Fajardo and Uturunco2016). Based on the current IUCN distribution map for this species, our records are c. 60 km west of the known range (Payan & Oliveira, Reference Payan and Oliveira2016).
Other large-bodied species of note detected that require large intact habitats include the lowland tapir and the giant anteater, suggesting a high degree of ecological integrity within the core Sira Reserve. The presence of many small, rare and cryptic species, including the margay, the short-eared dog and the pacarana, further underlines the importance of the Reserve in sustaining species of conservation significance (Bickford et al., Reference Bickford, Lohman, Sodhi, Ng, Meier and Winker2007). The Peruvian woolly monkey is a key species for seed dispersal, supporting carbon stores and maintaining ecosystem viability (Bello et al., Reference Bello, Galetti, Pizo, Magnago, Rocha and Lima2015; Estrada et al., Reference Estrada, Garber, Rylands, Roos, Fernandez-Duque and Di Fiore2017); it is also a favoured target for hunters, as we discovered, and is susceptible to local extirpation (Peres, Reference Peres, Bennett and Robinson2000).
In addition to the direct evidence of hunting in the Reserve recorded by the camera traps, informal interviews with members of local communities living within the buffer zone confirmed the disappearance of key bushmeat species in nearby lowland areas (in particular the Peruvian woolly monkey, the black-faced spider monkey Ateles chamek and the white-lipped peccary Tayassu pecari). This loss of game vertebrates is driving hunting pressure into the upper reaches of the forest within the core reserve area. Subsistence hunting is conducted when the hunter has no viable alternatives, especially where other food options are not readily available (Ripple et al., Reference Ripple, Abernethy, Betts, Chapron, Dirzo and Galetti2016). During our stay in the communities close to our study site we observed that they reared many animals for personal consumption (chicken, cows, ducks and pigs), and informal conversations confirmed that hunting is practised predominantly as a cultural legacy (enjoying bushmeat, collecting trophies and a social activity with family and friends), not for survival.
In 2016 we witnessed illegal logging inside the core area, at 1,250 m. Previously most anthropogenic impact had occurred outside the core area; however, our direct evidence, along with satellite imagery showing canopy loss in the northern reaches of the core zone (Novoa et al., Reference Novoa, Finer and Snelgrove2016), indicates this is a real threat to the protected core region. The National Service of Natural Protected Areas (SERNANP) is limited in its capacity to patrol large remote forested landscapes. In such cases, remote sensing technologies such as camera traps and acoustic monitoring devices have been proven to be effective as complementary tools for monitoring illegal activities in protected areas (Hossain et al., Reference Hossain, Barlow, Barlow, Lynam, Chakma and Savini2016).
The remoteness and rugged terrain of the Cerros del Sira has so far protected it from extensive human pressure within the core area of the Reserve. However, evidence of defaunation of other remote areas (Fa et al., Reference Fa, Peres and Meeuwig2002; Galetti & Dirzo, Reference Galetti and Dirzo2013) suggests that we cannot rely on isolation alone to protect this important conservation area. Fragmentation and isolation could be detrimental to the Cerros del Sira; surrounding areas must retain sufficient integrity and connectivity between key protected areas of the Oxapampa–Asháninka–Yánesha Biosphere Reserve to facilitate species migration and gene flow for viable populations.
Although the surrounding lowlands have been subject to widespread ecological degradation, the high-elevation areas still maintain a unique assemblage of lowland and highland tropical rainforest mammals, many of which are threatened or poorly known. The presence of these species indicates the Reserve has a high degree of ecological integrity and remains one of the few intact wilderness regions (Watson et al., Reference Watson, Evans, Venter, Williams, Tulloch and Stewart2018). Although the species in these highlands have thus far been protected from habitat loss, the increasing human population and demand for economic development are exerting increasing pressure on their habitats (Soh et al., Reference Soh, Sodhi and Lim2006). If such pressures are combined with climate change induced range shifts (Forero-Medina et al., Reference Forero-Medina, Terborgh, Socolar and Pimm2011), species losses may be catastrophic. There is a need for increased protection of the roadless, intact core area of the Reserve and to ensure the maintenance of connectivity to other key protected areas (Kearney et al., Reference Kearney, Adams, Fuller, Possingham and Watson2018). The development of plans in collaboration with local people and national park authorities to help create viable sustainable livelihoods that limit impacts on biodiversity could foster this, in addition to providing park rangers with the necessary facilities and resources to implement protection of the Reserve.
Acknowledgements
We thank all supporters of Exploration Sira: the Royal Geographical Society, with the Institute of British Geographers; the Neville Shulman Challenge Award; Idea Wild; crowdfunder campaign supporters (http://www.crowdfunder.co.uk/exploration-sira); the authorities of Sira Communal Reserve (SERNANP; permits N°004-2015-SERNANP-RCS & N°003-2016-SERNANP-RCS); Handykam; WildlifeKate; Bushnell Nature; the Scientific Exploration Society for the 2016 Cadogan Tate Explorer Award to AW; B.D. Patterson (Field Museum of Natural History, Chicago) for assistance in identification of Mustela frenata; and the Golondrinas community for providing us with expert local guides.
Author contributions
Study design and fieldwork: RPH, AW, CB, JSR; data analysis and writing: RPH, AW, CB.
Conflicts of interest
None.
Ethical standards
This research abided by the Oryx Code of conduct; the camera traps did not use flash, and the image of the hunter pictured in Plate 1 was anonymized.






