Introduction
Prior to global roll-out of rotavirus vaccines, rotavirus was the leading cause of severe diarrhoea in infants and children [Reference Parashar1, Reference Tate2]. Global surveillance estimates from 2009, before widespread vaccination, indicated the median prevalence of rotavirus among children hospitalised for gastroenteritis was 36% (range among countries: 12–68%) [3]. In the pre-vaccine era, almost every child in the world was thought to experience rotavirus infection [Reference Glass4, Reference Shaw5] and about one in every 260 children would die as a result of the infection [Reference Tate2]. Although the incidence of rotavirus gastroenteritis (RVGE) is similar in high-, middle- and low-income countries, 80–90% of rotavirus-associated deaths occur in the world's poorest countries [Reference Parashar1].
As of 2009, the World Health Organization recommended rotavirus vaccination for all infants [6]. There are two live, oral rotavirus vaccines used broadly across the globe: a monovalent (Rotarix™, GlaxoSmithKline Biologicals, Rixensart, Belgium) and pentavalent vaccine (RotaTeq™, Merck & Co., Inc.; Kenilworth, NJ, USA). While high protective 1-year efficacy (96–98%) against severe RVGE has been reported in high-income countries (HICs) [Reference Ruiz-Palacios7–Reference Vesikari9], the efficacy has been much lower (51–64%) in trials conducted in low- and middle-income countries (LMICs) [Reference Madhi10–Reference Zaman12].
One potential intervention that may increase the effectiveness of rotavirus vaccines in LMICs is altering the vaccine schedule (number and/or timing) of rotavirus vaccines doses. Recent research suggests delaying the start of the rotavirus vaccine series may result in some gains in vaccine efficacy [Reference Madhi10, Reference Armah13–Reference Gruber15], possibly due to less interference from transplacental antibodies [Reference Armah13, Reference Ali16]. However, first RVGE episode is thought to occur early in children in LMICs (median 6–9 months) [Reference Glass17]. Consequently, delaying vaccination could result in severe RVGE occurring prior to administration of the vaccine.
Therefore, it is important to understand the timing of severe RVGE among unvaccinated children to be able to weigh the potential advantages and disadvantages of delaying vaccination schedules. Although there have been a number of studies investigating the natural history of rotavirus [Reference Espinoza18–Reference Steele23], these studies have not provided data on the cumulative incidence of severe RVGE over the first few years of life. In this study, we analysed the placebo arms of two large rotavirus vaccine trials conducted in LMICs to better understand the timing and predictors of first severe RVGE episodes among unvaccinated children in LMICs.
Methods
Parent study data
This was an analysis of Phase III, placebo-controlled, multicentre randomised trials of monovalent (RV1) and pentavalent (RV5) rotavirus vaccines in LMICs [Clinical Trial Numbers: NCT00241644 (RV1) and NCT00362648 (RV5)]. Each trial has been described previously [Reference Madhi10, Reference Armah11, Reference Steele24]. A brief overview of each trial follows.
In the RV1 trial, healthy infants, 5–10 weeks of age, were randomly assigned to receive doses of vaccine or placebo at approximately 6, 10 and 14 weeks of age and were followed for 1–2 years of age. Beginning at enrolment, there was active surveillance of any gastroenteritis through weekly visits to parents or guardians to collect diary cards and through visits to health clinics that served the populations. Gastroenteritis was defined as three or more stools that were looser than normal within a 24 h period. Stool samples were collected during any episode of gastroenteritis and were analysed for the presence of rotavirus antigens using enzyme-linked immunosorbent assays (ELISA) (Rotaclone, Meridian Bioscience) with reverse transcription polymerase chain reaction (RT–PCR) confirmation. Severity was defined using the 20-point Vesikari clinical score for PCR-confirmed RVGE episodes [Reference Ruuska and Vesikari25].
In the RV5 trial, enrolled infants, 4–12 weeks of age, were randomly assigned to receive either three doses of pentavalent rotavirus vaccine or placebo at approximately 6, 10 and 14 weeks of age and were followed for approximately 2 years. There was active surveillance at local clinics and hospitals for any occurrence of gastroenteritis occurring after study entry. Gastroenteritis was defined as three or more stools that were watery or looser than normal within a 24-h period, or forceful vomiting. Stool samples and patient histories were collected from infants presenting with symptoms of gastroenteritis. Stool samples were analysed for the presence of rotavirus antigens using an enzyme immunoassay with RT–PCR confirmation. The severity of disease was determined using the 20-point modified Vesikari clinical score for infants with PCR confirmed RVGE [Reference Zaman12, Reference Ruuska and Vesikari25, Reference Armah26].
Study data
In this analysis, the objective was to describe the timing and predictors of first severe RVGE episodes among those not receiving the rotavirus vaccine; therefore, only the placebo arms of each trial were analysed. There were 1641 and 3753 infants randomised to receive only the placebo treatment in the RV1 trial (cohort 1) and the RV5 trial (cohort 2), respectively. In cohort 1, 27 (1.6%) infants were excluded because they were not randomised, their randomisation code was broken at the investigator site, the study vaccine dose was not administered according to the protocol, or they did not have at least one day of follow-up. In cohort 2, one infant (0.02%) was excluded because he or she received at least one dose of placebo. Each cohort was analysed separately, but the results are presented in parallel. This research was approved by the University of North Carolina at Chapel Hill Institutional Review Board.
Statistical analysis
Prior to analysis, we categorised all available data for variables measured at enrolment including demographic information, breastfeeding and growth status, prior or current infection, prior or current antibiotic use, routine vaccinations and severe RVGE. Breastfeeding status was classified as exclusive vs. non-exclusive. We classified relevant nutrition indicators by using underweight, stunted and wasting criteria specified by the World Health Organization (WHO) [27]. Infant body length was not recorded in Bangladesh; therefore, stunted and wasting status were not determined for Bangladeshi infants. We also classified prior or current antibiotic use using data collected in medical histories taken at baseline (e.g. enrolment). Topical antibiotics were not included in prior or current antibiotic use. Routine vaccines were also classified to determine the number of doses received prior to or at enrolment for all vaccines except Bacillus Calmette–Guérin vaccine (BCG), which was classified based on the receipt before enrolment. Severe RVGE was defined as a Vesikari or modified-Vesikari score of >11. We also determined the age at first severe RVGE episode. For cohort 1, the age of enrolment was provided in weeks; therefore, the age of severe RVGE may not be exact, but within 6 days of the actual age at which the episode occurred.
To describe the timing of first severe RVGE episodes among children in LMICs, we estimated the incidence rates and cumulative incidence of first episodes of severe RVGE for each country as well as the age distributions of first severe RVGE episodes by cohort. Specifically, rates and exact 95% confidence intervals [Reference Ulm28] were estimated as the number of first severe RVGE episodes from enrolment through one to 2 years of follow-up divided by the person-time accumulated. To estimate the cumulative incidence and 95% confidence interval of first severe RVGE episodes, we obtained the complement of the extended Kaplan–Meier survival curve overall and stratified by country. Use of the extended Kaplan–Meier survival curve allowed for late entry on an age-specific time scale [Reference Klein and Moeschberger29]. Follow-up began at 6 weeks of age and continued until the first episode of severe RVGE occurred or the infant was censored. Any infant who was recruited into either study before 6 weeks of age began accumulating person-time at 6 weeks of age. Follow-up time in cohort 1 is within 6 days of the exact number of days followed from 6 weeks of age because age at enrolment was provided in weeks and follow-up was provided as days from enrolment. Finally, among those experiencing a severe RVGE episode, we described the median and interquartile range (IQR) of the age of onset of first severe RVGE episodes by cohort.
We were interested in what baseline patient characteristics affected the rate of severe RVGE using the data that were available in each cohort. To be evaluated as a potential predictor of first severe RVGE episodes, we required at least ten severe RVGE episodes within each stratum of each factor. Baseline factors considered in both cohorts were sex (female/male), underweight status (yes/no), prior or current infection (yes/no) at enrolment, prior or current antibiotic use (yes/no) at enrolment, and receipt of other vaccines. Other vaccines included were BCG receipt prior to enrolment (0 vs. at least one); diphtheria–tetanus–pertussis–Hepatitis B and –Haemophilus influenza B vaccine (DTP–HB/HIB) or diphtheria and tetanus toxoids and acellular pertussis vaccine (DTaP) and Hepatitis B vaccine (HB) receipt prior or at enrolment (0 vs. at least one); and oral poliovirus vaccine (OPV) receipt prior or at enrolment (one or fewer doses vs. two). Stunted (yes/no) and wasting (yes/no) were considered as potential predictors in only cohort 1 while exclusive breastfeeding (yes/no) was considered as a potential predictor in only cohort 2.
We estimated the association between the baseline factors described above and rates of first severe RVGE episodes using a Cox proportional hazards model with the exact method to analyse tied episodes. We assessed the proportional hazards assumption by inspecting the plot of log(time) and log[−log(Survival)] for each variable. Similar to the methods described above for estimating the incidence rates, follow-up began at 6 weeks of age for both cohorts with late entry adjustment for those enrolled later. If more than 10% of participants in either cohort discontinued follow-up, we examined the potential for differential dropout (right censoring) within each level of each predictor to determine if censoring could be informative.
Crude and adjusted hazard ratios (HR) and 95% confidence intervals were estimated and were considered statistically significant at a cut-off of α = 0.05. Due to the low number of severe RVGE episodes, we analysed each cohort separately, adjusted for the country in the multivariable model, rather than fit individual models for each country. As a sensitivity analysis, we estimated the crude and adjusted HRs for cohort 2 excluding Mali and Kenya, because there were problems with gastroenteritis surveillance in those countries [Reference Sow30].
All analyses were performed using SAS Clinical Trial Data Transparency (Version 4.5.2; SAS Institute Inc., Cary, NC, USA).
Results
There were 1614 and 3752 children included in the analysis from cohort 1 and two, respectively (Table 1). The median lengths of follow-up to censoring or first severe RVGE episode were 327 and 518 days for cohorts 1 and 2, respectively (Table 2). The majority of infants in both cohorts were African and enrolled at 6–7 weeks of age. In cohort 1, about 20% of children were stunted and about 5% were underweight and 5% had wasting. In cohort 2, about 10% were stunted, 10% were underweight and about 20% had wasting. Prior or current infection at enrolment was reported for about 5% and 20% of infants in cohorts 1 and 2, respectively. Prior or current antibiotic use at enrolment was reported for about 10% and 5% of cohorts 1 and 2, respectively.
Table 1. Characteristics of the placebo arms (cohorts 1 and 2) at enrolment
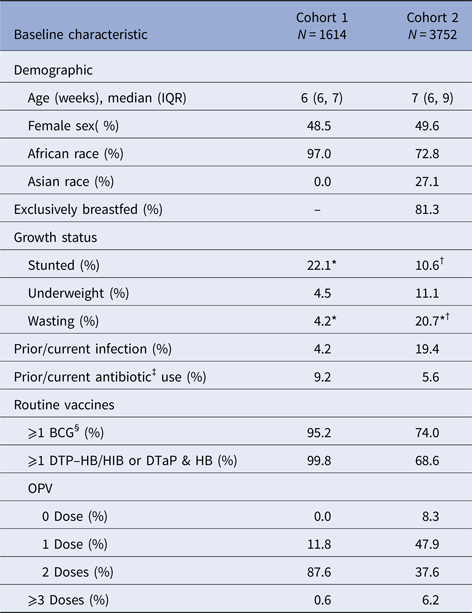
IQR, interquartile range; BCG, Bacillus Calmette–Guérin vaccine; DTP–HB/HIB, diphtheria–tetanus–pertussis–Hepatitis B and –Haemophilus influenza B vaccines; DTaP, diphtheria and tetanus toxoids and acellular pertussis vaccine; HB, Hepatitis B vaccine; OPV, oral polio vaccine; GMC, geometric mean concentration.
* Missing one to 15 observations.
† Excluding Bangladesh.
‡ Excluding topical antibiotics.
§ Administered prior to enrolment.
Table 2. Rates of first severe RVGE by cohort and country
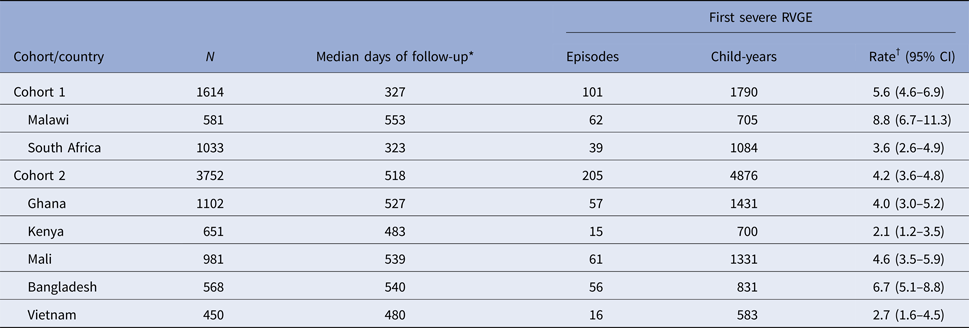
RVGE, rotavirus gastroenteritis.
* From enrolment.
† Per 100 Child-years.
There were 101 and 205 first episodes of severe RVGE in cohorts 1 and 2, respectively (Table 2). The overall incidence rates of first severe RVGE episodes were 5.6 (95% CI 4.6–6.9) and 4.2 (95% CI 3.6–4.8) per 100 child-years in cohorts 1 and 2, respectively. The cumulative incidence and 95% confidence interval (CI) of first severe RVGE episodes at different ages are presented in Table 3. The median and IQR of the age of onset for first severe RVGE episodes was 33.3 weeks (IQR: 23.6, 47.3) in cohort 1 and 52.9 weeks (IQR: 34.1, 69.1) in cohort 2. There was variability in the cumulative incidence by country (Fig. 1).
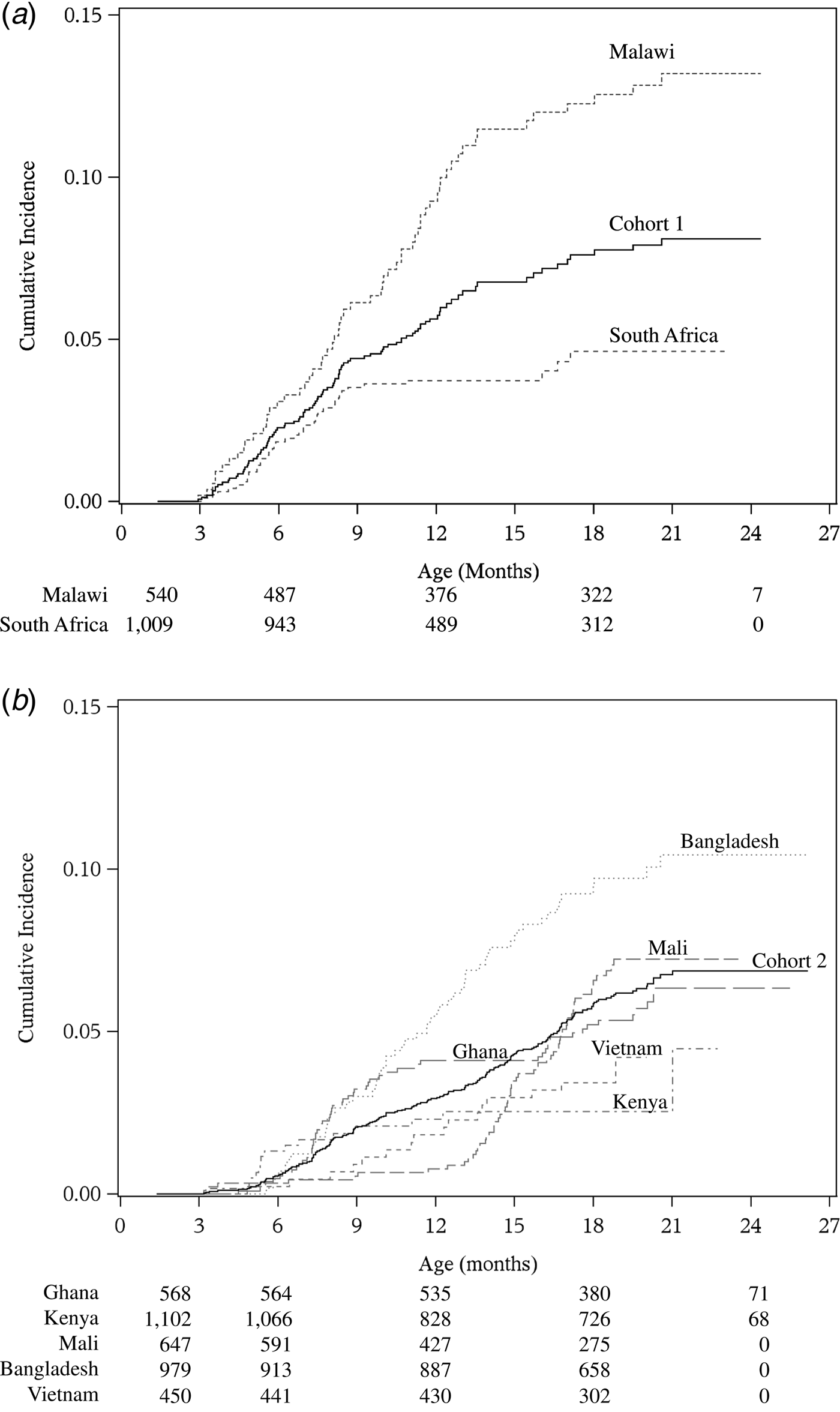
Fig. 1. Cumulative incidence of severe rotavirus gastroenteritis from 6 weeks of age in cohort 1 (a) and cohort 2 (b). Number at risk at the start of follow-up and at 6 months intervals is labelled at corresponding time points for each country below the x-axis.
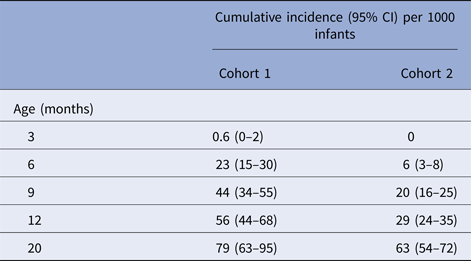
Table 3. Cumulative incidence of first severe RVGE episodes at different ages in cohorts 1 and 2
Prior or current antibiotic use at enrolment was associated a twofold increase in the rate of first episodes of severe RVGE [adjusted HR: 2.03 (95% CI 1.18–3.48)] compared with those with no use in cohort 1 (Table 4). In cohort 2, the direction of the association was similar with the rate of first severe RVGE episodes estimated at about one and half times the rate in those with prior or current antibiotic use [adjusted HR: 1.41 (95% CI 0.80–2.51)] compared with those with no use. In addition, in cohort 1, females had a higher rate of severe RVGE compared with males [adjusted HR: 1.43 (95% CI 0.96–2.12)]. No variables in cohort 2 were strongly associated with first episodes of severe RVGE, but there was a lower rate of first severe RVGE episodes among those who did not receive BCG before enrolment compared with those who received one or more doses of BCG [adjusted HR: 0.65 (95% CI 0.35–1.21)]. Results including and excluding Kenya and Mali in the analysis were similar (Supplementary Table S1).
Table 4. Predictors of first severe RVGE episodes in cohorts 1 and 2
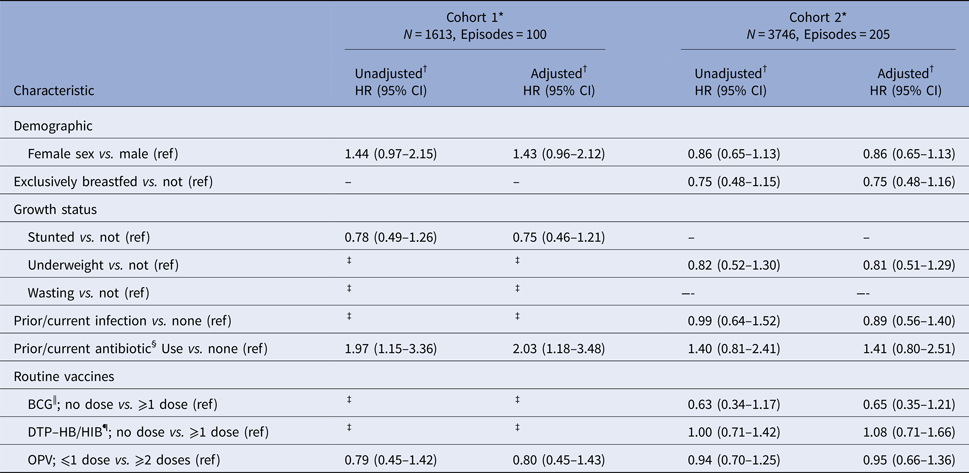
BCG, Bacillus Calmette–Guérin vaccine; DTP–HB/HIB, diphtheria–tetanus–pertussis–Hepatitis B and –Haemophilus influenza B vaccines; DTaP, diphtheria and tetanus toxoids and acellular pertussis vaccine; HB, Hepatitis B vaccine; OPV, oral polio vaccine.
* One infant in cohort 1 experienced an episode prior to 6 weeks of age and 6 infants from cohort 2 entered and exited the study before 6 weeks of age.
† Adjusted for country.
‡ Less than ten episodes in each strata.
§ Administered prior to enrolment.
‖ Excluding topical antibiotics.
¶ Or DTaP & HB, which were the standard vaccines given in Asian countries.
In cohort 1, 17% of the study population dropped out or were lost to follow-up prior to the end of the study. In cohort 2, 3.9% of participants discontinued follow-up. Additional information on loss to follow-up in cohort 1 can be found in Supplementary Table S2.
Discussion
We analysed data from the placebo arms of two large trials conducted in LMICs to describe the timing and predictors of first severe RVGE episodes. The cumulative incidence of first severe RVGE episodes was low at 6 months of age. Early antibiotic use was associated with an increase in the rate of first severe RVGE episodes in both cohorts.
The cumulative incidence of first severe RVGE episode increased slowly but steadily over the first 1–2 years of life with a low risk of rotavirus at 6 months of age. In cohort 1, almost all severe RVGE cases occurred in the first year of life. This could be due to fewer cases occurring in the second year of life or because of decreased surveillance of gastroenteritis in that period. By contrast, severe RVGE events occurred steadily over about the first 24 months of age in cohort 2. The differences in the original clinical trials may account for these and any other differences observed. For example, gastroenteritis and severity had slightly different definitions in each trial, and there were different mechanisms for capturing episodes of gastroenteritis (households vs. health facilities). In addition, the study sites and inclusion criteria were different for the two trials. Any of these factors may be a partial or complete cause of any differences observed between the two cohorts.
Although some studies have described age-specific rates of RVGE or the distribution of age of RVGE onset [Reference Espinoza18, Reference Fischer19, Reference Gladstone21, Reference Reves31], these studies have not differentiated episodes of severe and non-severe RVGE or first vs. subsequent episodes of RVGE. Therefore, due to different methods of presenting data, it is difficult to compare the cumulative incidence estimates to those from prior studies. However, in this study, we found did find the overall rates of severe RVGE in cohorts 1 and 2 were similar or slightly higher than previously reported estimates. A study in India reported the incidence of severe RVGE in the first year of life to be five events per 100 child-years [Reference Chandola32], while another study in Pakistan reported approximately two events per 100 child-years [Reference Qazi33].
Given the low risk of severe RVGE at 6 months of age, a delay of 4 weeks in the start of rotavirus vaccination could result in fewer episodes of severe RVGE if delaying vaccination improved the performance of the vaccine. Approximately 0.6–2.3% of unvaccinated infants experienced severe RVGE by 6 months of age. Therefore, the decision to delay the start of vaccination would depend on the level of improvement in vaccine effectiveness expected to occur with delayed vaccination, taking into account a potential small increase in risk before 6 months of age. Assessing timing of vaccination, the proportion of infants who would be successfully vaccinated under the proposed schedule and incidence of severe RVGE would be needed to understand the potential benefits and harms of delaying vaccinations.
In both cohorts, we found that antibiotic use early in life was associated with earlier first episodes of severe RVGE. To our knowledge, antibiotic use has not been reported to be associated with severe RVGE but has been linked to increased risk of diarrhoeal disease. Recent studies conducted in India found an increased risk of diarrhoeal disease in children receiving antibiotics at less than 6 months of age compared with those who did not [Reference Rogawski34], and a shorter time to the subsequent diarrhoeal episode when the first episode was treated with antibiotics [Reference Rogawski35]. Antibiotics reduce the diversity of the gut microbiota and can affect the early development of the infant microbiota [Reference Johnson and Versalovic36]. It is likely that microbial colonisation and diversification play a critical role in susceptibility to diarrhoeal diseases and possibly severe RVGE. Conversely, it is also possible that antibiotic use is an indication of children who are sicker and exposed to more pathogens and are therefore more likely to develop severe RVGE. We were unable to assess antibiotic use after enrolment, nor the indication, duration and type of antibiotics received at enrolment. Further analyses addressing these aspects would be helpful to understand these results. Although antibiotic use being associated with severe RVGE may have biological implications, this finding will not likely have broad public health significance in administration in the post-rotavirus vaccine era.
There are some limitations to this research. These data were collected as part of clinical trials. Therefore, the participants may not be generalisable to the broader study population of infants in each country, because trials typically have strict inclusion and exclusion criteria that do not always represent all the children who will be routinely vaccinated. However, it should be noted that there were few exclusion criteria for the RV5 trial, and it seems plausible that the study population would be relatively representative of all infants being vaccinated. In addition, the study inclusion and exclusion criteria for both cohorts 1 and 2 did not specifically prohibit children who received the placebo from being in the same household or neighbourhood as children who were vaccinated. If vaccinated children were nearby, there may have been potential for herd protection, which would decrease the number of unvaccinated (placebo) children with RVGE. As a result, the incidence of first episodes of severe RVGE in this study would not represent a completely unvaccinated cohort. Also, we were unable to estimate the rates and cumulative incidence of first severe RVGE episodes from birth, because the trial recruited children around 6 weeks of age. This means we have likely underestimated the incidence of first episodes of severe RVGE in early childhood by missing severe RVGE episodes occurring between birth and 6 weeks of age. Similarly, due to staggered enrolment by age, the earliest time period we had sufficient sample size to analyse the study populations was at 6 weeks of age. This resulted in the exclusion of one severe RVGE episode from the cumulative incidence estimates and predictor analysis because the episode occurred prior to 6 weeks of age in cohort 1. Early episodes would be essential to include when weighing the option of beginning vaccination prior to 6 weeks of age. However, most routine schedules begin vaccination at 6 weeks or later, which means episodes occurring prior to 6 weeks could not be prevented through regular or delayed vaccination. Limited covariate data were also collected, and as a result, we were restricted in our ability to identify predictors of first severe RVGE episodes. There are likely other unmeasured factors that predict the occurrence of first episodes of severe RVGE. For example, recent results from the Etiology, Risk Factors, and Interactions of Enteric Infections and Malnutrition and the Consequences for Child Health and Development (MAL-ED) multisite study found that overcrowding (>two persons per room) and high pathogen load were associated with RVGE. These factors may have also been important in our study, but data were not available to assess these risk factors. Finally, there appeared to be differential dropout for some covariates in cohort 1. This could have resulted in some factors not being identified as predictors because these factors were associated with early dropout.
There are also several strengths of this analysis. The large sample size of infants prospectively followed allowed us to analyse severe RVGE episodes, which is often unfeasible in small studies. With these data, we could analyse the timing and predictors of first severe RVGE episodes to understand the potential impact of delaying the start of rotavirus vaccine schedules. In addition, the study participants came from a broad geographic area representing several different LMICs including both urban and rural areas. Also, severe RVGE outcomes were wild-type PCR-confirmed episodes of RVGE. Finally, we analysed data focusing on the age of severe RVGE and used methods that allowed for late-entry into the study such that the time scale could be age instead of time since enrolment, which is more relevant for understanding the timing of severe RVGE episodes and the potential impact of altering vaccine schedules.
In conclusion, the cumulative incidence of first episodes of severe RVGE in both cohorts was low at 6 months of age, indicating a delay up to 4 weeks in the start of vaccination may not result in a large number of severe RVGE episodes occurring prior to vaccination. In addition, we found early antibiotic use was associated with an increase in the rate of first severe RVGE episodes. These data provide important insights regarding the epidemiology of rotavirus in LMICs in the pre-vaccine era that may inform the use of rotavirus vaccines in LMICs.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0950268818000626
Acknowledgements
Dr Gruber was supported by the University of North Carolina Graduate School Dissertation Completion Fellowship. Dr Becker-Dreps was supported by the National Institute of Allergy and Infectious Diseases at the National Institutes of Health (NIH) [grant number 1R56A1108515-01]. Dr Jonsson Funk receives investigator-initiated research funding and support as Principal Investigator from the National Heart Lung and Blood Institute at the NIH [grant no. R01 HL118255]; as a Co-Investigator from the NIH National Institute on Aging [grant no. R01 AG023178]; the NIH National Center for Advancing Translational Sciences [grant no. 1UL1TR001111]; and AstraZeneca. Dr Jonsson Funk receives salary support from the Center for Pharmacoepidemiology in the Department of Epidemiology, Gillings School of Global Public Health (current members: GlaxoSmithKline, UCB BioSciences, Merck). Dr Brookhart was supported by a grant from the National Institutes of Health [grant no. 1R21HD080214-01A1].
Declaration of Interest
Data for this manuscript were provided by GlaxoSmithKline (GSK), Rixensart, Belgium through a third party, ClinicalStudyDatarequest.com, and by Merck and Co., Inc., Kenilworth, NJ, USA. Neither company had involvement in this study design and analysis. Both Merck and Co., Inc., Kenilworth, NJ, USA and GSK, Rixensart, Belgium were given the opportunity to comment on this manuscript prior to publication. All decisions regarding the content of this manuscript were made by the authors. Dr Gruber was employed by Merck and Co., Inc., Kenilworth, NJ, USA as a graduate research assistant from January–December 2014 to work on research related to their rotavirus vaccine. Dr Jonsson Funk is a member of the Scientific Steering Committee (SSC) for a post-approval safety study of an unrelated drug class funded by GSK. All compensation for services provided on the SSC is invoiced by and paid to UNC Chapel Hill. Dr Jonsson Funk receives salary support from the Center for Pharmacoepidemiology in the Department of Epidemiology, Gillings School of Global Public Health (current members: GSK, UCB BioSciences, Merck and Co., Inc.). Dr Jonsson Funk does not accept personal compensation of any kind from any pharmaceutical company. Dr Brookhart has received investigator-initiated research funding from the NIH and through contracts with the AHRQ's DEcIDE program and the PCORI. Within the past three years, he has received research support from Amgen and AstraZeneca and has served as a scientific advisor for Amgen, Merck and Co., Inc., GSK, UCB BioSciences, and RxAnte. Within the past three years, Dr Brookhart has received research support from Amgen and AstraZeneca and has served as a scientific advisor for Amgen, Merck, GSK, Genentech, TargetPharma, and RxAnte. Dr Brookhart owns equity in NoviSci, LLC, a data sciences company.
Ethical Standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.








