The transition from childhood to adolescence and early adulthood is particularly important for the study of attention-deficit hyperactivity disorder (ADHD), a childhood-onset neurodevelopmental disorder that has long-term impact throughout the lifespan. 1 Despite high rates of persistence of ADHD, around a third of individuals no longer meet diagnostic criteria for ADHD during young adulthood and appear free of clinical impairment. Reference Faraone, Biederman and Mick2,Reference Biederman, Mick and Faraone3 Yet, there is a scarcity of research investigating the cognitive and neurobiological processes relating to the developmental pathways towards persistence or remission of ADHD. Reference Loo4 Identifying the mechanisms of ADHD remission may inform development of novel treatment strategies that improve the outcomes in ADHD.
Although longitudinal cognitive data on ADHD are limited and inconclusive, Reference van Lieshout, Luman, Buitelaar, Rommelse and Oosterlaan5 cross-sectional data on cognitive impairments and their aetiology in ADHD point to an aetiological separation of cognitive impairments in ADHD. Genetic model fitting analyses on two independent samples of ADHD and control sibling pairs consistently identified two familial cognitive impairment factors in ADHD: the first factor capturing increased reaction time variability (RTV) Reference Kuntsi, Wood, Rijsdijk, Johnson, Andreou and Albrecht6 and intra-individual variability of responses, Reference Frazier-Wood, Bralten, Arias-Vasquez, Luman, Ooterlaan and Sergeant7 and the second executive function impairments, including response inhibition Reference Kuntsi, Wood, Rijsdijk, Johnson, Andreou and Albrecht6 and working memory. Reference Frazier-Wood, Bralten, Arias-Vasquez, Luman, Ooterlaan and Sergeant7 Recent twin analyses further confirm the aetiological separation between RTV and inhibition. Reference Kuntsi, Pinto, Price, van der Meere, Frazier-Wood and Asherson8 In addition to the two (at least partially) separable familial cognitive impairment factors in ADHD, further largely separable aetiological influences underlie the association between ADHD and lower IQ. Reference Wood, Asherson, van der Meere and Kuntsi9–Reference Rommelse, Altink, Oosterlaan, Buschgens, Buitelaar and Sergeant11 Neurophysiological studies reveal atypical patterns of electroencephalogram (EEG) oscillatory activity Reference Snyder and Hall12 and attenuated event-related potential (ERP) activity, including measures of inhibition (nogo-P3 amplitudes), preparation (contingent negative variations, CNVs) and attention orientation (cue P3 amplitudes) in both children and adults with ADHD, Reference Albrecht, Brandeis, Uebel, Valko, Heinrich and Drechsler13–Reference McLoughlin, Asherson, Albrecht, Banaschewski, Rothenberger and Brandeis15 but data on EEG/ERP markers of persistence and remittance are as yet lacking.
The present study follows up individuals with childhood ADHD, who during childhood assessments demonstrated impairment in cognitive measures of RTV, Go/No-Go (GNG) task commission error (CE) and omission error (OE), Reference Kuntsi, Wood, Rijsdijk, Johnson, Andreou and Albrecht6,Reference Kuntsi, Pinto, Price, van der Meere, Frazier-Wood and Asherson8,Reference Andreou, Neale, Chen, Christiansen, Gabriels and Heise16,Reference Uebel, Albrecht, Asherson, Borger, Butler and Chen17 IQ Reference Wood, Rijsdijk, Johnson, Andreou, Albrecht and Arias-Vasquez10 and digit spans, Reference Cheung, Fazier-Wood, Asherson, Rijsdijk and Kuntsi18 and had a higher mean and variability of objectively measured actigraph movement intensity and count. Reference Wood, Asherson, Rijsdijk and Kuntsi19 We now aim to identify markers of underlying behavioural, cognitive and neurophysiological processes that relate to (a) an enduring deficit that continues to be impaired in those with childhood ADHD, irrespective of whether their ADHD symptoms have improved; and (b) remission of ADHD symptoms and associated impairments during the transition from childhood to adolescence/early adulthood. In addition to cognitive performance and actigraph measures, we focus on EEG frequency bands (delta, theta, alpha and beta) and ERP measures from the cued continuous performance task (CPT-OX) (CNV, cue-P3 and nogo-P3 amplitudes), which have previously demonstrated sensitivity to ADHD. Reference Albrecht, Brandeis, Uebel, Valko, Heinrich and Drechsler13,Reference McLoughlin, Asherson, Albrecht, Banaschewski, Rothenberger and Brandeis15 As well as defining ADHD outcome using a categorical diagnosis of persistence, we also examine ADHD symptoms and related impairments at follow-up as continuous traits.
Method
Participants
The sample consists of 279 participants, who were followed up on average 5.8 years (s.d. = 1.1) after initial assessments: 110 had a diagnosis of DSM-IV combined type ADHD in childhood (10 sibling pairs and 90 singletons) and 169 were control participants (76 sibling pairs and 17 singletons; online Table DS1).
Participants with ADHD were initially recruited from specialised ADHD clinics in the UK. Reference Kuntsi, Wood, Rijsdijk, Johnson, Andreou and Albrecht6,Reference Chen, Zhou, Sham, Franke, Kuntsi and Campbell20 Research diagnosis of DSM-IV combined type ADHD was established using the Parental Account of Children's Symptoms (PACS), a semi-structured, standardised, investigator interview with high interrater reliability. Reference Chen, Zhou, Sham, Franke, Kuntsi and Campbell20 The control group was initially recruited from schools in the UK, aiming for an age and gender match with the clinical sample. All participants were aged between 6 and 17 at the initial assessment. The same exclusion criteria were applied for all participants at the baseline childhood assessment: IQ<70, autism, epilepsy, general intellectual difficulties, brain disorders and any genetic or medical disorder associated with externalising behaviours that might mimic ADHD. Parents of all participants gave informed consent following procedures approved by the London-Surrey Borders Research Ethics Committee (09/H0806/58).
At follow-up, six control participants met DSM-IV ADHD criteria based on the parent-rated Barkley Informant Rating Scale Reference Kooij and Francken21 and six participants with ADHD had missing parent ratings of clinical impairments; these participants were therefore excluded from the analysis. Two participants with childhood ADHD, who did not meet ADHD symptom criteria but met clinical levels of impairment at follow-up, had different cognitive profiles compared with the other individuals from the remitted group, and were also excluded to minimise heterogeneity in the sample.
Among those with childhood ADHD diagnosis, 87 (79%) continued to meet clinical (DSM-IV) levels of ADHD symptoms and impairment and were classified as ADHD persisters, 23 (21%) were below the clinical cut-off and were classified as ADHD remitters. Of the ‘remitted’ individuals, 14 displayed five or more items on either the inattention or hyperactivity-impulsivity symptom domains, but did not show functional impairment (less than two domains). At follow-up, ADHD persisters, remitters and controls did not differ in age, but there were significantly more males in the remitted group than in the other two groups (Table 1). The follow-up duration for the persistent group ranged from 4.41 to 9.08 years (mean = 6.56; s.d. = 0.80), with 75% of participants assessed between 5 and 7 years. The remittent group were assessed between 4.50 and 8.54 years (mean = 6.39; s.d. = 0.94), with 70% of participants followed up within 5–7 years. The follow-up duration was not significantly different between the two groups (z = 0.80, P = 0.43).
Table 1 Group comparisons on age, gender, IQ, digit span, cognitive performance, ERP, EEG and actigraph measures

| F (d.f.) P | Cohen's d' | Cohen's d'
(IQ controlled) |
|
|---|---|---|---|
| Mean age (s.d.) | 1.45 | ||
| (2, 192) | |||
| 0.15 | |||
| Male, n (%) | 7.65 | ||
| (2, 192) | |||
| 0.02 | |||
| Cognitive measures | |||
| IQ | 22.35 | −0.99 a ** | |
| (2, 192) | −0.58 b * | ||
| <0.01 | −0.41 c | ||
| Digit span forward | 7.40 | −0.55 a ** | −0.23 a |
| (2, 192) | −0.36 b | −0.14 b | |
| <0.01 | −0.19 c | −0.04 c | |
| Digit span backward | 13.01 | −0.70 a ** | −0.34 a * |
| (2, 192) | −0.31 b | −0.08 b | |
| <0.01 | −0.40 c | −0.21 c | |
| RTV (CPT-OX) | 10.86 | 0.68 a ** | 0.48 a ** |
| (2, 192) | 0.55 b ** | 0.44 b * | |
| <0.01 | −0.08 c | −0.09 c | |
| RTV (fast task) | 31.57 | 1.23 a ** | 0.83 a ** |
| (2, 190) | 0.62 b ** | 0.44 b * | |
| <0.01 | 0.29 c | 0.19 c | |
| CE (CPT-OX) | 10.28 | 0.69 a ** | 0.47 a ** |
| (2, 191) | 0.28 b | 0.17 b | |
| <0.01 | 0.24 c | 0.19 c | |
| OE (CPT-OX) | 18.88 | 0.93 a ** | 0.63 a ** |
| (2, 191) | 0.77 b ** | 0.59 b ** | |
| <0.01 | 0.10 c | 0.04 c | |
| ERPs (CPT-OX) | |||
| CNV | 6.52 | 0.54 a ** | 0.45 a ** |
| (2, 190) | 0.42 b * | 0.39 b | |
| <0.01 | 0.07 c | 0.05 c | |
| Cue P3 | 3.68 | −0.37 a ** | −0.39 a ** |
| (2, 190) | −0.27 b | −0.30 b | |
| 0.03 | −0.02 c | −0.05 c | |
| No-Go P3 | 8.09 | −0.61 a ** | −0.57 a ** |
| (2, 188) | −0.22 b | −0.23 b | |
| <0.01 | −0.27 c | −0.27 c | |
| EEG frequency bands (CPT-OX) |
|||
| Delta | 4.84 | 0.44 a ** | 0.18 a |
| (2, 189) | 0.45 b * | 0.35 b | |
| <0.01 | −0.11 c | −0.18 c | |
| Theta | 3.89 | 0.35 a * | 0.12 a |
| (2, 189) | 0.50 b * | 0.36 b | |
| 0.02 | −0.14 c | −0.20 c | |
| Alpha | 3.28 | 0.39 a * | 0.29 a |
| (2, 189) | 0.22 b | 0.17 b | |
| 0.04 | 0.14 c | 0.13 c | |
| Beta | 2.36 | 0.33 a * | 0.18 a |
| (2, 189) | 0.15 b | 0.13 b | |
| 0.10 | 0.08 c | 0.04 c | |
| Actigraph movement | |||
| Mean intensity | 10.77 | 0.71 a ** | 0.59 a ** |
| (2, 169) | 0.60 b ** | 0.53 b * | |
| <0.01 | 0.04 c | 0.00 c | |
| Mean count | 13.77 | 0.87 a ** | 0.59 a ** |
| (2, 143) | 0.80 b ** | 0.70 b * | |
| <0.01 | 0.01 c | −0.03 c | |
ERP, event-related potential; EEG, electroencephalogram; CPT-OX, continuous performance task; RTV, reaction time variability; CE, commission errors; OE, omission errors; CNV, continuous negative variation.
a. ADHD persisters v. controls.
b. ADHD persisters v. ADHD remitters.
c. ADHD remitters v. controls.
Cohen's effect sizes (d') are presented without and with IQ included as a covariate.
Significant group differences are indicated in bold.
* P<0.05,
** P<0.01.
Almost half (47%) of the participants were under medication treatment for ADHD at the time of the follow-up assessment. Those who were on medication had significantly higher ADHD symptoms (F = 11.34, P<0.01) and exhibited more functional impairment (F = 5.22, P<0.01) than those who were not on medication at follow-up. However, the proportion of participants on medication at follow-up did not differ between the persistent and remittent groups (χ = 1.95, P = 0.16).
Procedure
Participants were re-contacted by telephone and scheduled for a follow-up clinical interview and a cognitive-EEG assessment with simultaneous actigraph assessment at the same research centre where the initial assessment took place. For those prescribed stimulants (n = 52), a 48-hour ADHD medication-free period was required prior to cognitive-EEG assessments. The total length of the test session, including breaks, was approximately 4 h. Participants received verbal and written information about the aims and procedures of the study and gave written informed consent.
Measures
The Diagnostic Interview for ADHD in adults (DIVA) Reference Kooij and Francken21
The DIVA is a semi-structured interview designed to evaluate the DSM-IV criteria for both adult and childhood ADHD symptoms and impairment. The DIVA was conducted by trained researchers with parents of the ADHD proband. Reference Barkley and Murphy22
The Barkley Functional Impairment Scale (BFIS) Reference Barkley and Murphy22
This 10-item scale is used to assess the levels of functional impairments commonly associated with ADHD symptoms in five areas of everyday life: family/relationship; work/education; social interaction; leisure activities; and management of daily responsibilities. Each item ranged from 0 (never or rarely) to 3 (very often).
Participants were classified as ‘affected’ at follow-up if they scored a ‘yes’ on ⩾ 6 items in either the inattention or hyperactivity-impulsivity domains on the DIVA, and they scored ⩾ 2 on two or more areas of impairments on the BFIS.
IQ and digit span
The vocabulary and block design subtests of the Wechsler Abbreviated Scale of Intelligence (WASI) Reference Wechsler23 were administered to all participants to derive an estimate of IQ. The digit span subtest from the Weschler Intelligence Scale for Children (WISC-III) Reference Wechsler24 or the Weschler Adult Intelligence Scale (WAIS-III) Reference Wechsler25 was administered to participants aged below 16 and aged 16 or above respectively, to obtain digit span forward (DSF) and backward (DSB). The forward test requires the participant to verbally repeat a sequence of digits in the straightforward order, and is a measure of short-term verbal memory.
Actigraph measures of activity level
The actigraph readings used in the current analyses were taken during the clinical interviews and cognitive-EEG assessments. The total length of the testing session was approximately 3 h, excluding a 30-minute unstructured break given approximately halfway through the session when actigraph measurements were not analysed. Two actigraph measures, which we previously showed to reliably distinguish between ADHD probands and controls (receiver operating characteristic-area under the curve = 0.61–0.79), Reference Wood, Asherson, Rijsdijk and Kuntsi19 were obtained from the dominant ankle of each participant: the mean intensity of movements (mean intensity), and the mean number of movements (mean count).
The Fast Task
The baseline condition consists of 72 trials, which followed a standard warned four-choice reaction time task. Reference Andreou, Neale, Chen, Christiansen, Gabriels and Heise16 Four empty circles (warning signals, arranged horizontally) first appeared for 8 s, after which one of them (the target) was coloured in. Participants were asked to press the response key that directly corresponded to the position of the target stimulus. Following a response, the stimuli disappeared from the screen and a fixed inter-trial interval of 2.5 s followed. Speed and accuracy were emphasised equally. If the participant did not respond within 10 s, the trial terminated. A comparison condition with a fast event rate (1 s) and incentives followed the baseline condition. Reference Andreou, Neale, Chen, Christiansen, Gabriels and Heise16 Cognitive performance measure of RTV from the baseline condition was included in this analysis, as this condition is more sensitive to ADHD. Reference Kuntsi, Frazier-Wood, Banaschewski, Gill, Miranda and Oades26
The cued flanker CPT-OX
This CPT includes rare cued Go and No-Go conditions embedded in a vigilance task with frequent distractors to assess both attentional and inhibitory processes. Reference Doehnert, Brandeis, Straub, Steinhausen and Drechsler27,Reference Valko, Doehnert, Muller, Schneider, Albrecht and Dreschler28 The test consists of 400 letters presented for 150 ms with an SOA (stimulus onset asynchrony) of 1.65 s in a pseudorandomised order at the centre of a computer monitor. The viewing distance to the monitor measured 120 cm at a vertical visual angle of approximately 0.5°. The cue letter O occurred with 20% probability (80 cue stimuli), signalled a GNG task, and induced response preparation. Participants pressed a mouse button with the index finger of their dominant hand as fast as possible every time the cue was followed directly by the letter X (O-X) target sequence (10% probability, 40 Go stimuli) but had to withhold responses to O-not-X sequences (No-Go trials, also 10%, 40 No-Go stimuli). Cognitive performance measures of RTV, CE, OE; EEG measures of delta, theta, alpha and beta power; and ERP amplitude measures of CNV, cue-P3 and nogo-P3 were obtained from this task.
EEG recording and processing
The EEG was recorded from 62 channels DC-coupled recording system (extended 10–20 montage), with a 500 Hz sampling rate, impedances kept under 10 kΩ, and FCz as the reference electrode. The electro-oculograms (EOGs) were recorded from electrodes above and below the left eye and at the outer canthi.
The EEG data were analysed using Brain Vision Analyser (2.0) (Brain Products, Germany). After down-sampling the data to 256 Hz, the EEG data were re-referenced to the average and filtered offline with digitally band-pass (0.1–30 Hz, 24 dB/oct) Butterworth filters. Ocular artefacts were identified from the data using Independent Component Analysis (ICA). Reference Jung, Makeig, Humphries, Lee, McKeown and Iragui29 The extracted independent components were manually inspected and ocular artefacts were removed by back-projection of all but those components. Data with other artefacts exceeding ± 100μV in any channel were rejected. No baseline subtraction was applied in line with previous ERP analyses on this task. Reference Albrecht, Brandeis, Uebel, Valko, Heinrich and Drechsler13–Reference McLoughlin, Asherson, Albrecht, Banaschewski, Rothenberger and Brandeis15 All averages contained at least 20 sweeps.
ERP analyses
The CNVs were analysed as mean amplitudes between 1300 and 1650 ms following cues over the central electrode (Cz). The cue-P3 had a parietal maximum and was defined as the most positive peak between 250 and 600 ms following cue trials at electrode Pz. The nogo-P3 was defined as the most positive peak between 250 and 600 ms following No-Go trials at electrode Cz.
EEG frequency analyses
We estimated the mean EEG power (μV2) in the delta (0.5–3 Hz), theta (4–7 Hz), alpha (7–12 Hz) and beta (12–30 Hz) bands using the Fast Fourier Transform (FFT). To reduce the number of statistical comparisons, we analysed the frontal location only, which has consistently been reported as sensitive to ADHD impairment, by computing the mean activity of electrodes (F1–F8, Fz).
Statistical analyses
We ran regression models with dummy variables to identify which measures showed an overall effect of group (ADHD persisters v. ADHD remitters v. controls), with controls as the reference group. On measures that indicated differences between ADHD persisters and controls, post hoc t-tests were conducted to examine the differences between ADHD persisters and remitters on these measures. As all three groups were matched on age at follow-up, this variable was not included as a covariate. We explored the effect of gender by re-running all analyses with the females (n = 15) removed; the pattern of results remained the same. Cohen's d effect sizes are presented along with means, standard deviations and test statistics for the group analyses (Table 1), where 0.2 is considered a small effect, 0.5 a medium effect and 0.8 a large effect. Pearson correlations were also conducted on these measures to examine their associations with DIVA ADHD symptom scores, and clinical impairment within those who had a childhood ADHD diagnosis, with age and gender included as covariates.
All analyses were conducted first without controlling for IQ; we then re-ran the analysis covarying for IQ to examine its potential effects. All cognitive measures and EEG frequency measures were skewed and log-transformed to normal in STATA version 10 (StataCorp, College Station, Texas, USA). We also controlled for genetic relatedness of the sibling pairs using the ‘robust cluster’ command in STATA. Reference Tye, Rijsdijk, Greven, Kuntsi, Asherson and McLoughlin30
Results
Means and standard deviations on all the measures in the ADHD-persistent, ADHD-remittent and control groups are reported in the data supplement (Table DS1).
Which measures show ADHD-control differences at follow-up?
ADHD-persistent and control group differences were observed on all measures (Table 1). For delta, theta, alpha and beta activity, as well as DSF, the ADHD-persistent v. control group difference was no longer significant, when IQ was included as a covariate (all P>0.05).
RTV (Fast Task), IQ, OE and actigraph mean count discriminated between ADHD-persistent and controls with a large effect size, whereas medium effect sizes were observed for actigraph mean intensity, RTV (from CPT-OX), digit spans (forward and backward), CE, nogo-P3 and CNV (Table 1). Other ERP and EEG measures, including cue-P3, delta, theta, alpha and beta activity had small effect sizes (Table 1). Controlling for IQ led to some reduction in effect sizes for most variables (Table 1); effect size was large now only for RTV from Fast Task.
Which processes are markers of recovery that distinguish between ADHD persisters and remitters?
ADHD remitters were significantly different from ADHD persisters, and not significantly different from controls, on measures of IQ, RTV, OE, CNV, delta and theta activity, actigraph intensity and count (Table 1, Fig. 1(a)). In addition, for cue-P3 amplitudes we observed a similar but non-significant pattern of findings: ADHD remitters were not significantly different from controls (Table 1 and Fig. 1(b)), and both the comparisons between the ADHD-persistent v. ADHD-remittent and between ADHD-persistent v. controls were of medium effect sizes, although the former was not significant (P = 0.18).

Fig. 1 Waveform event-related potentials and topographical maps for the (a) contingent negative variation (CNV) at central electrode (Cz) and (b) cue-P3 amplitudes at parietal electrode (Pz) in attention-deficit hyperactivity disorder (ADHD) persisters (dash), ADHD remitters (dot) and controls (solid).
As ADHD persisters had a lower IQ than ADHD remitters (Table 1), we re-ran the analyses while controlling for effects of IQ for all variables. The group differences between ADHD persisters and remitters remained significant for RTV (P = 0.03), OE, actigraph intensity and count (all P<0.01), but controlling for IQ diminished the group effects for CNV amplitude, delta and theta power which were no longer significant (Table 1). The effect sizes remained similar for alpha power and cue-P3 amplitude when controlling for IQ.
Which processes continue to be impaired in those with childhood ADHD diagnosis, irrespective of current ADHD status?
The full requirement for an enduring deficit would be a significant ADHD-remittent v. control group difference but no ADHD-persister v. ADHD-remittent group difference. Here, although ADHD remitters were not significantly different from ADHD persisters on several of the measures, none of the measures showed significant differences between the ADHD remitters and controls (Table 1). Therefore, none of the processes investigated in this study fulfilled the strict criteria for enduring deficits, when using categorical diagnoses.
However, several variables did not differ significantly between ADHD persisters and remitters, and the effect size for the ADHD remittent v. control comparison was comparable to the effect size of the ADHD persistent-remittent comparison (around 0.30). Such a pattern, where the ADHD remitters are in the middle, in between the other two groups, was observed for DSB, CE and nogo-P3 (Fig. 2). With IQ as a covariate, the pattern remained unchanged for CE and nogo-P3 amplitudes, although the effect size for the ADHD persistent-remittent comparison on DSB reduced from 0.31 to 0.08 (Table 1).
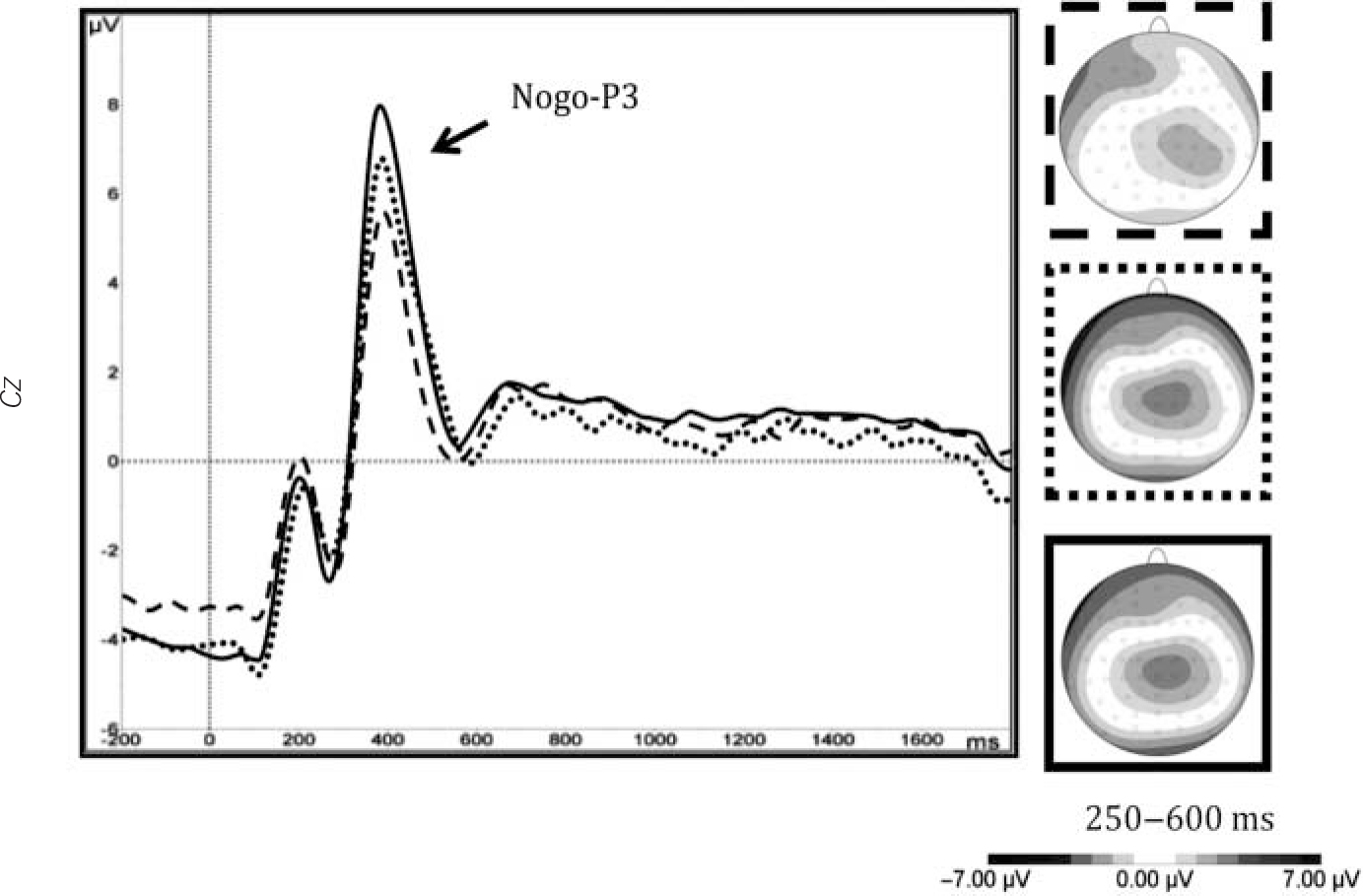
Fig. 2 Waveform event-related potentials and topographical maps for nogo-P3 at central electrode (Cz), in attention-deficit hyperactivity disorder (ADHD) persisters (dash), ADHD remitters (dot) and controls (solid).
Which processes are associated with ADHD symptoms and clinical impairment at follow-up within those who had a childhood ADHD diagnosis?
ADHD symptoms and impairment at follow-up correlated significantly with IQ, RTV (from both tasks), OE, delta activity and actigraph count, whereas actigraph intensity was associated only with impairment (Table 2). No other significant associations were observed.
Table 2 Pearson correlations (two-tailed) of IQ, digit span, cognitive performance, ERP, EEG and actigraph measures with interview-based DIVA ADHD symptoms and clinical impairment within the ADHD group only (n = 110), without controlling for IQ

| ADHD symptoms | Impairment | |||
|---|---|---|---|---|
| r | P | r | P | |
| IQ | −0.26 | <0.01 | −0.27 | <0.01 |
| Digit span forward | −0.07 | 0.50 | −0.11 | 0.24 |
| Digit span backward | −0.12 | 0.20 | −0.13 | 0.19 |
| RTV (CPT-OX) | 0.25 | <0.01 | 0.19 | 0.05 |
| RTV (fast task) | 0.26 | 0.01 | 0.26 | <0.01 |
| Commission errors | −0.01 | 0.99 | 0.17 | 0.08 |
| Omission errors | 0.19 | 0.05 | 0.27 | <0.01 |
| CNV | 0.03 | 0.80 | 0.12 | 0.24 |
| Cue P3 | −0.10 | 0.36 | −0.12 | 0.23 |
| No Go P3 | −0.07 | 0.48 | −0.04 | 0.72 |
| Delta | 0.21 | 0.04 | 0.27 | 0.01 |
| Theta | 0.08 | 0.43 | 0.07 | 0.46 |
| Alpha | 0.06 | 0.56 | 0.07 | 0.45 |
| Beta | −0.06 | 0.53 | 0.09 | 0.37 |
| Movement intensity | 0.20 | 0.07 | 0.23 | 0.03 |
| Movement count | 0.33 | <0.01 | 0.36 | <0.01 |
ERP, event-related potential; EEG, electroencephalogram; DIVA, Diagnostic Interview for ADHD in adults; ADHD, attention-deficit hyperactivity disorder; CPT-OX, continuous performance task; RTV, reaction time variability; CNV, continuous negative variation. Significant correlations are indicated in bold.
When we re-ran the analysis with IQ as a covariate in addition to gender and age, RTV from the CPT-OX was no longer significantly associated with impairment, and OE and delta were no longer associated with ADHD symptoms (online Table DS2). All the other correlations remained significant. Of the variables on which ADHD remitters were in-between ADHD persisters and controls, the expected lack of association with ADHD symptoms was observed for No-Go P3 amplitudes, CE and DSB, with correlations non-significant and low at −0.01 to −0.12.
Discussion
This follow-up study of 110 adolescents and young adults with childhood combined type ADHD and 169 controls identified three cognitive processes in relation to ADHD outcome. The first process encompasses preparation-vigilance measures (OE, RTV, CNV, delta and theta activity, and a trend for cue-P3 amplitude and alpha activity), as well as objectively measured physical activity (actigraph intensity and count), which are no longer impaired in individuals whose ADHD symptoms improve and represent markers of remission. As these processes are associated with improvement in ADHD, they may also potentially mediate ADHD outcome; further studies are required to investigate this possibility. The second process involves executive control processes of inhibition and working memory (commission errors (CE), nogo-P3 and DSB), on which ADHD remitters lie intermediate between ADHD persisters and controls, though were not significantly different from either group. These markers of executive control were not associated with follow-up ADHD symptoms or impairment.
IQ represents a third process, as a potential moderator of ADHD outcome. We previously found childhood IQ to predict future ADHD outcome in the present sample, whereas other cognitive variables, such as RTV and CE, did not. Reference Cheung, Rijsdijk, McLoughlin, Faraone, Asherson and Kuntsi31 In this analysis, moderator variables reflect baseline characteristics that predict change in ADHD symptoms over development. This is different from mediating variables, which reflect mediating effects that explain the change in ADHD symptoms such that change in a mediator would predict change in ADHD. Our findings suggesting that IQ is a moderator rather than a mediator of ADHD outcome is also consistent with findings from longitudinal treatment studies, which report positive associations between childhood IQ in ADHD and treatment response. Reference Handen, Janosky and McAuliffe32,Reference Owens, Hinshaw, Kraemer, Arnold, Abikoff and Cantwell33 In the current analyses we further demonstrate that ADHD remitters have a higher IQ than ADHD persisters at follow-up. Aetiological influences on ADHD and IQ have also been shown to largely separate from those on the other cognitive impairments in ADHD. Reference Wood, Asherson, van der Meere and Kuntsi9–Reference Rommelse, Altink, Oosterlaan, Buschgens, Buitelaar and Sergeant11 Overall, the convergent findings emphasise the role of IQ in the developmental course of ADHD, and demonstrate the potential risk of poor outcome in children with concurrent ADHD symptoms and low IQ. In the present analyses IQ differences between the groups accounted also for some of the observed group differences on verbal short-term memory (DSF) and EEG activity across the frequency bands.
With regard to the first two processes, our results are largely consistent with the previously observed separation of ADHD-related impairments into executive function v. preparation-vigilance processes. Reference Kuntsi, Wood, Rijsdijk, Johnson, Andreou and Albrecht6,Reference O'Connell, Dockree, Bellgrove, Turin, Ward and Foxe34,Reference Johnson, Kelly, Bellgrove, Barry, Cox and Gill35 Although a distinction between potential top-down cognitive control and bottom-up arousal regulation was also made in a previous developmental ADHD model, Reference Halperin and Schulz36,Reference Halperin, Trampush, Miller, Marks and Newcorn37 the pattern observed in our data is not consistent with the model's predictions of how the impairments map onto persistence and remittance. The present study adds to existing knowledge by drawing direct comparisons between ADHD persisters and remitters, with an inclusion of both cognitive and neurophysiological measures, as well as a long follow-up period. Our data suggest that the preparation-vigilance markers, rather than executive control processes, are markers of remission in ADHD.
Previous observations of ADHD-sensitive improvement in RTV but not in inhibitory deficits following incentives Reference Uebel, Albrecht, Asherson, Borger, Butler and Chen17,Reference Banaschewski, Jennen-Steinmetz, Brandeis, Buitelaar, Kuntsi and Poustka38,Reference Kuntsi, Wood, Van Der Meere and Asherson39 are also consistent with our findings that, relative to executive control processes such as inhibition, RTV and related measures may reflect a more malleable process and show a stronger association with the improvement of ADHD symptoms. An important direction for future research will be to link the cognitive and EEG markers of remission and persistence to the interdependent but partially separate neural networks identified in functional magnetic resonance imaging studies on ADHD, which include the frontal-parietal network, the default-mode network and the ventral-attentional network. Reference Castellanos and Proal40,Reference Cortese, Kelly, Chabernaud, Proal, Di Martino and Milham41
Our further analyses on continuous measures of ADHD outcome confirmed the association of IQ, RTV, OE, delta and actigraph movement count with both ADHD symptoms and impairment at follow-up, and the lack of such an association for DSB, CE and nogo-P3 amplitudes. Exceptions to the pattern expected based on group comparisons were obtained for theta activity and CNV. The findings for OE in relation to the underlying process that it captures are less consistent than for other cognitive performance variables: whereas the present data on OE merging with RTV rather than CE is consistent with previous studies on the arousal-attention model, Reference Johnson, Kelly, Bellgrove, Barry, Cox and Gill35 in our two-factor familial model OE merged with CE at the familial level (although at the level of individual-specific environmental influences OE loaded both onto the ‘RTV’ and ‘CE’ factors). Reference Kuntsi, Wood, Rijsdijk, Johnson, Andreou and Albrecht6
A limitation of this study is that the sample covers only adolescence and young adults, where some younger individuals are still undergoing fundamental changes in brain development. Although, importantly, our study groups were matched for age, it would be informative to examine the hypotheses again in future follow-up assessments when all participants have reached adulthood and more ADHD participants may have remitted.
Overall, our findings and evidence from earlier research raise the possibility that cognitive impairments in ADHD reflect (at least) three processes: markers of recovery, potential moderators of ADHD outcome and processes that are not significantly associated with ADHD outcome in adolescence and early adulthood. Although these possibilities await rigorous testing in future studies, the pattern would fit with the previously identified aetiological separation of the cognitive impairments in ADHD into three main groups (response variability, lower IQ and executive function impairments), Reference Kuntsi, Wood, Rijsdijk, Johnson, Andreou and Albrecht6,Reference Frazier-Wood, Bralten, Arias-Vasquez, Luman, Ooterlaan and Sergeant7 and raises intriguing questions on possible links to the neuroimaging networks identified in ADHD. Reference Castellanos and Proal40,Reference Cortese, Kelly, Chabernaud, Proal, Di Martino and Milham41 For both researchers and clinicians, these findings highlight the importance of a developmental approach to ADHD. Based on these data, the strongest candidates for the development of non-pharmacological interventions involving cognitive training and neurofeedback are the preparation-vigilance processes that we identified as markers of ADHD remission.
Funding
This project was supported by generous grants from Action Medical Research and the Peter Sowerby Charitable Foundation (grant GN1777 to J.K.). Initial cognitive assessments of the ADHD and control groups in childhood, and the recruitment of the control sample were supported by UK Medical Research Council grant G0300189 to J.K. Initial sample recruitment of the ADHD group was supported by NIMH grant R01MH062873 to SV Faraone.
Acknowledgements
We thank all who make this research possible: our participants and their families; Jessica Deadman, Hannah Collyer and Sarah-Jane Gregori.







eLetters
No eLetters have been published for this article.