Introduction
The first Vibrio parahaemolyticus outbreak occurred in Japan in 1950, with 272 total cases including 20 deaths [Reference Fujino1], and was associated with boiled and semi-dried young sardine (Engraulis japonica Houttuyn) products (~50 kg) from a manufacturer in Japan. A new pathogen isolated from patients' samples and semi-dried young sardines were initially classified as Pasteurella parahaemolyticus but later as V. parahaemolyticus. Although V. parahaemolyticus has been a major foodborne pathogen for decades in Japan [2, 3], recently, the incidence of infections has been increasing in various countries of Asia [Reference Bag4–Reference Ottaviani9], Europe [Reference Martinez-Urtaza8, Reference Ottaviani9], North America [Reference Daniels10–Reference Newton13], South America [Reference González-Escalona14], and other countries [Reference Nair15].
The population of pathogenic V. parahaemolyticus, comprising thermostable direct haemolysin (TDH)- and/or TDH-related haemolysin (TRH)-positive strains, is generally smaller than that of non-pathogenic strains. In addition, it is difficult to distinguish pathogenic strains from non-pathogenic strains because their characteristics are similar. This contributes to the difficulties associated with surveying contamination and isolating pathogenic V. parahaemolyticus from seafood and environmental samples. However, since the mid-1990s, many studies have reported detection methods for pathogenic V. parahaemolyticus [Reference Bej16–Reference Ward and Bej19] and investigated contamination in various countries [Reference Bilung20–Reference Terzi, Büyüktanir and Yurdusev24]. Novel approaches to control V. parahaemolyticus infections were developed using data from such studies [25, 26]. In Japan, a council for V. parahaemolyticus control was established in 1998 to investigate effective regulatory actions to prevent V. parahaemolyticus infection, and the numbers of infections were successfully reduced. In this review, we describe the trends of V. parahaemolyticus infections and the regulations instituted by the Ministry of Health, Labour, and Welfare (MHLW) of Japan. Investigations on changes in V. parahaemolyticus contamination in seafood and the characteristics of V. parahaemolyticus isolates provide support for the positive impacts of such regulations.
Trends of V. parahaemolyticus infections in Japan
V. parahaemolyticus has been a dominant cause of foodborne infections in Japan since the 1960s, and infections have been continuously reported (Fig. 1). The most recent increase, since 1997, was coincident with a large wave of infections involving V. parahaemolyticus in Japan. The highest number (839) of outbreaks per year was recorded, and the number of cases each year reached 12 318 at the peak of infection in 1998. From 1999 to 2001, the MHLW discussed and established step-by-step regulations for seafood safety from the production stage to the consumption stage (Fig. 1). Subsequently, the number of cases and outbreaks of V. parahaemolyticus infections decreased by 99- and 93-fold, respectively, from 1998 to 2012 (Fig. 1). Such a marked decrease has never previously been recorded for V. parahaemolyticus infections.
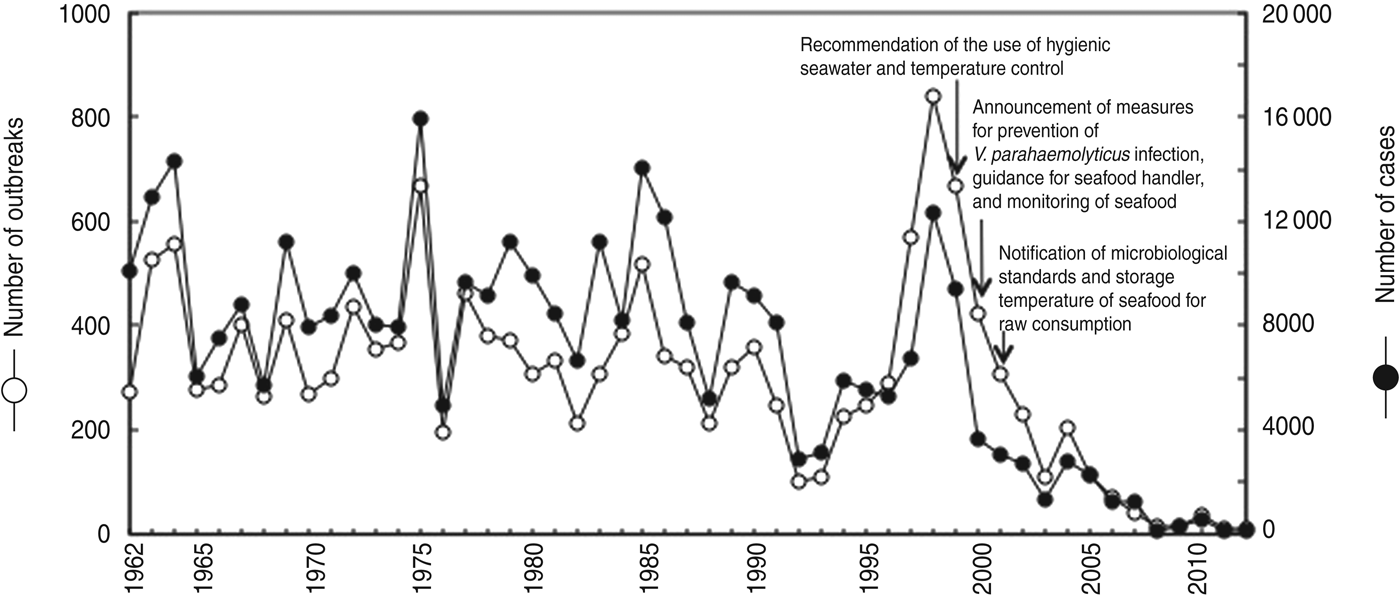
Fig. 1. Annual incidence of V. parahaemolyticus infections between 1962 and 2012. This figure was prepared from data published in the annual Handbook of Health and Welfare Statistics from the Ministry of Health, Labour, and Welfare, Japan.
Based on analysis of 3955 V. parahaemolyticus outbreaks from 1989 to 1999 by the Committee of Milk, Meat and Seafood for the Food Sanitation Investigation Council, 2391 food-related outbreaks were identified, and 92% of the 2391 outbreaks were related to seafood [Reference Kumagai27]. In particular, 49% of the 2391 outbreaks were associated with sashimi (pieces of raw fish fillet) and sushi (vinegary rice balls with raw fish fillet); outbreaks were also caused by shellfish (16%), cooked seafood (12%) and boiled seafood such as crab (10%) and sea urchin (5%). Seven large-scale outbreaks of more than 500 cases have been reported, although they occurred far less frequently than small-scale outbreaks (1002 outbreaks of 1–10 cases). In 1996, a large outbreak including 703 cases occurred in association with boiled crab sold by a seafood shop. In 1997, a large outbreak including 527 cases occurred in association with boxed lunches prepared by a restaurant. In 1998, two large outbreaks were reported; one including 1167 cases associated with boxed lunch served by a restaurant, and the other including 742 cases associated with boxed lunch. In 1999, two large outbreaks were reported; one including 674 cases associated with sushi prepared by a food manufacturer, and the other including 509 cases associated with boiled crab cooked by a food manufacturer. In 2007, a large outbreak including 620 cases occurred in association with salted squid produced by a food manufacturer. Furthermore, facilities related to 2391 outbreaks were analysed, and 48%, 18%, 12%, 12%, 4% and 6% were restaurants, hotels, caterers, shops making boxed lunches, home, seafood facilities and other facilities, respectively.
Based on a surveillance report of infections that occurred over 22 years in Shizuoka prefecture, Japan since 1963, the major serotypes initially involved in infections were O2:K3, O3:K7, O4:K8, O4:K12, O5:K15, and O4:K63 although other serotypes accounted for more than half of the subsequent infections [Reference Akahane28]. Nationwide surveillance by the National Institute of Infectious Disease (NIID) of Japan identified the serotypes of isolates from patients between 1990 and 1999. Until 1995, O1:K56, O4:K4, O4:K8, O4:K11, O4:K12, and O4:K63 were the predominant serotypes, and other serotypes accounted for more than half of the infections (Fig. 2a ) [Reference Hara-Kudo29]. The proportion of cases with serotype O3:K6 rapidly increased beginning in 1996, and this serotype became the most predominant serotype between 1997 and 1999. Serotype O3:K6 accounted for more than half of the total cases between 1997 and 1999, which was the first time that a particular serotype accounted for more than half of the total incidents. From 1998 to 2007, 31 local governments collected serotype data as part of a research project focusing on the V. parahaemolyticus serotypes involved in outbreaks [Reference Hara-Kudo29]. Serotype O3:K6 was determined to be responsible for about 50% of the total number of outbreaks reported each year (Fig. 2b ) [Reference Hara-Kudo29]. Serotypes O1:K25, O1:K56, O4:K8, and O4:K68 were also relatively dominant among more than 60 serotypes during this time. However, various serotypes were associated with each outbreak, and there was a decrease in the number of outbreaks independent of serotype. Thus, the increase in V. parahaemolyticus infections from 1996 to 1998 reflected mainly the increase in serotype O3:K6 infections, but the following decrease in V. parahaemolyticus infections reflected the decrease not only in serotype O3:K6 but also in other serotypes infections. The regulations by MHLW of Japan for seafood safety in 1999–2001 might achieve unprecedented results in the reduction of V. parahaemolyticus infections. In the next section, the regulations and the scientific foundation are introduced.

Fig. 2. Serotypes of V. parahaemolyticus isolates from humans between 1990 and 2008. (a) Nationwide surveillance by the National Institute of Infectious Disease in Japan between 1990 and 1999, and (b) surveillance by 31 local governments in Japan between 1998 and 2007.
Regulations for seafood safety
MHLW of Japan established step-by-step regulations for seafood safety from the production stage to the consumption stage in 1999–2001, and the four main points of these regulations were as follows:
-
Regulation 1: Hygienic seawater. Seafood handlers were advised to use disinfected or artificial seawater, or potable water to wash and process shellfish and finfish.
-
Regulation 2: Temperature control. The temperature for seafood during distribution and storage was recommended to be ⩽4°C, but was ultimately set at ⩽10°C.
-
Regulation 3: V. parahaemolyticus levels in seafood. The microbiological standards of V. parahaemolyticus levels in seafood were set at ⩽100 most probable number (MPN)/g for raw consumption, and non-detectable (ND) levels/25 g for ready-to-eat boiled seafood.
-
Regulation 4: Rapid consumption. Consumers were advised to consume seafood within 2 h after removal from the refrigerator. Restaurants were advised to serve seafood to consumers immediately after taking the seafood out of the refrigerator.
Scientific findings in surveys of V. parahaemolyticus contamination and experiments in vitro were used to formulate these regulations.
Regulation 1: Hygienic seawater
In 1999, 256 seafood markets throughout Japan responded to a questionnaire conducted by the MHLW on the seawater used in their facilities. The results showed that 90 (35%) of 256 seafood markets had used natural seawater obtained from the seacoast for washing, soaking, and preserving fish, and 72 (80%) of the 90 markets had used such natural seawater without sanitation treatment [Reference Kumagai30, Reference Hara-Kudo and Kumagai31]. Therefore the seafood handled in 28% of the seafood markets was considered to be possibly contaminated with V. parahaemolyticus derived from natural seawater.
Coastal seawater used in seafood markets was surveyed for the presence of the tdh gene and the total V. parahaemolyticus level in 1999 [32, Reference Shimada33]. The results showed that the frequency of tdh-positive V. parahaemolyticus increased depending on the total V. parahaemolyticus level. Over 70% of 29 seawater samples were positive for tdh when the total V. parahaemolyticus level was higher than 104 MPN/l. However, 28% of 157 seawater samples were positive for tdh even at low levels of total V. parahaemolyticus, from ND levels to 102 MPN/l. These findings suggested that coastal seawater was the most likely source of contamination of seafood with tdh-positive V. parahaemolyticus, of which the frequency may increase with total V. parahaemolyticus levels. Many outbreaks of V. parahaemolyticus were associated with boiled seafood such as crab. Because coastal seawater is often used to cool boiled seafood in processing plants, the boiled food might be contaminated with V. parahaemolyticus.
Regulation 2: Temperature control
Because temperatures <5°C are known to reduce V. parahaemolyticus population [34], temperatures ⩽4°C were initially recommended for seafood storage by the MHLW. However, this temperature was difficult for the seafood industry to accept because of the extra costs needed to install equipment for cooling at ⩽4°C instead of ⩽10°C. Therefore, to study the growth of V. parahaemolyticus including serotype O3:K6 strains at 10°C, ten strains of TDH-producing V. parahaemolyticus, five strains of serotype O3:K6, and one strain each of serotypes O1:K25, O1:K56, O4:K8, O4:K68, and O5:K15 were cultured in buffered peptone water (BPW) in triplicate with 3% NaCl at 10°C and 12°C, and the number of bacteria in the BPW was determined at 8 h, 24 h, 48 h, and 72 h. The growth results were compared between strains cultured at 10°C and 12°C. V. parahaemolyticus rapidly grew at 12°C after 8 h, and increased to 106·5 c.f.u./ml at 72 h, while growth at 10°C was slower and increased by 10- and 100-fold at 48 h and 72 h, respectively. There were significant differences between the growth of 10 strains of V. parahaemolyticus at 10°C and 12°C (P < 0·01). Seafood for raw consumption is generally consumed while the seafood is fresh, usually within 48 h, and therefore keeping fresh seafood at ⩽10°C was considered acceptable for raw consumption.
Regulation 3: V. parahaemolyticus levels in seafood
It is known that the population of TDH-producing V. parahaemolyticus in environments including seawater and seafood is much smaller than that of non-pathogenic V. parahaemolyticus, and therefore it is difficult to distinguish TDH-producing V. parahaemolyticus strains from a large number of non-pathogenic V. parahaemolyticus strains. Therefore, total V. parahaemolyticus was considered as an indicator to conduct an inspection test for contamination with TDH-producing V. parahaemolyticus. The level of total V. parahaemolyticus in seafood was surveyed in 2000. Most seafood samples (99·3% of 3641 samples), including seafood for raw consumption and boiled crab/boiled octopus, contained ⩽50 V. parahaemolyticus MPN/g food, and 0·6% samples contained >100 MPN/g [35]. It is indicated that the ⩽50 MPN/g regulation level would limit the sale and consumption of most seafood distributed in Japan.
To set up a microbiological standard for V. parahaemolyticus in seafood for raw consumption, the levels of total V. parahaemolyticus in seafood were sought based on data of tdh-positive and total V. parahaemolyticus contamination in seawater and seafood, and based on the assumptions that an infectious dose of TDH-producing V. parahaemolyticus is 100 cells/serving and that the amount of consumption of raw seafood is equivalent to 100 g/serving. In a study on the level of V. parahaemolyticus in seawater, the ratios of tdh-positive V. parahaemolyticus to total V. parahaemolyticus in six tdh-positive seawater samples were 1/14, 1/23, 1/150, 1/500, 1/700 and 1/1000 [Reference Sugiyama36]. The data on seawater were extrapolated to seafood contamination. If seafood contained total V. parahaemolyticus at a level of 101 cells/g, the levels of tdh-positive V. parahaemolyticus in seafood were calculated as 7·2, 4·4, 0·7, 0·2, 0·1 and 0·1 MPN/g (721, 439, 67, 20, 10 and 10 MPN/100 g). This indicates that 2/6 seafood samples cause V. parahaemolyticus infections because they contained over 100 tdh-positive V. parahaemolyticus cells/100 g. Therefore, a microbiological standard set at the level of 100 cells/g was expected to prevent V. parahaemolyticus infections caused by seafood contaminated with total V. parahaemolyticus at the level of 100 cells/g, although a microbiological standard set at 1000 cells/g would not prevent the infections at all. In another study using seven tdh-positive seawater samples, the ratios of tdh-positive V. parahaemolyticus to total V. parahaemolyticus were 1/23, 1/63, 1/600, 1/830, 1/1000, 1/5010 and 1/21400 [Reference Hara-Kudo and Kumagai31]. From calculations, it is indicated that 2/7 seafood samples cause V. parahaemolyticus infections if seafood contained total V. parahaemolyticus at a level of 100 cells/g. Therefore, this level of microbiological standard was also expected to reduce V. parahaemolyticus infections. The results of both studies suggest that a large number of V. parahaemolyticus infections would be prevented by setting a microbiological standard of total V. parahaemolyticus at a level of 100 cells/g, and also suggest that the impact of a level of ⩽1000 total V. parahaemolyticus cells/g as a microbiological standard would be far smaller than setting a level of 100 cells/g.
Furthermore, total V. parahaemolyticus levels in seafood associated with 11 outbreaks from 1998 were analysed. The contamination levels in 8/11 outbreaks were >100 V. parahaemolyticus MPN/g food (Table 1) [37], which suggested that the regulatory level of ⩽100 V. parahaemolyticus MPN/g is effective for food control. A level of ⩽10 MPN/g may be more effective, but huge amounts of seafood free from tdh-positive strains would have to be removed from raw consumption.
Table 1. Levels of total V. parahaemolyticus in seafood associated with outbreaks
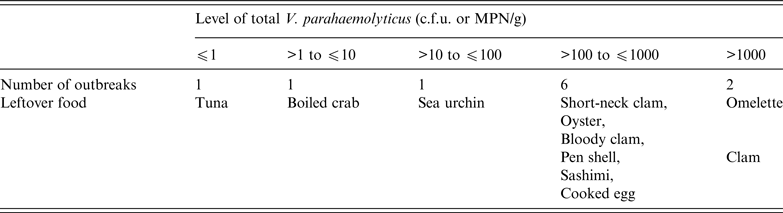
c.f.u., Colony-forming unit; MPN, most probable number.
Thus, the microbiological standard of V. parahaemolyticus levels for raw consumption was set at ⩽100 MPN/g seafood. In addition, the microbiological standard of V. parahaemolyticus levels for ready-to-eat boiled seafood were set at a ND level/25 g seafood because the population of V. parahaemolyticus in seafood markedly reduces to ND levels by boiling.
Changes in hygienic practices in seafood handling facilities
A total of 300 seafood retail shops, food service facilities, and restaurants in Shizuoka prefecture, Japan, were invited to complete a questionnaire, of which 244 shops responded to a survey on changes in food hygiene practices implemented after 2001 for securing seafood safety [Reference Kumagai38]. In total, 224/244 (91·8%) facilities responded to questions on alterations in the control by food safety agencies (Table 2, question a). Two-thirds of the facilities responded that the control of food safety agencies was strengthened after 2001. In addition, 231/244 (94·7%) facilities responded to questions on reforming or renovating kitchens (Table 2, question b). One-quarter of these had reformed or renovated their kitchens after 2001 but three-quarters of these answered that they performed no change. There may be financial constraints for improvements pertaining to seafood hygiene. Furthermore, 133/244 (55·2%) facilities responded to questions on seafood preservation methods (Table 2, question c). Half of the respondents stored seafood in a refrigerator just before serving. This indicates that importance of rapid consumption was widely known. A total of 168/244 (68·9%) facilities responded to questions related to cooking equipment (Table 2, question d). Four-fifths of these respondents had changed their cooking equipment, in particular replacing wooden cutting boards with plastic cutting boards, separating cutting boards for raw seafood from those used for other seafood, or using separate cooking knives for each kind of food item. A total of 114/244 (46·7%) facilities responded to questions on methods used to handle and cook seafood (Table 2, question e). Half of these respondents had changed their seafood purchasing and kitchen sanitation practices; they purchased only cleaned fish and often washed and hot water-sterilized their cutting boards and knives. The results of questions on cooking equipment, and methods used to handle and cook seafood suggest that preventing cross-contamination by hygienic cooking was implemented. The overall results of the survey suggested that food safety regulations led to improvements in food hygiene at seafood retail shops, food service facilities, and restaurants.
Table 2. Questionnaire on seafood regulations by prefecture governments in Japan, that was sent to seafood retail shops, food service facilities and restaurants
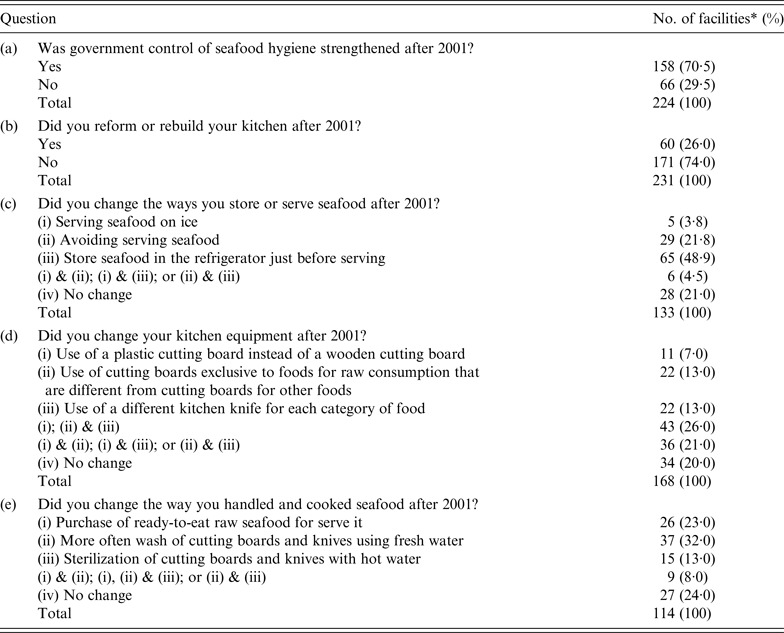
* A total of 244 facilities responded.
Changes in V. parahaemolyticus contamination in seafood after regulations were proposed
Frequencies of total and tdh-positive V. parahaemolyticus
Two studies comparing V. parahaemolyticus contamination in seafood between V. parahaemolyticus epidemic and non-epidemic periods were conducted with similar seafood targets using the same methods. A total of 329 samples in 2001 [Reference Hara-Kudo39] and 842 samples from 2007 to 2009 [Reference Hara-Kudo29] were qualitatively and quantitatively tested for tdh. Although seafood in Japan was highly contaminated with total V. parahaemolyticus in both periods (95·4% in 2001 and 85·2% from 2007 to 2009), contamination with tdh-positive V. parahaemolyticus was observed in 33 samples (10·0%) in 2001 and 65 samples (7·7%) from 2007 to 2009.
There were no significant differences between the frequencies of tdh-positive seafood samples in 2001 and those from 2007 to 2009. However, the number of cases and outbreaks decreased by 18- and 17-fold from 2001 to 2007–2009, respectively.
In a report on V. parahaemolyticus contamination in the western region of Japan between 2002 and 2004, about 80% of the seafood tested was found to be contaminated with V. parahaemolyticus, and 10·6% and 14·9% of the seafood was positive for tdh and trh, respectively [Reference Fukushima40]. TDH-producing V. parahaemolyticus and TRH2-producing bacteria were isolated from 3·7% and 6·2% of seafood samples, respectively. In another report on contamination in the central region of Japan, 11·7% of seafood samples tested was positive for tdh [Reference Miwa41]. However, both reports indicated that molluscs, such as short-neck clams, were highly tdh-positive, showing 27–35% positivity rates. In 2010, 5·1%, 18·6%, and 5·6% of seafood from various regions in Japan were positive for tdh, trh, and both tdh and trh, respectively (S. Saito et al., unpublished data). Although the surveys of these reports were conducted with samples from different regions, the frequency of trh was consistently higher than that of tdh.
Relationship between total and tdh-positive V. parahaemolyticus levels
The relationship between total and tdh-positive V. parahaemolyticus levels in seafood purchased in markets in 2001 [Reference Hara-Kudo39] and 2007–2009 [Reference Hara-Kudo29] was investigated in two studies.
The density of tdh-positive V. parahaemolyticus ranged from <0·3 to 9·3 MPN/g in the results in 2001 and from <0·3 to 2·1 MPN/g in the results from 2007 to 2009. The density of tdh-positive V. parahaemolyticus did not exactly correlate with the number of total V. parahaemolyticus. However, the incidence of tdh-positive V. parahaemolyticus was higher in samples contaminated with relatively high levels of total V. parahaemolyticus. The frequencies of tdh-positive V. parahaemolyticus in samples contaminated with total V. parahaemolyticus at a level >100 MPN/g (25·2% in 2001, 15·7% from 2007 to 2009) were about 10 and three times higher than those of tdh-positive samples below this level (2·5% in 2001, 5·8% from 2007 to 2009) (Table 3). The data suggest that regulating the levels of total V. parahaemolyticus to 100 MPN/g would reduce the consumption of seafood contaminated with tdh-positive V. parahaemolyticus. The rate of samples for which the total V. parahaemolyticus level exceeded 100 MPN/g was 31·8% (55/173) and 19·7% (166/842) in 2001 and 2007–2009, respectively. The reduction of rate was smaller than the decrease of V. parahaemolyticus foodborne infections. Seafood exceeding the suggested levels of V. parahaemolyticus is still distributed, but regulations such as temperature control might help to inhibit tdh-positive V. parahaemolyticus in the steps prior to consumption.
Table 3. The frequencies of tdh-positive seafood samples in food with total V. parahaemolyticus at levels of <100 MPN/g and >100 MPN/g in 2001 and 2007–2009
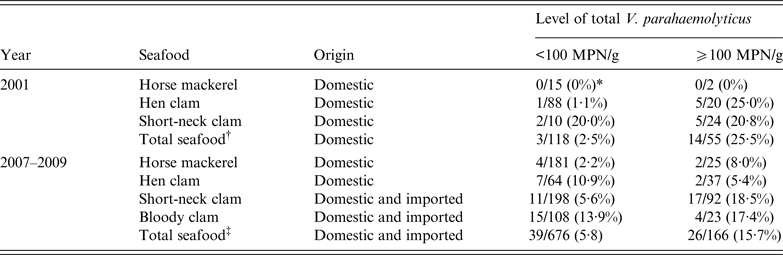
MPN, Most probable number.
* The number of tdh-positive samples/the number of samples tested.
† Total samples, including horse mackerel, hen clam, short-necked clam, rock oysters, scallops, and other molluscan shellfish.
‡ Total samples, including horse mackerel, hen clam, short-necked clam, bloody clam, rock oysters, scallops, and other molluscan shellfish.
Characteristics of V. parahaemolyticus isolates
The characteristics of tdh-positive, trh-negative and pandemic O3:K6 strains isolated from seafood and patients from 2007 to 2009 were analysed and compared to those of O3:K6 strains isolated from 1997 to 2006 [Reference Hara-Kudo29]. The strains were classified using the NotI and SfiI restriction profiles in pulsed-field gel electrophoresis analysis (Table 4). Four combinations of NotI and SfiI restriction profiles (NotI: 4, SfiI: b; NotI: 5, SfiI: b; NotI: 5, SfiI: f; NotI: 13, SfiI: g) were identical in strains isolated from seafood and patients. These findings demonstrate that the pandemic O3:K6 strains continued to inhabit areas in or around Japan up to 2009.
Table 4. Analysis of the characteristics of TDH-producing V. parahaemolyticus serotype O3:K6 from seafood and patients
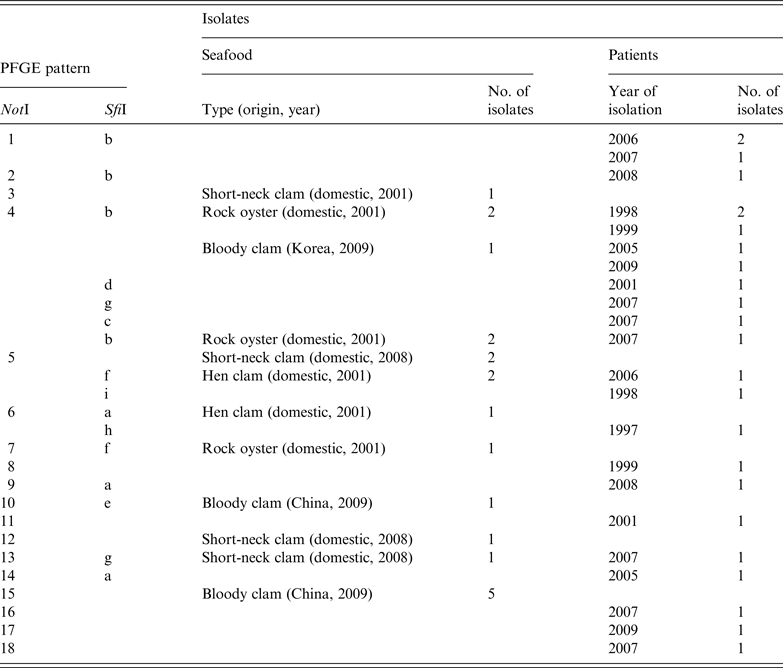
Furthermore, a study conducted in 2010 (S. Saito et al., unpublished data) showed that the serotype O3:K6 was still present in Japan because TDH-producing O3:K6 was isolated from 7/8 TDH-producing V. parahaemolyticus samples. All of these samples were from molluscs, such as the short-neck clam, and were from various regions throughout Japan. Therefore, it appears that TDH-producing O3:K6-contaminated seafood is currently being consumed in Japan.
Conclusions
V. parahaemolyticus has continued to cause many foodborne infections since the 1960s in Japan, and a large pandemic of O3:K6 infections in 1998 and a rapid decrease of V. parahaemolyticus by all serotypes including O3:K6 since 1999 was observed. We demonstrated that the rapid and considerable decrease in V. parahaemolyticus infections was not due to changes in the levels of V. parahaemolyticus contamination in seafood in association with possible natural changes in the prevalence of pathogenic V. parahaemolyticus. Instead, this marked decline can be attributed to a series of governmental regulations implemented from 1999 to 2001 that may have facilitated improvements in hygiene conditions throughout the supply chain, spanning the distribution to consumption stages of seafood. These regulations have probably contributed to reductions in V. parahaemolyticus infections caused by not only serotype O3:K6 but also other serotypes in Japan. Therefore, this review suggests that broadly implementing similar food regulations would help control foodborne infections with Vibrio spp., especially V. parahaemolyticus, throughout the world.
Acknowledgements
We thank the governments of the prefectures and cities, and research centres in Japan for their collaborations.
Declaration of Interest
None.









